Abstract
The risk of Helicobacter pylori infection is highest in childhood, but the colonization process of the stomach mucosa is poorly understood. We used anesthetized Mongolian gerbils to study the initial stages of H. pylori colonization. Prandial and postprandial gastric conditions characteristic of humans of different ages were simulated. The fraction of bacteria that reached the deep mucus layer varied strongly with the modelled postprandial conditions. Colonization success was weak with fast gastric reacidification typical of adults. The efficiency of deep mucus entry was also low with a slow pH decrease as seen in pH profiles simulating the situation in babies. Initial colonization was most efficient under conditions simulating the postprandial reacidification and pepsin activation profiles in young children. In conclusion, initial H. pylori colonization depends on age-related gastric physiology, providing evidence from an in vivo infection model that suggests an explanation why the bacterium is predominantly acquired in early childhood.
More than half of the world population become colonized by the Gram-negative bacterium Helicobacter pylori and remain infected for most of their life time1. Infection with H. pylori causes chronic gastritis2 which can give rise to peptic ulcers, gastric adenocarcinoma, or gastric lymphoma of the mucosa-associated lymphoid tissue3,4,5. H. pylori is thus one of the most important human pathogens and responsible for at least one half of a million deaths per year6,7. Efforts to control H. pylori infection are hampered by the lack of a vaccine as well as by gaps in our knowledge about its transmission. Several studies have shown that the infection is commonly acquired in childhood, before the age of five, both in developing as well as in developed countries8,9,10 (for a review see11,12). These data suggest that the transmission process is favoured by a stomach environment that exists during childhood over that prevailing later in adult life when the acquisition of H. pylori is relatively rare. However, reasons why children appear to incur a greater risk to acquire H. pylori than adults are unknown.
Studies in different animal models have demonstrated that H. pylori depends on flagellar motility to achieve colonization13 of the gastric mucus layer adjacent to the epithelium (juxtamucosal mucus), the physiological habitat of the pathogen14,15. The bacteria use a pH gradient for spatial orientation in the gastric mucus in vivo15. Motility of H. pylori is strongly inhibited by low gastric luminal pH and resulting high activities of pepsins in vivo16,17. Highly acidic conditions such as those usually prevailing between meals rapidly immobilize H. pylori, which would most likely provide a strong barrier to colonization. We therefore hypothesized at the outset of this study that H. pylori infection might chiefly occur either during food intake or in the postprandial period, immediately following a meal. During these periods, the pH in the gastric lumen is higher than under fasting conditions, in part due to the buffering capacity of the meal18,19. If an H. pylori infection occurred in the prandial or postprandial period, this might also account for the differences in susceptibility to H. pylori infection between adults and children, since age groups have been reported to differ markedly with respect to their pH profiles between and during food intake18,19,20,21. In adults and older children, food intake leads only to a partial neutralization of the acidic pH in the stomach lumen, and food then triggers a strong increase in gastric acid secretion which quickly reacidifies the gastric lumen18,19, followed by a strong activation of antibacterial pepsin. In comparison, in young children who have eaten a milk-based meal and in milk-fed babies, the gastric lumen pH was shown to reach close to neutral values during the meal, and the subsequent process of luminal reacidification is much slower than in adults20,21. This slower reacidification process also may have the consequence that the concentration of active pepsin in the gastric lumen remains relatively low for an extended period of time20,21.
We have now used a novel experimental approach to test the hypothesis that conditions in the stomach during the transmission process may affect the likelihood that ingested H. pylori bacteria reach the mucus layer of the stomach close to the mucosa, which is thought to be a pivotal first step during the colonization process. In our experimental model in anesthetized Mongolian gerbils, the intragastric conditions were tightly controlled in order to reflect the projected spatiotemporal changes of gastric physiology during and after a meal. This process was modelled in three different ways, to simulate the conditions extrapolated from physiological studies for humans of three different age groups (baby, young child and adult). During this physiological simulation, live H. pylori was added into the stomach lumen as to model the first minutes of natural entry of H. pylori into the stomach. After the application of a suspension containing H. pylori to the gerbil stomach, acid or base was added to the gastric lumen using an autotitrator, leaving the composition of the gastric juice otherwise unaltered. The success of H. pylori to reach its proposed natural ecological niche in the depth of the mucus layer15 during the simulated meals was measured by counting the bacterial numbers using digital microscopy in the anesthetized animal. Motile and non-motile H. pylori in the gastric lumen and at the mucosa as well as pepsin activity during the simulated changes was determined. In parallel, experiments in explanted gastric mucosae of guinea pigs were performed in order to determine the pH changes in the mucus layers closer to the gastric lumen. Using pH-sensitive ultrafine microelectrodes that were able to measure pH at the level of the mucosal cells, the pH at different depths of the gastric mucus layer was measured simultaneously to the titrated luminal pH profiles. The data show that the proportion of H. pylori cells that reach the deep mucus layer varies strongly between the different pH profiles. Our study suggests that dynamic pH conditions in the stomach may have a strong effect on the numbers of H. pylori actively reaching the lower mucus layers, and provides a biologically plausible model and a possible explanation for the different susceptibilities of children as compared to adults for the initial colonization with H. pylori.
Results
A model to study the first minutes of H. pylori colonization in anesthetized Mongolian gerbils
The process how H. pylori reaches the deep gastric mucus layer during the initial phase of colonization, and how gastric conditions such as the luminal pH value influence this process are poorly understood. We developed an experimental model that permits to study this process in anesthetized Mongolian gerbils under tightly controlled gastric conditions. The experiments were designed to test the central hypothesis that temporal changes of the luminal pH might affect the ability of the bacteria to enter the mucus and to reach the deep mucus layer where they multiply during a chronic infection.
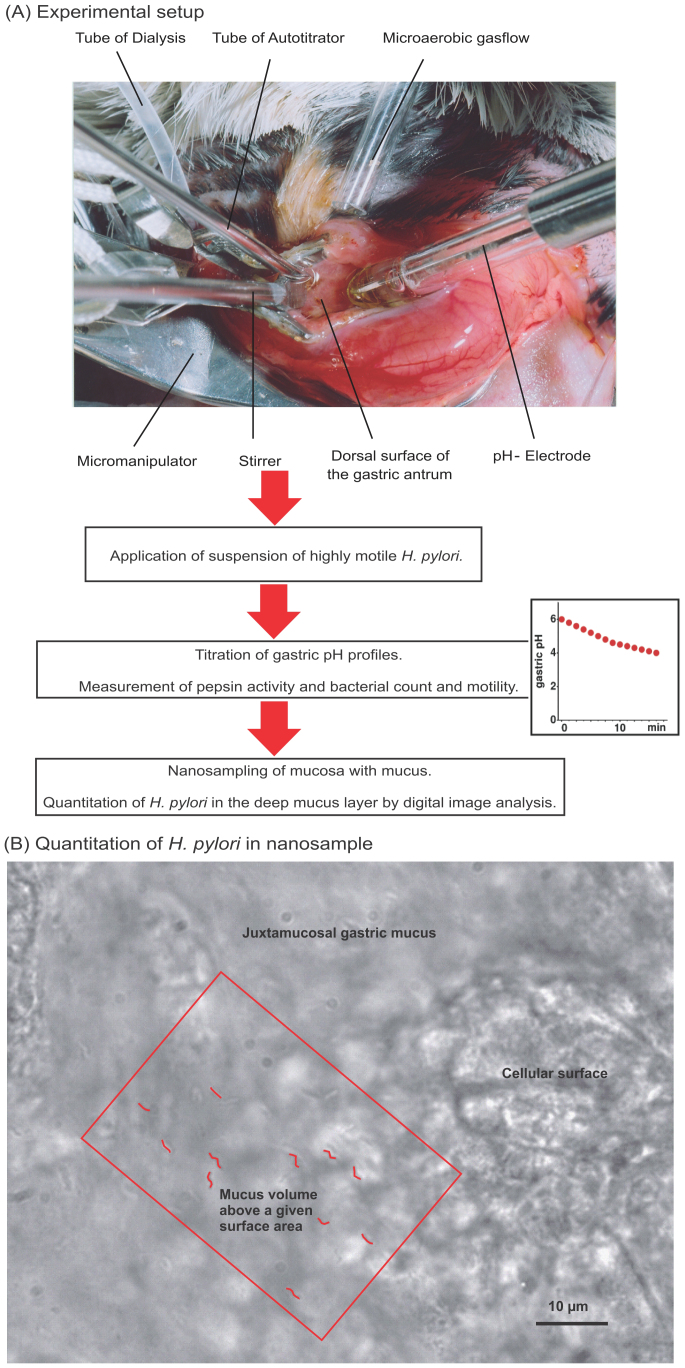
For each experiment, the ventral body wall of an anesthetized gerbil was opened microsurgically and the stomach was lifted onto a micromanipulator. A small incision was made into the stomach wall, the gastric chymus was partially removed, and a microaerobic atmosphere was generated by an appropriate gas flow over the opened stomach. A standardized inoculum of highly motile H. pylori (strain SS1) was then added to the gastric juice (Fig. 1, see Materials and Methods for a detailed description of the experimental setup and Supplemental Fig. S1). Immediately after addition of the bacteria, the pH in the lumen was set to a defined start value by addition of acid or base using an autotitrator, and then changed progressively in order to simulate temporal pH profiles in the human gastric lumen during or after a meal. These pH profiles were modelled to simulate the prandial and postprandial conditions extrapolated from physiological studies for humans of three different age groups (baby, young child and adult). After completion of the pH profile titration, the numbers of H. pylori bacteria in the deep mucus layer close to the gastric epithelium were determined by digital microscopy, and a “colonization rate” was calculated as the ratio between the luminal numbers of bacteria at the onset of the experiment and the bacterial numbers close to the epithelium after completion of the pH profile simulation (Fig. 1).
Figure 1. Flow diagram of the key steps of the Mongolian gerbil in vivo model system.
(A) Photograph of opened gerbil stomach and setup for the simulation of prandial and postprandial conditions. Luminal gastric conditions can be manipulated through a small incision within the wall of the Mongolian gerbil stomach. A micro-pH-electrode measured the luminal pH value continuously, while the small stirrer ensured efficient mixing of the gastric content. Defined pH profiles were generated by an autotitrator, which applies 0.25 M HCl and 100 mM urea or 0.15 M NaOH. A gas flow of 5% O2, 10% CO2 and 85% N2 ensured a microaerobic atmosphere. 50 µl bacterial suspension was applied into the gastric lumen, which contained approx. 0.5 ml of gastric juice. Each animal received the same number of highly motile Helicobacter pylori. After simulation of defined pH profiles representing different gastric conditions of certain age groups, bacterial numbers in the mucosa can be sampled by the nanosampling method. (B) Micrograph of H. pylori in the juxtamucosal mucus layer. Microscopical detection of H. pylori in the mucus volume above a given surface area. The volume of gastric juice in the lumen above the gastric surface in the setup constellation is known. Spatial distribution of H. pylori can be identified in the mucus layer near the epithelium. Colonized H. pylori cells were coloured red (see also supplemental Figure S2 (B) for the original image).
H. pylori mucus entry under gastric conditions simulating the phase of food intake
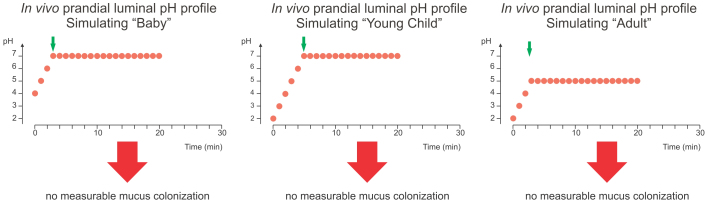
The first series of measurements was designed to simulate the conditions in the stomach during the intake of food – the prandial phase. Three different luminal pH profiles were designed to model the extrapolated gastric conditions during a meal for breast-milk fed babies (Fig. 2, left panel), young children up to schoolchildren after a milk-based meal (Fig. 2, middle panel), and adults (Fig. 2, right panel). Although a large bacterial inoculum (>2 × 108 motile H. pylori) was used in each gerbil stomach, we detected no bacteria in the mucus layer close to the epithelium at the end of the experiment under all three conditions (detection limit: 1 bacterium per 20 picoliter of mucus). This suggested that H. pylori colonization may not be possible under these conditions simulating the stomach during food intake (n = 4 animals per profile, 5 – 10 nanosamples per animal -in all experiments-).
Figure 2. In vivo colonization under simulated prandial gastric conditions.
The three panels show the luminal pH profiles (red bullets) that were titrated to simulate the prandial phase in breast-fed babies (left), young children (middle), and adults (right). The H. pylori inoculum is given during the simulation of the meal, as administration at the beginning of the profile would lead to rapid loss of bacterial motility due to the acidic fasting pH in profiles A and C. In profile B, the pH is remaining at 4 between the meals. The time points when an inoculum of 108 motile H. pylori was added in the in vivo experiments in the Mongolian gerbil are marked by green arrows.
H. pylori gastric mucus entry under conditions simulating the phase after food intake
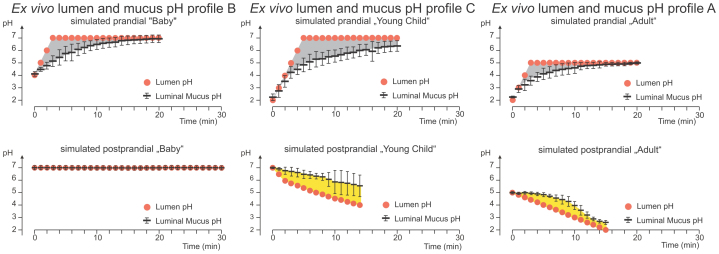
We subsequently used a similar experimental setting to model and investigate H. pylori colonization in the phase directly following food intake (postprandial phase). Here, also three different model conditions were used that we designed to reflect different age groups (see above). The pH profiles after a meal have been reported to vary strongly with age18,19,20,21. We performed an extensive review of the available literature on pH measurements in the human stomach after food intake. Much of this literature dates from the early 20th century, so that the H. pylori status of the tested individuals is not available. A summary of this review with a full list of references is provided as Supplemental Material. Based on the published measurements, we designed three different pH profiles that simulate salient features of pH profiles of the different age groups. Each of the respective pH profiles was simulated for 15 or 30 minutes, and pepsin activity in the gastric lumen and the number of motile H. pylori in the luminal fluid were measured during this period at regular intervals. The initial infectious dose used was 2.1 ± 0.8 × 108 motile H. pylori / 0.5 ml luminal stomach juice (427 ± 159 motile H. pylori per nl of lumen content; n = 20 animals). Figure 3 shows microscopic samples of the gastric lumen from the beginning of the experiment, and the numbers of H. pylori close to the mucus at the end of the experiment.
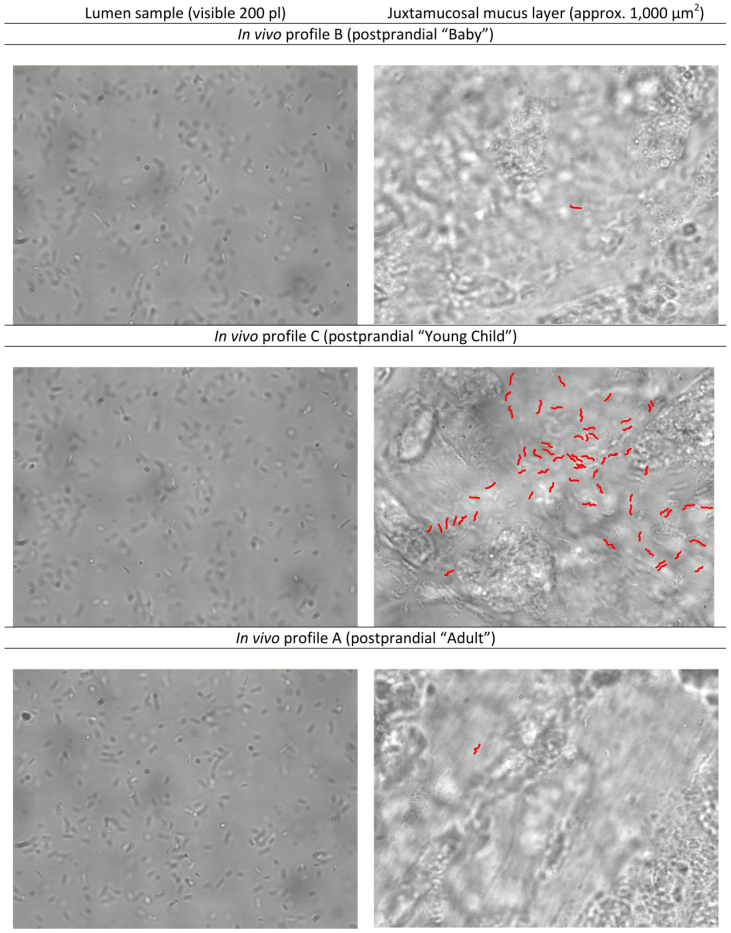
Figure 3. Luminal and juxtamucosal densities of H. pylori after application of three age-dependent postprandial gastric pH profiles.
Left panels: Luminal samples analyzed in a heated chamber. The visible volume is 200 picoliters. Right panels: Nanosamples from the anesthetized gerbil, with different numbers of H. pylori that have entered the juxtamucosal mucus. The profile simulating the postprandial gastric conditions in young children causes a remarkably higher colonization. To improve contrast and readability, all sharply visible bacteria (± 2,5 µm from focus plane) were coloured in red. A composite with the original (uncoloured) micrographs is included as supplemental figure S2 (A). Five nanosamples of juxtamucosal mucus were averaged to determine the colonization density of H. pylori in each animal.
H. pylori mucus entry under conditions simulating the postprandial phase in babies (profile B)
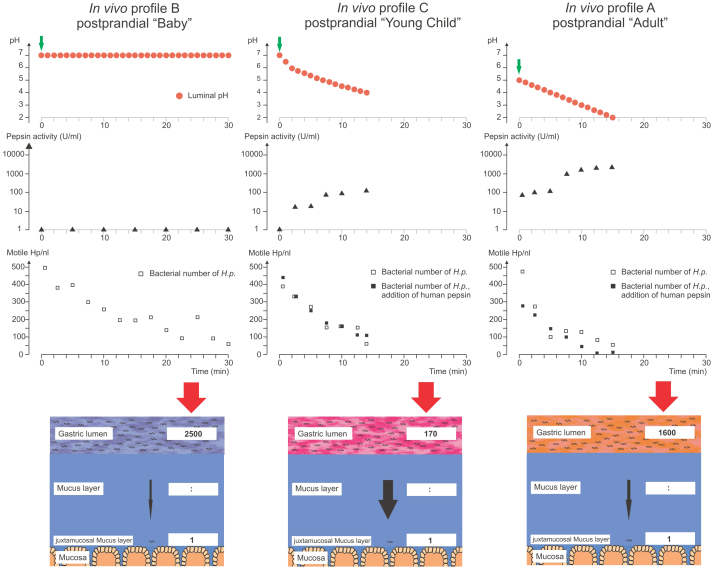
The pH profile of the milk-fed baby after a meal is supposed to consist of a longer period of a neutral pH and subsequent slow pH decrease20,21. During these conditions, pepsins are thought to remain largely inactive as a result of the neutral pH value. To simulate these conditions in the gerbil, the chymus was kept at a pH of 7.0 over 30 minutes. As expected at neutral pH and in the absence of pepsin activity16,17, the bacteria only slowly lost motility in the gastric lumen during the experiment (Fig. 4, profile B). However, in spite of the conditions which apparently preserved viability and motility of H. pylori, bacterial numbers in the mucus layer close to the epithelium at the end of this simulation were very low. On average, only one out of 2,500 motile bacteria present in the lumen at the start of the experiment had reached the juxtamucosal mucus layer at the end of the profile (colonization ratio; mean ± SD: 2,500∶1 ± 3,200∶1: range: 790∶1 to 11,000∶1; n = 8 animals).
Figure 4. Postprandial gastric conditions strongly affect H. pylori colonization.
The figure shows the effects of three different postprandial pH profiles on pepsin activity, H. pylori motility, and H. pylori mucus entry in anesthetized Mongolian gerbils. pH profiles simulate postprandial conditions in breast-fed babies (profile B, left column), young children (profile C, middle column), and adults (profile A, right column). The three top panels show the titrated profiles (red bullets). The time points of H. pylori inoculation are marked by green arrows. The next two rows of graphs depict pepsin activity in logarithmic scale (triangles) and the number of motile H. pylori during the in vivo experiment (Mongolian gerbil), open squares indicate values measured during experiments performed without addition of exogenous pepsin (i.e. the gastric juice contains native gerbil pepsin only), black squares are values determined in experiments where human pepsin was added. The values depicted here are mean values of at least three independent experiments for each experimental group. The lowest panel visualizes the number of motile H. pylori in the lumen required for one bacterium to reach the juxtamucosal mucus.
H. pylori mucus entry under stomach conditions simulating the postprandial phase in young children (profile C)
A different titration protocol was performed to simulate the postprandial pH profile in young children in the age range between 1 year and school age, after drinking milk or eating a milk-based meal (profile C). Milk or milk-based meals initially buffer the gastric content of young children to pH 6 to 720,21. The milk clots immediately, and the pH decreases22. This projected fast pH decrease is thought to stop above a pH of around 423,24. To simulate these projected conditions, we started the profile at pH = 7.0, titrated within 2 min to a pH of 6.0, then within 5 min to a pH of 5.0, and subsequently within 7 min to a pH of 4.0.
At the beginning of this simulation, comparable to profile B, a high number of bacteria were found to remain motile in the lumen, however, within 15 minutes, more than 85% of the bacteria lost motility (initial bacterial numbers; mean ± SD: 378 ± 61 motile H. pylori / nl versus 60 ± 66 motile H. pylori / nl after 15 min, P<0.001, n = 5, Fig. 4). As a result of the only moderately acidic luminal pH, the activity of pepsins was weak (~100 U/ml at pH 4; Fig. 4). The bacterial numbers in the deep mucus layer at the end of the profile C simulation were much higher as compared with profile B. One out of 170 H. pylori applied to the gastric lumen was able to reach its main physiological habitat14,15, the deepest 25 µm of the mucus layer, adjacent to the tissue surface. This represents an almost 10-fold increase in bacterial numbers in the lower mucus layers as compared to profile B (colonization ratio; mean ± SD: 170∶1 ± 80∶1; range: 100∶1 to 330∶1; P <0.05; n = 10 animals).
H. pylori mucus entry under gastric conditions simulating the postprandial phase in adults (profile A)
The third profile was designed to model postprandial conditions in healthy adults (profile A). In adults, food intake has been reported to cause a rise of pH to ~5, but did not lead to complete pH neutralization18,19. This change was rapidly followed by the quick reacidification of the gastric lumen18,19. To simulate this, a titration from a pH of 5 to 2 in the gastric lumen was performed over a period of 15 minutes. Starting from a pH of 5, the pH values of pH 4.0, 3.0, and 2.0 were reached at 5, 10, and 15 minutes. With the observed rapid acidification, pepsin activity is predicted to rise already a few minutes after the simulated meal. The number of motile bacteria in the lumen decreased rapidly during this titration (with 294 ± 75 motile H. pylori / nl after only 2.5 min versus initial motility of 467 ± 30 H. pylori / nl; P<0.05, n = 3), which corroborated data from earlier studies16. The number of H. pylori counted in the juxtamucosal mucus layer under these conditions was constantly low. The colonization ratio for profile A was 1,600∶1 ± 1,200∶1 (range 390∶1 – 3,700∶1; n = 5 animals), significantly different (P<0.01) from postprandial profile C. In contrast, the difference between postprandial profiles A and B was not significant (Fig. 4 and Table 1).
Table 1. Entry of H. pylori into the deep mucus layer (juxtamucosal mucus) under three simulated postprandial conditions.
| Initial luminal bacterial number | Initial luminal bacterial number above 10,000 µm2 tissue (50 nl volume) | Number of H. pylori in juxtamucosal mucus above 10,000 µm2 tissue | Colonization ratio | ||
|---|---|---|---|---|---|
| motile H. pylori / nl | motile H. pylori / 10,000 µm2 | juxtamuscosal H. pylori / 10,000 µm2 | luminal H. pylori : juxtamucosal H. pylori | ||
| Gastric conditions | Animals | mean ± SD from all samples | mean ± SD all samples from each animal accumulated | ||
| profile B (“baby”) | n = 8 | 466 ± 198 | 23,281 ± 11,038 | 16 ± 8 | 2,500∶1 ± 3,200∶1 |
| profile C (“child / toddler”) | n = 10 | 393 ± 136 | 19,655 ± 6,822 | 128 ± 54 | 170∶1 ± 80∶1 |
| profile A (adult) | n = 5 | 475 ± 71 | 23,730 ± 3,559 | 27 ± 20 | 1,600∶1 ± 1,200∶1 |
| Significance | No statistically significant difference between all samples | C vs.B P = 0.0001 C vs.A P = 0.016 B vs.A n.s. | C vs.B P = 0.034 C vs.A P = 0.0036 B vs.A n.s. | ||
Note. The bacterial counts are expressed as mean ± SD across all samples. Mean ± SD of colonization rate was calculated from all samples of each experiment/ animal accumulated, in order to compare animals. Therefore the ratio of the average of displayed bacterial counts can differ from the average of all colonization rates for mathematical reasons.
Taken together, the data from the three different profiles simulating postprandial conditions suggested that the numbers of H. pylori bacteria that actively reach the deep mucus layers varied strongly with the conditions in the stomach (Table 1).
Activity and antibacterial effects of human and gerbil pepsin against H. pylori are similar under model conditions
The gastric protease pepsin has antibacterial activity16,17. Consequently, the outcome of the gerbil model experiments might depend on the fact that the susceptibility of the human pathogen H. pylori to gerbil pepsin could differ from its susceptibility to human pepsin. A separate set of experiments was therefore performed for the profiles C and A in which a mixture of human pepsins A and C was added to the lumen liquid. The data obtained from this set of experiments are shown in Fig. 4 and in more detail in Table S1. The addition of human pepsins had no significant effects on the experimental outcome (decrease of numbers of motile H. pylori in the lumen; colonization ratio) as described above for experiments performed without addition of human pepsin.
Effect of luminal pH changes on the pH values within the mucus layer
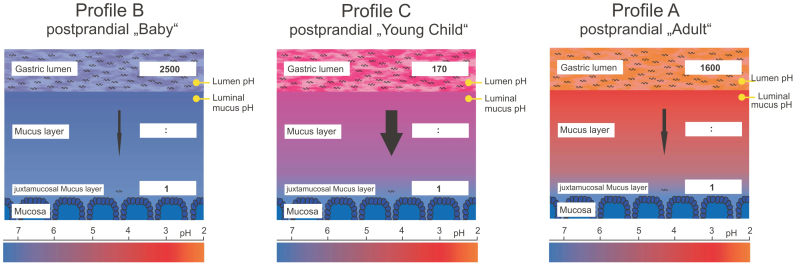
H. pylori has been shown to rely on a pH gradient across the mucus for spatial orientation25. In order to generate hypotheses why the luminal pH profile had such a strong effect on the numbers of H. pylori reaching the lower mucus layers, it was of interest to measure the pH within the mucus under these model conditions. pH measurements within the mucus layer require the use of ultrafine double-barrelled microelectrodes, whose tips can be positioned to precisely defined locations within the mucus layer25. Due to technical obstacles, these measurements cannot be performed simultaneously with the in vivo experiments. They also could not be performed with gerbil mucosa at all (see Methods for a technical explanation). In order to still be able to obtain measurements from within the mucus under the simulation conditions in the lumen, we used explanted gastric mucosa of the guinea pig, which is a highly standardized and well-documented model system for the study of gastric mucosal physiology25. We exposed four explanted guinea pig mucosae per profile to the same luminal pH changes as used for the in vivo experiments and measured the resulting pH values within the mucus at a depth of 20–40 µm below the luminal mucus surface. The measurements are shown in Figure 5 (simulations of prandial and postprandial phase). The data demonstrated that the pH in the mucus layer was more acidic than the pH in the lumen during the simulations of conditions during food intake, regardless of which of the three profiles was applied. Under the conditions simulating the situation after a meal, clear differences were measured for the three model profiles. Lumen and mucus pH both remained neutral during profile B. For profiles A, and C, the within-mucus pH decreased more slowly than the luminal pH, so that a pH gradient was detected between the lumen and the interior of the mucus. The differences between lumen and mucus pH were distinct for the profiles A and C. For profile C, the pH difference between lumen and mucus measurement appeared to be most pronounced. Figure 6 shows a model of colonization under the three different simulated conditions, combining the results of the different experimental settings used in this study.
Figure 5. Luminal pH profiles and the pH values within the mucus layer.
The figure shows the effects of three different prandial and postprandial pH profiles on mucus pH as measured by ultrafine tipped double-barrelled microelectrodes in explanted guinea pig mucosa. pH profiles simulate prandial (top panel) and postprandial (bottom panel) conditions in breast-fed babies (left column), young children (middle column), and adults (right column). Black horizontal lines represent measurements of the resulting pH within the mucus layer (mean ± SD, n = 3 each) in the antral guinea pig mucosa. The pH difference between lumen and mucus is indicated by grey shading following prandial pH profiles. Under all three prandial profiles, the mucus pH was more acidic than the lumen pH. The three bottom panels show the titrated postprandial profiles and the resulting mucus pH values following postprandial profiles (B, C, A). The yellow-shaded area represents the difference between lumen pH and the pH in the mucus. Note that, by contrast to the prandial conditions, the pH is lower in the lumen than in the mucus in postprandial profiles A and C.
Figure 6. Schematical model summarizing the results from both experimental setups.
Depicted is the pH gradient within the mucus layer and the luminal pH by coloration gradient (as measured in guinea pig experiments), as well as the colonization ratio following postprandial conditions (from Mongolian gerbil experiments).
Discussion
Except for the few documented cases of experimental infections of volunteers26,27,28, acute H. pylori infections are almost never diagnosed. This is one of the major reasons, why the conditions under which H. pylori achieves efficient colonization after transmission are unknown. Likewise, it is not known why transmission of H. pylori infection to children is apparently more efficient than transmission to adults.
In this study, we have used state–of-the-art in vivo measurement and systems manipulation technology to study how conditions in the gastric lumen affect the entry of H. pylori into the mucus, and thus to model the initial phase of H. pylori infection that has until now not been amenable to systematic analysis. The Mongolian gerbil is widely used as an experimental model for H. pylori infection of humans due to similarity in gastric physiology, pathological outcome of infection and pharmacology of eradication therapy29,30. The animal model used here is, however, very different from classical H. pylori infection models in gerbils or mice31,32. While the latter models permit the study of the complete infection cycle over several months, including pathological sequelae, the possibilities to exactly control experimental parameters (such as, in our case, the luminal pH) and to directly observe the behaviour of microbes in the host environment are limited. The central experimental approach of this study was to generate defined pH profiles in the gastric lumen of anesthetized Mongolian gerbils that were designed to simulate the temporal changes of pH in the human stomach during and after food intake. The impact of these different pH profiles on the efficiency of H. pylori mucus entry could then be measured with high reproducibility.
As a first important result of our experiments, H. pylori only entered the mucus when there was a pH gradient between a more acidic lumen and a less acidic mucus layer. H. pylori did not (prandial profiles) or very inefficiently (postprandial profile B) enter the deep gastric mucus layer under conditions where the lumen was less acidic than the mucus (prandial) or had approximately the same pH as the mucus (postprandial profile B). We propose that in the absence of a suitable pH gradient between lumen and mucus layer, the H. pylori orientation system is unable to guide the swimming bacteria towards the deeper mucus layers. Only a very small percentage of bacteria moving about randomly may enter the mucus gel serendipitously under those conditions, but are probably unable to find the deeper mucus layers against the continuous mucus flow and turnover in order to establish a stable infection. This observation is consistent with our previous results that the orientation of H. pylori in the stomach mucus depends on a transmucus pH gradient, which guides the bacteria within the mucus to zones where the pH is near neutral15. Recently, two different behavioural sensors of H. pylori, the proposed pH sensor TlpB33 and TlpD as part of the energy sensing system34 were identified in vitro which could be responsible for tactic behaviour of the bacteria away from acidic and energetically unfavourable conditions.
Mucus entry was also strongly impaired in our model, when the initial luminal pH was very low (postprandial profile A, which started at pH 5 and quickly acidified further). The most likely explanation is that acid- and pepsin-mediated loss of bacterial motility occurred too quickly and the bacteria thus become immobilized and trapped17. This explanation is consistent with our previous results showing that H. pylori loses its motility in less than one minute at pH values of 2 and 3, and in less than 2 minutes at pH 416. Even at pH 5, less than 20% of the bacteria were still motile after 2 minutes. At pH 6, the period of time during which most bacteria stayed motile increased to 15 minutes. To further test the hypothesis that the acidic starting pH and the thereby activated pepsins were responsible for the low efficiency of mucus entry, we tested an additional profile that started at pH 7 and decreased to pH 3. Under these conditions, H. pylori could enter the deeper layers of the gastric mucus (data not shown).
By contrast, among the conditions tested, H. pylori was best able to enter the mucus layer when we simulated the pH profile during the postprandial phase in young children, which is characterized by a moderate food-induced increase of pH and relatively slow reacidification. Under these conditions, the change over time of luminal pH generated a pH difference between stomach lumen and mucus, with the lumen being more acidic than the mucus.
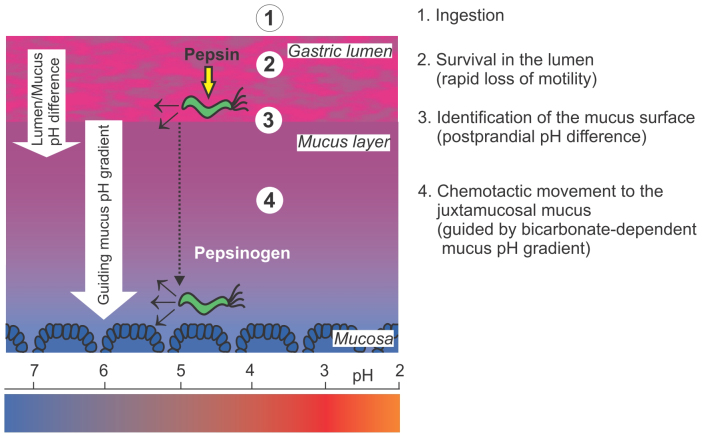
Figure 7 schematically illustrates the proposed steps required for a successful Helicobacter pylori mucus entry, the crucial initial step of bacterial transmission and colonization.
Figure 7. A hypothetical model of the steps required for a successful Helicobacter pylori colonization of the stomach.
-1- Oral ingestion -2- H. pylori has to remain motile in the gastric lumen, where active pepsins, particularly pepsin C, can cause a rapid loss of motility and could therefore prevent an acute colonization16,17.-3- As deduced from the presented experimental series, a postprandial pH difference between lumen and mucus caused by a dynamic decrease of the gastric lumen pH might be essential for H. pylori to be guided toward the mucus surface for colonization. -4- In the gastric mucus, chemotactic movement guided by the bicarbonate-dependent mucus pH-gradient appeared to be one condition required for H. pylori orientation to reach the mucus adjacent to the tissue (juxtamucosal mucus)15. Within this deep mucus layer, the secreted pepsinogen is not activated and the surrounding pH is less acidic.
Our data suggest that the acidic lumen and mucus lining of the stomach represent a formidable barrier, even to H. pylori, a bacterium that has coevolved with humans for at least 50,000 years35. Even under the most favourable of the conditions tested, less than one percent of bacteria in the lumen succeeded in entering the juxtamucosal mucus layer. With a single infectious dose below 107 bacteria, measurable bacterial counts in the deep mucus layer could not be achieved under any condition (data not shown). On the other hand, none of the postprandial conditions completely blocked mucus entry, and the 10–15 fold differences of colonization efficiency could in reality be either compensated by a high infectious dose, or by conditions affecting acid secretion (e.g. viral infections). It has to be noted that adults do get infected, but apparently with far lower frequency36,37. Gastric acidity in adults can be neutralized and reacidification be slowed down by administration of antacids or other medication38,39. In fact, pH profiles of adults after antacid or proton pump inhibitor intake strongly resemble our profile C39. Diminished activation of pepsins under conditions of reduced acid secretion might increase the risk of H. pylori acquisition in persons taking such inhibitors.
Due to the high technical requirements of our experimental approach, only a small number of pH profiles could be evaluated in this study. While acidity profiles in the human stomach can vary extensively with the buffer capacity of the food, the three profiles tested were carefully chosen to be representative of conditions observed in the three age groups. In particular, it is rare that the gastric pH rises above 6 in a healthy adult, even after a meal containing strongly buffering components39. Likewise, pH profiles showing complete neutralization of acid and slow reacidification after the meal as modelled in our profile C are well documented for young children after weaning who receive meals based on cow’s milk40.
In conclusion, our data suggest that temporal changes of pH have a very strong influence on the likelihood that ingested H. pylori bacteria enter the deep layer of the gastric mucus and identified features of a pH profile associated with a higher risk of H. pylori infection. These findings could provide a biologically plausible explanation for the epidemiological observations8,9,12 that young children between one year and the age of young schoolchildren appear to have the highest risk of acquiring H. pylori. The acquisition may occur after the ingestion of meals with high buffering capacity and an intrinsic pH value of 6–7, such as milk. Rowland et al. have shown that delayed weaning from the feeding bottle and consumption of more than two milk formula bottles per day in toddlers of 24–48 months significantly increased the risk of H. pylori acquisition10. Together with having an H. pylori-infected mother or sibling, these habits were identified as critical risk factors favouring the acquisition of H. pylori10 (see also supplemental Fig. S3). Additional studies in animal models, epidemiological studies, and possibly interventional trials might all contribute to a better understanding of the situations that are associated with a high risk of H. pylori transmission and ultimately lead to preventive strategies.
Methods
Helicobacter pylori culture
H. pylori strain SS1 was cultured as described15, until the bacterial suspension had reached a density of approx. 109 bacteria/ml. The bacterial motility was tested by microscopy of wet mounts for 20 min at pH 5. The bacteria were only used for the experiments if more than 70% of H. pylori remained motile.
Anaesthesia, operation, peritoneal dialysis
Male Mongolian gerbils (Meriones unguiculatus) of ~65 g (8–10 weeks of age) were anesthetized as previously described15,16. Subsequently, the ventral body wall was opened microsurgically and the stomach was lifted onto a micromanipulator. A peritoneal perfusion worked as a dialysis with a slightly hyposmolar solution to ensure a stable blood supply to the gastrointestinal tract. A small incision was made into the stomach wall using a radiofrequency electrocauterizer. Without touching the mucosal surface, a portion of gastric chymus was extracted. The amount of gerbil pepsin in the extracted volume was measured, and in an additional series of experiments it was substituted with 2000 U/ml (pH 2) of human pepsins A and C (in a ratio of 5 ∶ 1).
To maintain a microaerobic atmosphere, a gas flow of 5% O2, 10% CO2 and 85% N2 was applied over the opened stomach.
To simulate the luminal pH changes during and after meals for three different human age groups (baby, young child and adult), acid or base were instilled into the lumen with the help of an autotitrator.
Setup for postprandial profiles
A total volume of 50 µl of the bacterial suspension (> 108 Helicobacter pylori) was inserted into the lumen of the stomach. A pH microelectrode, the tube of an autotitrator, and a small stirrer were mounted dipping into the gastric lumen (Fig. 1). The pH electrode measured the pH value continuously, while the autotitrator was used to administer the defined pH profiles by applying appropriate amounts of 0.25 M HCl and 100 mM urea or 0.15 M NaOH. The stirrer ensured efficient mixing of the gastric content. The resulting concentration of urea in the gastric lumen was approx. 10 mM.
Experimental protocol
In preliminary tests, the average time period in which more than 90% of the luminal bacteria lose motility in the gastric lumen was ascertained for each of the three different pH profiles. Loss of motility occurred after 30 min with the pH profile simulating postprandial conditions in the baby, and after 15 min with the profiles simulating postprandial conditions in young children and adults. As motile bacteria are indispensable for mucus entry or colonization, the pH profiles were limited to 30 or 15 minutes, respectively.
Bacterial motility in the lumen
During the titration of each pH profile, directly after insertion of the bacteria and after every 2.5 min, samples of 2 µl were taken to measure the bacterial motility. Motility was expressed as the ratio of the number of motile bacteria out of the total bacteria counted in a 37°C heated chamber under the microscope.
Determination of mucosal bacterial numbers
After the pH profile was completed, nanosamples of the gastric mucosa together with gastric mucus were taken from the antrum region as previously described15. Briefly, a specific device working with a piezomotor-driven high acceleration pipette is used to aspirate small volumes of mucus and mucosa in the correct spatial orientation, the pipette is then withdrawn from the mucus and the collected sample is expelled, immediately cooled to 4°C and analyzed for the presence of H. pylori in the gastric mucus of the anesthetized animal using digital microscopic imaging15. Digital microscopic images were analyzed for colonizing H. pylori and the volume of the sample was calculated by the dhs evaluation software (dhs, Dietermann & Heuser Solution GmbH, Greifenstein-Beilstein, Germany). The detection limit was one bacterium per 20 picoliter mucus. The data were used to calculate the number of H. pylori within the mucus above a tissue surface area of 100 µm × 100 µm (10,000 µm2). In the experimental setting, we found a mean volume of 100 nl gastric juice above 10,000 µm2 mucosal surface. The ratio of the bacterial densities in 100 nl gastric juice and in the corresponding juxtamucosal mucus volume above the given surface, was used to quantify the number of successfully colonizing H. pylori resulting from a defined pH profile in the gastric lumen.
Lumen pH and luminal mucus pH in the explanted gastric mucosa of the guinea pig
In another setup, we performed pH measurements using ultrafine double-barrelled microelectrodes in an explant of the gastric mucosa of the guinea pig to analyze the effects of the defined luminal pH profiles on the pH value in the luminal side of the mucus layer (for a detailed description see24). In a small series of preliminary experiments, using the mucosae of gerbils, we found that the essential technique of “mucosal stripping” causes small damages and therefore, we decided to use the well-established model of the explanted guinea pig mucosa. The gastric mucosa was fixed in a chamber consisting of a lower serosal section perfused by a solution similar to blood plasma and an upper luminal section superfused by an isotonic NaCl/pepsin solution. The pH value within this luminal solution was titrated following the tested postprandial pH profiles. Ultrafine tipped double-barrelled microelectrodes were positioned in the luminal portion of the gastric mucus in a depth of 20–40 µm from the luminal mucus surface. While the pH was changed according to one of the three defined luminal profiles, the pH inside the mucus was recorded. Thus, the effect of the defined luminal pH profile on the pH values within the luminal part of the mucus was measured.
Statistical analysis
Data are expressed as mean values ± standard deviation (SD). Additionally the range of colonization ratio is given in the text next to the mean values. Statistical analysis was performed using two-tailed Student’s t-test, adjusted by Bonferroni-Holm correction for multiple comparisons. P<0.05 was considered to be statistically significant.
Ethics Statement
Animal experiments were approved by the local governmental authorities (Bezirksregierung Arnsberg, Germany).
Author Contributions
SS, SSch, CJ & RB designed the experiments, analyzed the data and wrote the manuscript, SSch & RB prepared the figures, RB, MAV, CG & DG performed the experiments, all authors revised the ms, SS & SSch share the senior authorship equally.
Supplementary Material
Supplemental Material
Acknowledgments
This work was partly supported by a Bennigsen-Foerder Award to Christine Josenhans and Sören Schreiber.
References
- Suerbaum S. & Michetti P. Helicobacter pylori infection. .N Engl J Med. 347, 1175–1186 (2002). [DOI] [PubMed] [Google Scholar]
- Dooley C. P. et al. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Engl J Med. 321, 1562–1566 (1989). [DOI] [PubMed] [Google Scholar]
- Kuipers E. J., Thijs J. C. & Festen H. P. The prevalence of Helicobacter pylori in peptic ulcer disease. Aliment Pharmacol Ther. 9 Suppl 2, 59–69 (1995). [PubMed] [Google Scholar]
- Eck M. et al. MALT-type lymphoma of the stomach is associated with Helicobacter pylori strains expressing the CagA protein. Gastroenterology 112, 1482–1486 (1997). [DOI] [PubMed] [Google Scholar]
- Brenner H. et al. Is Helicobacter pylori infection a necessary condition for noncardia gastric cancer? Am J Epidemiol. 159, 252–258 (2004). [DOI] [PubMed] [Google Scholar]
- Forman D. The prevalence of Helicobacter pylori infection in gastric cancer. Aliment Pharmacol Ther. 9 Suppl 2, 71–76 (1995). [PubMed] [Google Scholar]
- Axon A. & Forman D. Helicobacter gastroduodenitis: a serious infectious disease. BMJ 314, 1430–1431 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenbacher D. et al. Acquisition of Helicobacter pylori infection in a high-risk population occurs within the first 2 years of life. J Pediatr. 136, 744–748 (2000). [PubMed] [Google Scholar]
- Perez-Perez G. I. et al. Transient and persistent Helicobacter pylori colonization in Native American children. J Clin Microbiol. 41, 2401–2407 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland M. et al. Age-specific incidence of Helicobacter pylori. Gastroenterology 130, 65–72 (2006). [DOI] [PubMed] [Google Scholar]
- Brown L. M. Helicobacter pylori: epidemiology and routes of transmission. .Epidemiol Rev. 22, 283–297 (2000). [DOI] [PubMed] [Google Scholar]
- Magalhaes Queiroz D. M. & Luzza F. Epidemiology of Helicobacter pylori infection. Helicobacter 11 Suppl 1, 1–5 (2006). [DOI] [PubMed] [Google Scholar]
- Eaton K. A. et al. Colonization of gnotobiotic piglets by Helicobacter pylori deficient in two flagellin genes. Infect Immun. 64, 2445–2448 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S. et al. In vivo distribution of Helicobacter felis in the gastric mucus of the mouse: experimental method and results. .Infect Immun. 67, 5151–5156 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S. et al. The spatial orientation of Helicobacter pylori in the gastric mucus. Proc Natl Acad Sci U S A 101, 5024–5029 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S. et al. Rapid loss of motility of Helicobacter pylori in the gastric lumen in vivo. Infect Immun. 73, 1584–1589 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S. et al. Gastric antibacterial efficiency is different for pepsin A and C. .Arch Microbiol 184, 335–340 (2006). [DOI] [PubMed] [Google Scholar]
- Savarino V. et al. 24-hour study of intragastric acidity in duodenal ulcer patients and normal subjects using continuous intraluminal pH-metry. Dig Dis Sci. 33, 1077–1080 (1988). [DOI] [PubMed] [Google Scholar]
- Cilluffo T. et al. Reproducibility of ambulatory gastric pH recordings in the corpus and antrum. Effect of food, time, and electrode position. Scand J Gastroenterol 25, 1076–1083 (1990). [DOI] [PubMed] [Google Scholar]
- Mason S. Some aspects of gastric function in the newborn. Arch Dis Child. 37, 387–391 (1962). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. J., McClure B. G. & Tubman T. R. Simultaneous monitoring of gastric and oesophageal pH reveals limitations of conventional oesophageal pH monitoring in milk fed infants. Arch Dis Child. 84, 273–276 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennemann J. The coagulation of cow's milk in the human stomach. Archives of Pediatrics 34, 81–117 (1917). [Google Scholar]
- Babbott F. L. et al. Hydrogen-ion concentration of gastric contents of infants. Am J Dis Child. 26, 475–485 (1923). [Google Scholar]
- Wolman I. J. Gastric phase of milk digestion in childhood. Am J Dis Child. 7, 394–422 (1946). [DOI] [PubMed] [Google Scholar]
- Schreiber S. et al. In situ measurement of pH in the secreting canaliculus of the gastric parietal cell and adjacent structures. Cell Tissue Res. 329, 313–320 (2007). [DOI] [PubMed] [Google Scholar]
- Marshall B. J. et al. Attempt to fulfil Koch’s postulates for pyloric Campylobacter. Med. J. Austr. 42, 436–439 (1985). [DOI] [PubMed] [Google Scholar]
- Graham D. Y. et al. Challenge model for Helicobacter pylori infection in human volunteers. Gut. 53(9), 1235–43 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebischer T. et al. Correlation of T cell response and bacterial clearance in human volunteers challenged with Helicobacter pylori revealed by randomised controlled vaccination with Ty21a-based Salmonella vaccines. .Gut. 57(8), 1065–72 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keto Y., Ebata M. & Okabe S. Gastric mucosal changes induced by long term infection with Helicobacter pylori in Mongolian gerbils: effects of bacteria eradication. J Physiol Paris 95, 429–436 (2001). [DOI] [PubMed] [Google Scholar]
- Rieder G., Merchant J. L. & Haas R. Helicobacter pylori cag-type IV secretion system facilitates corpus colonization to induce precancerous conditions in Mongolian gerbils. .Gastroenterology 128, 1229–1242 (2005). [DOI] [PubMed] [Google Scholar]
- Marchetti M. et al. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 267(5204), 1655–1658 (1995). [DOI] [PubMed] [Google Scholar]
- Watanabe T. et al. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. .Gastroenterology. 115(3), 642–648 (1998). [DOI] [PubMed] [Google Scholar]
- Croxen M. A. et al. The Helicobacter pylori chemotaxis receptor TlpB (HP0103) is required for pH taxis and for colonization of the gastric mucosa. J Bacteriol. 188, 2656–2665 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinitzer T. et al. Functional characterization and mutagenesis of the proposed behavioral sensor TlpD of Helicobacter pylori. J Bacteriol. 190, 3244–3255 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz B. et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature 445, 915–918 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammermeister I. et al. Elevated risk of Helicobacter pylori infection in submarine crews. Eur J Clin Microbiol Infect Dis. 11(1), 9–14 (1992). [DOI] [PubMed] [Google Scholar]
- Gisbert J. P. et al. Role of partner's infection in reinfection after Helicobacter pylori eradication. Eur J Gastroenterol Hepatol. 14(8), 865–871 (2002). [DOI] [PubMed] [Google Scholar]
- Broicher H. & Gierlich G. Intragastric pH measurement of determination of efficacy of antacids in peptic ulcer. Arztl Wochensch. 9, 471–473 (1954). [PubMed] [Google Scholar]
- Berstad A. et al. Reduction of postprandial gastric acidity and pepsin concentration by ranitidine and antacids in healthy volunteers. .Scand J Gastroenterol Suppl. 69, 67–73 (1981). [PubMed] [Google Scholar]
- Agunod M. et al. Correlative study of hydrochloric acid, pepsin, and intrinsic factor secretion in newborns and infants. Am J Dig Dis. 14, 400–414 (1969). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material