Abstract
Study Objectives:
Preeclampsia affects 5% to 7% of pregnancies, is strongly associated with low birth weight and fetal death, and is accompanied by sleep disordered breathing. We hypothesized that sleep disordered breathing may link preeclampsia with reduced fetal movements (a marker of fetal health), and that treatment of sleep disordered breathing might improve fetal activity during sleep.
Design, Setting, and Participants:
First, a method of fetal movement recording was validated against ultrasound in 20 normal third trimester pregnancies. Second, fetal movement was measured overnight with concurrent polysomnography in 20 patients with preeclampsia and 20 control subjects during third trimester. Third, simultaneous polysomnography and fetal monitoring was done in 10 additional patients with preeclampsia during a control night and during a night of nasal CPAP.
Intervention:
Overnight continuous positive airway pressure.
Measurements and Results:
Women with preeclampsia had inspiratory flow limitation and an increased number of oxygen desaturations during sleep (P = 0.008), particularly during REM sleep. Preeclampsia was associated with reduced total fetal movements overnight (319 [SD 32]) versus controls (689 [SD 160], P < 0.0001) and a change in fetal movement patterns. The number of fetal hiccups was also substantially reduced in preeclampsia subjects (P < 0.0001). Continuous positive airway pressure treatment increased the number of fetal movements and hiccups (P < 0.0001 and P = 0.0002, respectively).
Conclusions:
The effectiveness of continuous positive airway pressure in improving fetal movements suggests a pathogenetic role for sleep disordered breathing in the reduced fetal activity and possibly in the poorer fetal outcomes associated with preeclampsia.
Citation:
Blyton DM; Skilton MR; Edwards N; Hennessy A; Celermajer DS; Sullivan CE. Treatment of sleep disordered breathing reverses low fetal activity levels in preeclampsia. SLEEP 2013;36(1):15–21.
Keywords: Preeclampsia, continuous positive airway pressure, fetal movement, sleep disordered breathing
INTRODUCTION
Preeclampsia is an important and common complication of pregnancy, characterized by the onset of hypertension after 20 weeks gestation with accompanying proteinuria, affecting approximately 5% of pregnancies. It is a major cause of maternal mortality and a risk factor for fetal growth restriction.1 Women with preeclampsia have higher than normal sympathetic nervous activity,2 which in turn increases systemic vascular resistance. Obstructive sleep apnea and sleep disordered breathing (SDB) influence sympathetic nervous system activity, a pathway implicated in obstructive sleep apnea induced hypertension. In adults with obstructive sleep apnea, treatment with nasal continuous positive airway pressure (CPAP) reduces this activity.3
There is clear evidence that snoring increases during pregnancy, with a number of studies reporting data on self-reported snoring.4,5 Moreover, there is some evidence that snoring during pregnancy is associated with poorer fetal outcomes.6 Recent evidence with use of objective recordings of sleep and breathing has shown a high rate of mild SDB in pregnancy associated with pregnancy-induced hypertension.7–9 We and others have previously shown that preeclampsia is associated with SDB characterized by inspiratory airflow limitation,10–12 suggesting that SDB during pregnancy may be a pathogenetic factor involved in preeclampsia, with potential effects on preeclampsia-related fetal well-being. In particular, we have shown that when flow limitation and obstructed breathing is reversed with low levels of CPAP, there is a marked improvement in cardiovascular parameters, providing direct evidence of a causal relationship between SDB and hypertension. Specifically, CPAP reduced the otherwise markedly elevated peripheral vascular resistance during sleep in preeclampsia and reduced systemic blood pressure and increased maternal cardiac output.13
Fetal movements are a normal physiological component of healthy pregnancy, and have been used both formally and informally as a marker of fetal well-being. Reductions in fetal movements accompany several complications of pregnancy, including fetal growth restriction and oligohydramnios,14 and complete absence of fetal movements can precede stillbirth.15,16
The aim of the current set of studies was to determine if the characteristic pattern of SDB that occurs in preeclampsia (i.e., flow limitation and obstructed breathing without frank obstructive apnea) has adverse effect on fetal movements as a marker of fetal well-being, and if so, to determine if controlling the SDB with nasal CPAP could alleviate such fetal distress.
METHODS
Fetal Activity Monitoring
The fetal activity monitor was developed in the David Read Laboratory, University of Sydney. The fetal sensors consisted of shallow aluminum cylinders approximately 20 mm in diameter; with a film of piezoelectric bi-layer plastic (polyvinylidene fluoride - PVDF) stretched to form the equivalent of a stethoscope diaphragm. The amplification of the signal was provided by an AMLAB instrumentation computer.
Before each study, the subject's abdomen was palpated and fetal heart sounds auscultated to determine the lie and position of the fetus. Four piezoelectric fetal sensors were then placed on all quadrants of the maternal abdomen (supplementary material). Sensors were positioned over the anterior shoulder of the fetus where the fetal heart sounds were auscultated, two over the fetal legs, and at the top of the fetal axis. The sensors were firmly attached to the maternal abdomen using Elasto-Gel (Southwest Technologies Inc, USA). The signals were filtered between 0.3 and 200 Hz and digitized at 250 Hz and recorded onto the maternal polysomnogram using a separate digital instrumentation amplifier (AMLAB II system, AMLAB International, Sydney, Australia).
Both the total number of movements and the number per hour were calculated using software (Profusion PSG, Compumedics, Australia). In order to exclude the possibility of obtaining false positives for fetal movement as a result of maternal movement, fetal movements were not scored if they occurred in association with maternal movement, as indicated by 2 external maternal sensors, one attached to the anterior tibialis and one midway between the scapulas, or maternal electroencephalogram arousals. Fetal movements had to be ≥ 3 sec apart to be scored as separate events. Fetal hiccups were defined as a rhythmical pattern detected by all 4 fetal channels. Fetal hiccups and breathing events were not recorded as movements. Movements that occurred during an episode of fetal hiccups were discarded to ensure accuracy and to prevent overscoring.
Validation Study: Fetal Activity Monitor (Study 1)
Subjects
We studied 20 women who were in the third trimester of pregnancy. All women were recruited from the antenatal ward at King George V Hospital for Mothers and Babies, Sydney, Australia.
All women had cardiotocographic (CTG) monitoring for ≥ 20 min prior to the fetal monitoring validation study to ensure that the fetus was normal. All fetal heart rates and beat-to beat variability were within normal limits of 110–160 beats/min. There was no uterine activity recorded on the cardiotocograph or reported by the participants. The mothers reported normal fetal activity prior to the study.
Protocol
Fetal activity monitoring and simultaneous ultrasound (GE Healthcare, RT3000) were acquired for 20 min while the mother rested in a supine position. The ultrasound transducer was fixed in a stereotactic clamp for the duration of the scan, with the fetus viewed in longitudinal axis with the head, trunk, and legs visible. Maternal movements such as coughing, talking, or change of position were noted, and the corresponding time periods were excluded from analysis. Two observers, blinded to participant identity, counted the total number of movements from the fetal activity monitor and ultrasound recordings.
Fetal hiccups occurred in several subjects during the study. Fetal hiccups are well recognized by ultrasound17 and were clearly identifiable on the fetal movement sensors.
Simultaneous PSG and Fetal Monitoring in Preeclampsia and Control Participants (Study 2)
In study 2, women with moderate to severe preeclampsia (n = 20) and gestation-matched pregnant control subjects (n = 20) underwent overnight sleep studies in standard hospital beds. Studies were undertaken at the Nepean Hospital (Sydney, Australia).
Inclusion criteria were singleton pregnancy during the third trimester, nonsmokers, cephalic presentation, intact membranes, normal amniotic fluid volume, and normal fetal morphology as determined by an 18-week ultrasound. Participants with gestational diabetes, preexisting hypertension, or a previously identified sleep disorder, with the exception of snoring, were excluded. Moderate to severe preeclampsia was defined according to the Australasian Society for the Study of Hypertension in Pregnancy criteria: pregnancy ≥ 20 weeks' gestation, blood pressure ≥ 140/90 mm Hg on 2 consecutive occasions ≥ 6 h apart, and urinary protein ≥ 300 mg in a 24-h period.18 Control subjects were recruited from patients admitted with various complications of pregnancy, including placenta previa, threatened premature labor, antepartum hemorrhage, and back pain.
Fetal Monitoring in Preeclampsia with and without Nasal CPAP (Study 3)
In study 3, women with moderate to severe preeclampsia (n = 10) underwent overnight sleep studies on consecutive nights, the first without and the second with nasal CPAP treatment. Participants had not been screened for SDB during pregnancy prior to participation. Participants were recruited from the hypertension clinic at King George V Hospital for Mothers and Babies, Sydney, Australia. Inclusion criteria were the same as study 2.
Polysomnography
Full overnight polysomnography was performed on the subjects in study 2 and study 3 using the Compumedics S-series (Compumedics, VIC, Australia), as previously described.13 Recordings included standard neurophysiologic (electroencephalogram, electro-oculogram, electromyogram), cardiac (electrocardiogram), and respiratory (thoracic and abdominal excursion and nasal pressure) parameters. Maternal arterial oxyhemoglobin saturation (SpO2) was recorded with an oximeter probe affixed to the index finger, with desaturation defined as SpO2 > 3% below waking levels.
Maternal sleep stage scoring was performed according to Rechtschaffen and Kales criteria.19 Sleep staging was performed on the basis of visual analysis of a 30-sec period, with the period scored if ≥ 50% of it met the criteria for that particular stage, and defined as either NREM or REM. NREM sleep was further categorized into stages 1 through to 4.
The arousal analysis was performed according to criteria by the American Academy of Sleep Medicine,20 including abrupt change in the electroencephalogram frequency which may include theta, alpha and/or frequencies > 16 Hz excluding sleep spindles, for ≥ 3 sec and an increase in amplitude of the submental EMG.
Respiratory variables including the apnea-hypopnea index (AHI) and flow limitation were analyzed and scored as previously described.11 Briefly, the AHI was defined as the number of apneas/hypopneas that occurred per hour of sleep. Inspiratory airflow limitation, a lack of increase in airflow despite increasing respiratory effort, occurs due to partial collapse of the upper airway with consequent increase in upper airway resistance.21 Inspiratory airflow limitation is characterized by a flattening of the inspiratory curve on the nasal flow channel on the polysomnogram. A total of 10 random 30-sec periods during which there was a clear respiratory tracing, i.e., no mouth breathing or poor signal quality due to nasal prong dislodgement, were selected from each sleep stage. The area under the curve was calculated for each breath. The combined inspiratory airflow limitation for each stage and overall was expressed as a percentage of an average of 5 breaths measured during a period of stable wakefulness. We also measured breath-by-breath flow limitation for the entire polysomnography study in a subset of each group of participants.
Participants remained medically stable for 48 h prior to and throughout the sleep studies. Written informed consent was obtained from all of the subjects, and the studies were approved by the Wentworth Area Health Service Ethics Review Committee (Nepean Hospital; study 2), and the Central Sydney Area Health Service Ethics Review Committee (Royal Prince Alfred Hospital; study 1 and study 3).
Nasal CPAP
The women were given a practice session with nasal CPAP and were able to try a range of masks during the afternoon of the CPAP study. Because the treatment was intended to control or reduce flow limitation, we used automatic devices (ResMed Autoset T, Resmed, North Ryde, NSW, Australia) that respond to the shape of the flow pattern and increase pressure to attempt to normalize the flow curve. Our prior experience with this in pregnancy was that low levels of pressure of between 4 and 8 cm H2O largely reduced the flow limitation and reduced the associated snoring, and that these low levels also led to a better control of blood pressure.11 We therefore took the same approach in this study. While we did not attempt to intervene during the night, we reviewed the flow recordings the next morning to confirm that the CPAP had worked. Both the diagnostic and treatment studies were performed in hospital and were closely supervised.
Statistical Analysis
The Kolmogorov-Smirnov test was used to assess normality. Non-normally distributed data, including fetal hiccups, AHI, and minimum SpO2, were log transformed for analysis. Normally distributed data are reported as mean and standard deviation, and non-normally distributed data as geometric mean and interquartile range. A Bland-Altman plot was used to visualize the agreement between fetal activity assessed by the fetal activity monitor and ultrasound. Fetal activity measured by the two methods was compared using Pearson correlation and intraclass correlation. Interobserver reliability was studied using intraclass correlation coefficient. For study 2, comparisons of control and preeclampsia groups were made using independent samples t-tests for continuous variables and χ2 for categorical variables. For study 3, comparisons of continuous variables between the first and second nights were made using paired t-tests. Comparisons of the number of fetal movements per hour through the sleep study were made by 2-way repeated measures ANOVA. Statistical significance was inferred at 2P < 0.05.
RESULTS
Study 1: Fetal Activity Monitor Validation Study
Specific participant characteristics of the 20 women in whom the fetal movement sensor was validated are shown in the supplementary material. These women were in hospital for a range of problems including induction, placenta previa, threatened premature labor, and antepartum hemorrhage. However none of the patients had any evidence of fetal distress, and all had normal CTG, fetal heart rate, and heart rate variability in a 20-min test just prior to the fetal monitor validation study.
The mean age for this group was 29 y (SD 3; range 22–34) with a mean gestational age of 35 weeks (SD 4; range 31–42). There was excellent agreement between the fetal activity monitor and ultrasound for the measurement of fetal movements (intraclass correlations for 2 independent observers: 0.96 (95% CI 0.91, 0.99) and 0.94 (95% CI 0.85, 0.98). The number of fetal movements was similar when assessed by ultrasound or fetal activity monitor (observer 1: ultrasound 21 [SD 6], fetal activity monitor 23 [SD 6]; observer 2: ultrasound 22 [SD 5], fetal activity monitor 22 [SD 5]), and were tightly correlated (observer 1: r = 0.93, P < 0.001; observer 2: r = 0.89, P < 0.001; supplementary material). Bland-Altman plots derived from the measures undertaken by the 2 observers showed generally good agreement, with no evidence of systematic bias (supplementary material). The interobserver reproducibility was similar for ultrasound and the fetal activity monitor (intraclass correlations: ultrasound 0.91 [95% CI 0.77, 0.97], fetal activity monitor 0.91 [95% CI 0.78, 0.96]).
Study 2. Comparison of Control and Preeclamptic Pregnancy
Specific participant characteristics for the 40 women in study 2 are presented in the supplementary material and Table 1. Briefly, the 20 control participants included 10 with placenta previa, 7 with threatened premature labor, 2 with antepartum hemorrhage, and 1 with back pain; none had hypertension, diabetes, or any other illness, and all were nonsmokers. All preeclamptic subjects were on antihypertensive medication (methyldopa or hydralazine). All subjects were stable for 48 h prior and throughout the sleep studies with no report of uterine activity or further hemorrhage.
Table 1.
Participant and sleep characteristics for comparison of preeclampsia and pregnant controls (study 2)
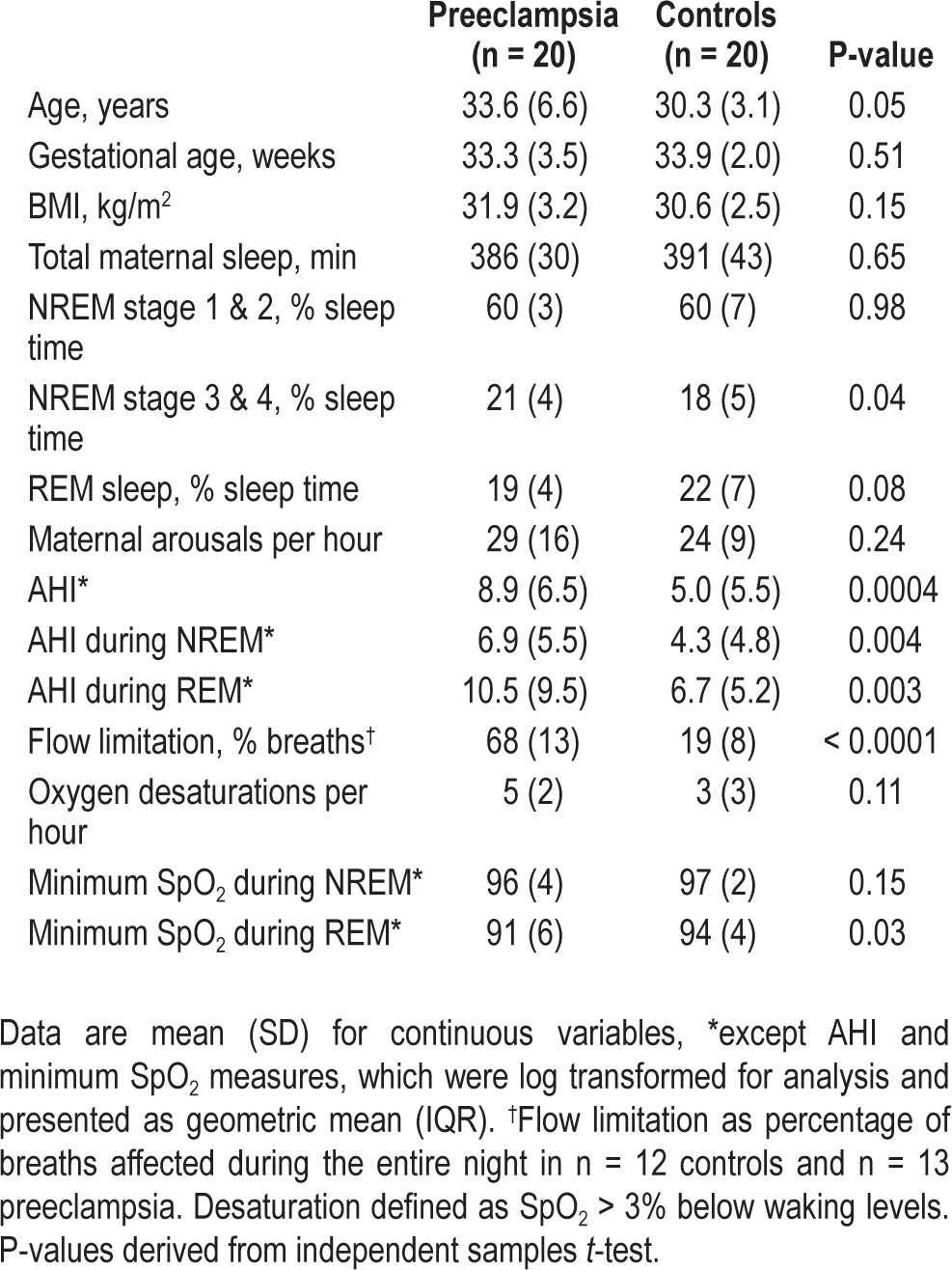
Sleep Characteristics and Sleep Disordered Breathing
Total time available for sleep was similar for both groups (443 min [SD 39] for control and 460 min [SD 18] for preeclampsia). Sleep and sleep disordered breathing characteristics are shown in Table 1. Briefly, there was some evidence that those with preeclampsia had increased time spent in NREM stage 3 and 4 and decreased time in REM sleep; however, there was no difference in the number of arousals per hour of sleep. The total AHI, and AHI during both NREM and REM sleep, was significantly lower in the control group than the preeclampsia group. Nine of the 20 preeclamptic subjects had an AHI > 10; however, none had major episodes of oxyhemoglobin desaturation.
In the control subjects, 15% of all breaths in the randomly selected epochs had flow limitation. In a subgroup of 12 control subjects, in whom all breath sounds were analyzed, the mean percent time spent in flow limitation was 19% (SD 8). The control subject with the highest value for flow limitation was 28% of total breaths for the whole sleep study. In contrast, flow limitation was present in 68% of all the randomly selected epochs in the preeclamptic subjects. In a subgroup of 13 preeclamptic subjects (in whom flow limitation of every breath was measured), the mean percent time spent in flow limitation was also 68% (SD 13). The preeclamptic subject with the least amount of flow limitation spent 50% of the sleep time in flow limitation. Thus, there was no overlap between the groups in the extent to which breathing in sleep was flow limited.
Fetal Activity
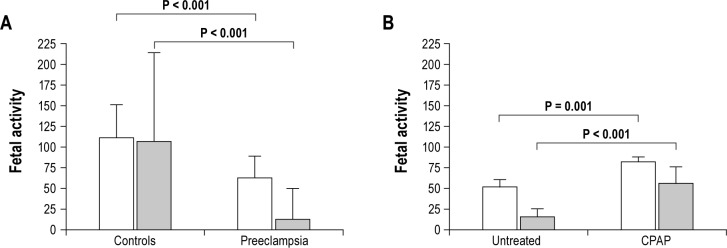
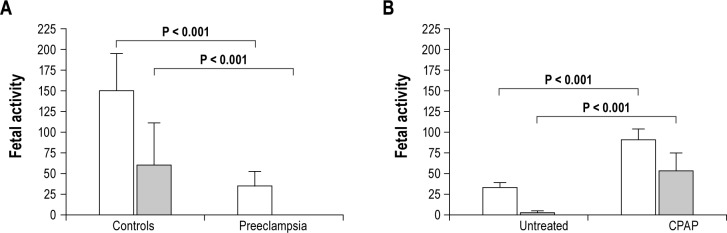
The number of fetal movements during maternal sleep was markedly lower in the preeclampsia group (289 [SD 121]) than the control group (689 [SD 160], P < 0.0001), evident during both NREM and REM sleep (Figures 1 and 2). In the control group, there were more fetal movements during REM than NREM sleep (P < 0.0001); whereas in those with preeclampsia there were fewer fetal movements during REM sleep (P < 0.0001).
Figure 1.
Fetal activity is (A) reduced during maternal NREM sleep in preeclampsia, which (B) can be partially reversed by nasal CPAP. Fetal activity data are mean (SD) for fetal movements per hour (white bars) and total fetal hiccups (gray bars).
Figure 2.
Fetal activity is (A) reduced during maternal REM sleep in preeclampsia, which (B) can be partially reversed by nasal CPAP. Fetal activity data are mean (SD) for fetal movements per hour (white bars) and total fetal hiccups (gray bars).
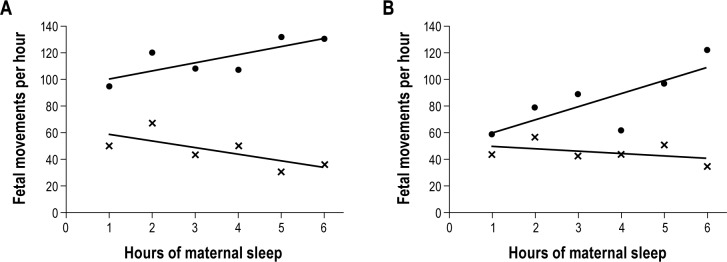
The number of fetal movements per hour of maternal sleep in the control group increased during the course of the night by 6.4 (SD 8.8) movements/h of maternal sleep, such that on average, the number of movements/h of maternal sleep during the seventh hour of the study was 43 more than the number per hour in the first hour of maternal sleep. By contrast, in the preeclampsia group the number of fetal movements per hour of sleep reduced by 1.9 (SD 8.8)/h as the night progressed; equating to 14 fewer fetal movements recorded during the last hour of sleep than the first hour of sleep (P < 0.001 vs controls; Figure 3).
Figure 3.
Fetal movements per hour of maternal sleep in (A) control and preeclampsia; and (B) preeclampsia with and without nasal CPAP. In panel A: (•) control, and (x) preeclampsia. In panel B: (•) nasal CPAP, and (x) untreated.
Episodes of fetal hiccups during maternal sleep were detected in only 2 (10%) of the preeclampsia group, compared with 85% of controls (P < 0.0001). The average number of hiccups for those in the control group was 83 (IQR 143), with 52%, 12%, and 36% occurring during NREM stage 1 and 2, NREM stage 3 and 4, and REM sleep, respectively. In the preeclampsia group the average number of hiccups was 1 (IQR 0; P < 0.0001 vs controls), occurring only during stage 1 and 2 NREM sleep.
Study 3: Effect of Nasal CPAP on Fetal Activity in Women with Preeclampsia
Ten women with preeclampsia underwent sleep studies on 2 consecutive nights, with nasal CPAP treatment on the second night. Specific participant characteristics are displayed in the supplementary material.
Sleep Characteristics and Sleep Disordered Breathing
Sleep characteristics for the 2 consecutive study nights are shown in Table 2. Briefly, there was some evidence for a change in sleep architecture, with a small but significant increase in NREM stage 3 and 4 on CPAP, but no change in REM sleep time.
Table 2.
Sleep characteristics for women with preeclampsia without and with nasal CPAP (study 3)
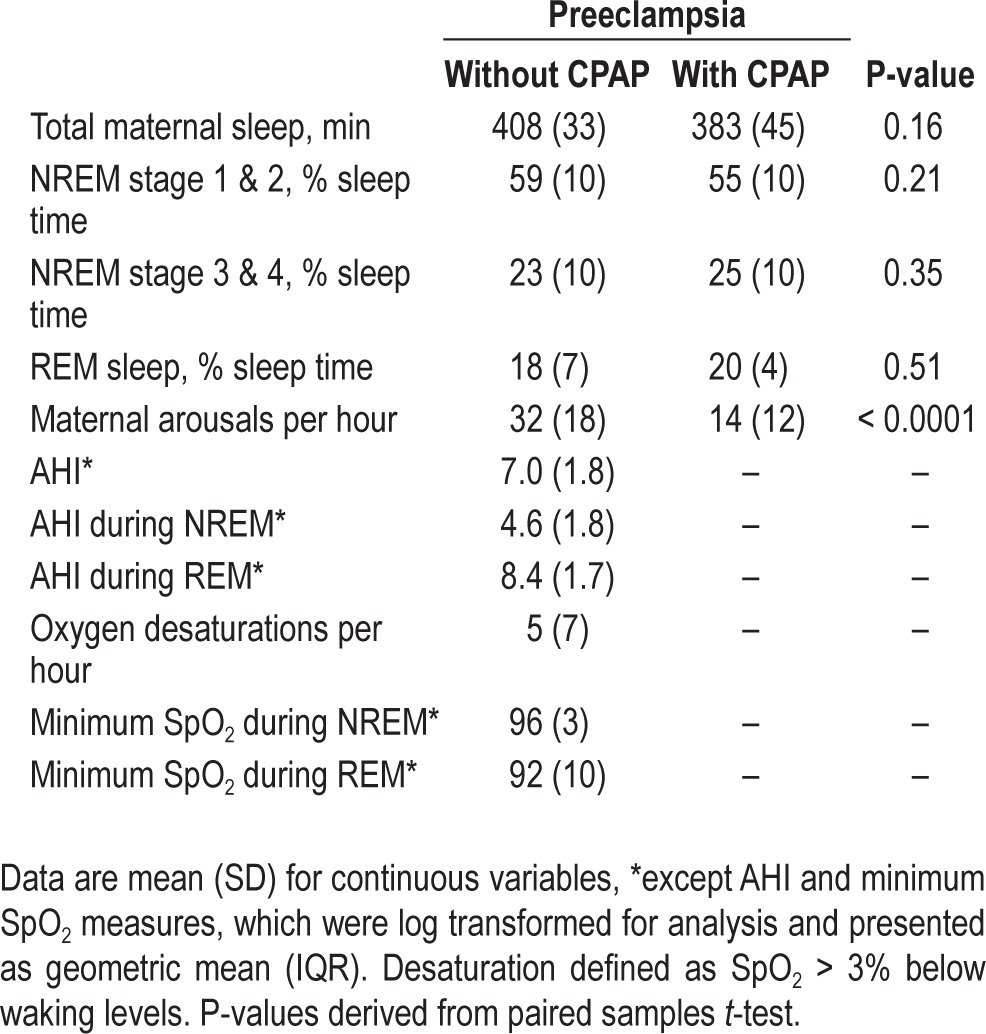
All of the patients displayed airflow limitation on the untreated night, occurring in approximately 65% of breaths. Minimal CPAP pressure was needed to eliminate upper airway obstruction and flow limitation (mean pressure 7 cm H2O [range 6–8]).
Fetal Activity
The average number of fetal movements on the night without CPAP treatment was 319 (SD 32). On the subsequent night with CPAP treatment, the number of fetal movements increased to 592 (SD 48; P < 0.0001). This increase in fetal movements with nasal CPAP treatment was seen in both NREM and REM sleep (Figures 1 and 2).
The number of fetal movements decreased steadily during the course of the night without CPAP treatment, by 7.4 (SD 8.4) movements per hour. In contrast, over the course of the night with CPAP treatment, the number of fetal movements increased by 12.6 (SD 14.2) per hour (P = 0.03 vs first night; Figure 3).
The average number of hiccups on the night without CPAP treatment was 12 (IQR 10), almost exclusively occurring during NREM sleep (Figures 1 and 2). This increased to 95 (IQR 87) on the night with CPAP treatment (P = 0.0002), split evenly between NREM and REM sleep (Figures 1 and 2).
DISCUSSION
Preeclampsia is an established risk factor for adverse fetal outcome. In this study we demonstrate that preeclampsia is accompanied by a reduction in fetal movements, a measure of fetal well-being. Women with preeclampsia also had marked inspiratory airflow limitation during sleep, resulting in a higher number of oxygen desaturations and a raised AHI. Treatment of this SDB with nasal CPAP increased the number and rate of fetal movements. This suggests that SDB, and consequent oxygen desaturations, may be a pathogenetic contributor to poorer fetal outcome in preeclampsia, and that this may be at least partially reversed by nasal CPAP treatment.
Our study confirmed previous reports, including our own, that women with preeclampsia have a high percentage of sleep linked upper airway flow limitation and a significant degree of mild SDB.7–9,11,12 While some of the non-preeclamptic women had significant amounts of flow limited breathing, there was no overlap in airflow limitation between the two groups. However, it is important to recognize that sleep apnea was not common, and although this group of preeclamptic women had some SDB, it was mild by current criteria. Most had audible snoring, however, its character was typically less vibratory than the typical heavy snoring male, and could readily be dismissed as trivial or “simple snoring.” However, we and others have shown that this level of upper airway flow limitation (i.e., obstructed breathing) is associated with elevations of systemic blood pressure and that this is controllable with low levels of CPAP11,13,22—experimental evidence that the otherwise apparently minor obstructed breathing has major effects on the vascular system.
The reason that pregnant women with preeclampsia are prone to develop high upper airway resistance in sleep is unknown. However, one mechanism might relate to tissue edema of pharyngeal tissue. Of considerable interest is the work of Friedman et al., who have shown that compression of the lower limbs in supine subjects leads to fluid shift into the neck and peripharyngeal tissue.23 Thus, in any state that involves tissue edema, including preeclampsia, there is the likelihood that fluid shift during prolonged periods of horizontal positioning may well lead to increased upper airway resistance.
The major goal of the present set of studies was to determine if this otherwise “minor” sleep induced obstructed breathing had adverse effects on the fetus. The first new finding was that the normal fetus is very active during maternal sleep and that activity increases during the course of the night sleep. In marked contrast, women with preeclampsia had fewer fetal movements, and the rate of movements tended to reduce as the night progressed. Furthermore, fetal movements increased during maternal REM sleep in the control group, whereas they decreased during maternal REM sleep in the preeclampsia group. These differences and the improvements found in fetal movements when we treated a small group of women with nasal CPAP provide direct evidence that the SDB contributes to the reduced fetal movements. How SDB affects the fetus is unclear; however, we believe that the most likely cause is that the SDB triggers a worsening of the already high peripheral vascular resistance and a decrease in cardiac output in these women,11 and thereby compromises uterine and placental blood flow. This occurs without major changes in maternal arterial oxyhemoglobin desaturation, and is most likely mediated by small rises in maternal arterial carbon dioxide. While maternal oxyhemoglobin desaturation was not a major issue in our patients, when frank sleep apnea does occur in pregnancy, the fetus is probably somewhat protected by the carrying capacity of fetal hemoglobin. However, our work suggests that the major challenge to the fetus is blood flow and hence oxygen delivery to the placenta.
We also studied the potential role of SDB and nasal CPAP treatment on the rate of fetal hiccups, the results of which mirrored our findings for fetal movements. The physiological relevance of fetal hiccups is poorly understood. It has been proposed that fetal hiccups may be an important isometric inspiratory muscle exercise in preparation for ex-utero life,24 and we believe that as such a reduction in the rate of hiccupping is further indication of poor fetal well-being, especially taken together with our findings concerning reduced fetal movements.
Whether or not sustained nasal CPAP treatment during maternal sleep would have a continued beneficial impact on fetal well-being and pregnancy outcome in pregnancies affected by preeclampsia and SDB requires further study. Delivery of the fetus resolves maternal risk associated with preeclampsia, with the decision to induce delivery dependent upon both maternal and fetal condition.25 Prolongation of pregnancy may have more beneficial outcomes for the fetus for those diagnosed early in pregnancy. Fetal monitoring to assess fetal well-being is an important component of such clinical management. Given our findings, it is plausible that nasal CPAP treatment of patients with preeclampsia also affected by SDB may potentially provide sufficient improvement in fetal well-being to allow for clinically relevant extensions of pregnancy; however, this has not yet been studied.
Limitations
Participants with and without preeclampsia may not have been representative of typical women. For example, those with gestational diabetes and preeclampsia, which frequently coexist, were excluded. Conversely, the control group consisted of women without preeclampsia, but with concurrent obstetric conditions including placenta previa, threatened premature labor, and antepartum hemorrhage. Nonetheless, one would expect that this participant profile would tend to result in a smaller observed difference between groups, than that for typical women with or without preeclampsia.
We used a novel technique for directly assessing the number of fetal movements during sleep, which we validated against an ultrasound-derived measure. This technique is able to overcome inherent limitations in maternal assessment of fetal movements during sleep, and provides an estimation of fetal movements both correlated with, and approximating in absolute terms, that derived by ultrasound. Although the validation of the method was done during daytime studies with the woman quietly awake, given the physical nature of the signals, we believe that the findings would not differ by maternal sleep/wake status. Accordingly, the period of time chosen to test against an ultrasound was based on practical requirements. While this could be done at night, it was impractical to try to achieve this while the mother slept. The reason for the 20-minute duration of the validation study is that longer duration ultrasound exposure is not recommenced because of potential physiological effects of ultrasound energy. In contrast, the movement sensors are useable over many hours without any concern about possible effects on the fetus.
In conclusion, preeclampsia is frequently complicated by SDB and reduced fetal activity during sleep. This decrease in fetal activity can be partially reversed by nasal CPAP therapy during maternal sleep. The effectiveness of CPAP in restoring fetal activity suggests a pathogenetic role for SDB in the reduced fetal activity associated with preeclampsia.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Sullivan was a co-founder of ResMed and former consultant. He still retains stock in the company. He is currently the director of a start-up company developing diagnostic sleep technology. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Dr. Skilton is supported by a National Health and Medical Research Council of Australia Career Development Fellowship (#1004474); there was no other financial support received for this study. Dr. Sullivan is the director of a start-up company developing diagnostic sleep technology; there are no other conflicts of interest to declare.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CPAP
continuous positive airway pressure
- NREM
non-rapid eye movement
- REM
rapid eye movement
- SpO2
arterial oxyhemoglobin saturation
- SDB
sleep disordered breathing
Footnotes
A commentary on this article appears in this issue on page 5.
SUPPLEMENTAL MATERIAL
Set-up for fetal monitor validation study. The mother's abdomen was palpated and the fetal heart sounds were auscultated to determine the position and lie of the fetus. Four fetal sensors were placed on the quadrants of the maternal abdomen, at least 2 sensors were placed over fetal limbs to detect maximum movement. The signals from the sensors were then isolated and recorded via the AMLAB instrument system. Simultaneous ultrasound recordings were acquired from the General Electric Healthcare ultrasound machine and recorded onto videotape.
Bland-Altman plot (A), and direct comparison of fetal movements assessed by ultrasound and fetal activity monitor (B). Panel A: (•) observer 1; (x) observer 2. Data in panel B are the mean from observers 1 – 2 of the total number of fetal movements in the 20 minute validation study.
Clinical presentation of each participant in the validation study
Clinical presentation of each participant with preeclampsia in study 1 (comparison with controls)
Clinical presentation of each control participant in study 1 (comparison with preeclampsia)
Clinical presentation of each participant in study 2 (preeclampsia with and without nasal CPAP)
REFERENCES
- 1.Odegard RA, Vatten LJ, Nilsen ST, Salvesen KA, Austgulen R. Preeclampsia and fetal growth. Obstet Gynecol. 2000;96:950–5. [PubMed] [Google Scholar]
- 2.Greenwood JP, Scott EM, Stoker JB, Walker JJ, Mary DA. Sympathetic neural mechanisms in normal and hypertensive pregnancy in humans. Circulation. 2001;104:2200–4. doi: 10.1161/hc4301.098253. [DOI] [PubMed] [Google Scholar]
- 3.Ziegler MG, Mills PJ, Loredo JS, Ancoli-Israel S, Dimsdale JE. Effect of continuous positive airway pressure and placebo treatment on sympathetic nervous activity in patients with obstructive sleep apnea. Chest. 2001;120:887–93. doi: 10.1378/chest.120.3.887. [DOI] [PubMed] [Google Scholar]
- 4.Pien GW, Fife D, Pack AI, Nkwuo JE, Schwab RJ. Changes in symptoms of sleep-disordered breathing during pregnancy. Sleep. 2005;28:1299–305. doi: 10.1093/sleep/28.10.1299. [DOI] [PubMed] [Google Scholar]
- 5.Loube DI, Poceta JS, Morales MC, Peacock MD, Mitler MM. Self-reported snoring in pregnancy. Association with fetal outcome. Chest. 1996;109:885–9. doi: 10.1378/chest.109.4.885. [DOI] [PubMed] [Google Scholar]
- 6.Franklin KA, Holmgren PA, Jonsson F, Poromaa N, Stenlund H, Svanborg E. Snoring, pregnancy-induced hypertension, and growth retardation of the fetus. Chest. 2000;117:137–41. doi: 10.1378/chest.117.1.137. [DOI] [PubMed] [Google Scholar]
- 7.Reid J, Skomro R, Cotton D, et al. Pregnant women with gestational hypertension may have a high frequency of sleep disordered breathing. Sleep. 2011;34:1033–8. doi: 10.5665/SLEEP.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guilleminault C, Querra-Salva M, Chowdhuri S, Poyares D. Normal pregnancy, daytime sleeping, snoring and blood pressure. Sleep Med. 2000;1:289–97. doi: 10.1016/s1389-9457(00)00046-0. [DOI] [PubMed] [Google Scholar]
- 9.Champagne K, Schwartzman K, Opatrny L, et al. Obstructive sleep apnoea and its association with gestational hypertension. Eur Respir J. 2009;33:559–65. doi: 10.1183/09031936.00122607. [DOI] [PubMed] [Google Scholar]
- 10.Izci B, Riha RL, Martin SE, et al. The upper airway in pregnancy and pre-eclampsia. Am J Respir Crit Care Med. 2003;167:137–40. doi: 10.1164/rccm.200206-590OC. [DOI] [PubMed] [Google Scholar]
- 11.Edwards N, Blyton DM, Kirjavainen T, Kesby GJ, Sullivan CE. Nasal continuous positive airway pressure reduces sleep-induced blood pressure increments in preeclampsia. Am J Respir Crit Care Med. 2000;162:252–7. doi: 10.1164/ajrccm.162.1.9905006. [DOI] [PubMed] [Google Scholar]
- 12.Connolly G, Razak AR, Hayanga A, Russell A, McKenna P, McNicholas WT. Inspiratory flow limitation during sleep in pre-eclampsia: comparison with normal pregnant and nonpregnant women. Eur Respir J. 2001;18:672–6. doi: 10.1183/09031936.01.00053501. [DOI] [PubMed] [Google Scholar]
- 13.Blyton DM, Sullivan CE, Edwards N. Reduced nocturnal cardiac output associated with preeclampsia is minimized with the use of nocturnal nasal CPAP. Sleep. 2004;27:79–84. doi: 10.1093/sleep/27.1.79. [DOI] [PubMed] [Google Scholar]
- 14.Froen JF, Tveit JV, Saastad E, et al. Management of decreased fetal movements. Semin Perinatol. 2008;32:307–11. doi: 10.1053/j.semperi.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Reinold E. Clinical value of fetal spontaneous movements in early pregnancy. J Perinat Med. 1973;1:65–72. doi: 10.1515/jpme.1973.1.1.65. [DOI] [PubMed] [Google Scholar]
- 16.Sadovsky E, Yaffe H. Daily fetal movement recording and fetal prognosis. Obstet Gynecol. 1973;41:845–50. [PubMed] [Google Scholar]
- 17.Zheng YT, Sampson MB, Soper R. The significance of umbilical vein Doppler changes during fetal hiccups. J Matern Fetal Investig. 1998;8:89–91. [PubMed] [Google Scholar]
- 18.Brown MA, Hague WM, Higgins J, et al. The detection, investigation and management of hypertension in pregnancy: executive summary. Aust N Z J Obstet Gynaecol. 2000;40:133–8. doi: 10.1111/j.1479-828x.2000.tb01136.x. [DOI] [PubMed] [Google Scholar]
- 19.Rechtschaffen A, Kales A. Washington, DC: NIH Publication No. 204; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [DOI] [PubMed] [Google Scholar]
- 20.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 21.Morgenstern C, Schwaibold M, Randerath WJ, Bolz A, Jane R. Assessment of changes in upper airway obstruction by automatic identification of inspiratory flow limitation during sleep. IEEE Trans Biomed Eng. 2009;56:2006–15. doi: 10.1109/TBME.2009.2023079. [DOI] [PubMed] [Google Scholar]
- 22.Poyares D, Guilleminault C, Hachul H, et al. Pre-eclampsia and nasal CPAP: part 2. Hypertension during pregnancy, chronic snoring, and early nasal CPAP intervention. Sleep Med. 2007;9:15–21. doi: 10.1016/j.sleep.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Friedman O, Bradley TD, Chan CT, Parkes R, Logan AG. Relationship between overnight rostral fluid shift and obstructive sleep apnea in drug-resistant hypertension. Hypertension. 2010;56:1077–82. doi: 10.1161/HYPERTENSIONAHA.110.154427. [DOI] [PubMed] [Google Scholar]
- 24.Kahrilas PJ, Shi G. Why do we hiccup? Gut. 1997;41:712–3. doi: 10.1136/gut.41.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lain KY, Roberts JM. Contemporary concepts of the pathogenesis and management of preeclampsia. JAMA. 2002;287:3183–6. doi: 10.1001/jama.287.24.3183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Set-up for fetal monitor validation study. The mother's abdomen was palpated and the fetal heart sounds were auscultated to determine the position and lie of the fetus. Four fetal sensors were placed on the quadrants of the maternal abdomen, at least 2 sensors were placed over fetal limbs to detect maximum movement. The signals from the sensors were then isolated and recorded via the AMLAB instrument system. Simultaneous ultrasound recordings were acquired from the General Electric Healthcare ultrasound machine and recorded onto videotape.
Bland-Altman plot (A), and direct comparison of fetal movements assessed by ultrasound and fetal activity monitor (B). Panel A: (•) observer 1; (x) observer 2. Data in panel B are the mean from observers 1 – 2 of the total number of fetal movements in the 20 minute validation study.
Clinical presentation of each participant in the validation study
Clinical presentation of each participant with preeclampsia in study 1 (comparison with controls)
Clinical presentation of each control participant in study 1 (comparison with preeclampsia)
Clinical presentation of each participant in study 2 (preeclampsia with and without nasal CPAP)