Abstract
Study Objectives:
Prolonged wakefulness leads to a progressive increase in sleep pressure, reflected in a global increase in slow wave activity (SWA, 0.5-4.5 Hz) in the sleep electroencephalogram (EEG). A global increase in wake theta activity (5-9 Hz) also occurs. Recently, it was shown that prolonged wakefulness in rodents leads to signs of “local sleep” in an otherwise awake brain, accompanied by a slow/theta wave (2-6 Hz) in the local EEG that occurs at different times in different cortical areas. Compelling evidence in animals and humans also indicates that sleep is locally regulated by the amount of experience-dependent plasticity. Here, we asked whether the extended practice of tasks that involve specific brain circuits results in increased occurrence of local intermittent theta waves in the human EEG, above and beyond the global EEG changes previously described.
Design:
Participants recorded with high-density EEG completed 2 experiments during which they stayed awake ≥ 24 h practicing a language task (audiobook listening [AB]) or a visuomotor task (driving simulator [DS]).
Setting:
Sleep laboratory.
Patients or Participants:
16 healthy participants (7 females).
Interventions:
Two extended wake periods.
Measurements and Results:
Both conditions resulted in global increases in resting wake EEG theta power at the end of 24 h of wake, accompanied by increased sleepiness. Moreover, wake theta power as well as the occurrence and amplitude of theta waves showed regional, task-dependent changes, increasing more over left frontal derivations in AB, and over posterior parietal regions in DS. These local changes in wake theta power correlated with similar local changes in sleep low frequencies including SWA.
Conclusions:
Extended experience-dependent plasticity of specific circuits results in a local increase of the wake theta EEG power in those regions, followed by more intense sleep, as reflected by SWA, over the same areas.
Citation:
Hung CS; Sarasso S; Ferrarelli F; Riedner B; Ghilardi MF; Cirelli C; Tononi G. Local experience-dependent changes in the wake EEG after prolonged wakefulness. SLEEP 2013;36(1):59–72.
Keywords: High-density EEG, theta activity, slow wave activity
INTRODUCTION
Experience-dependent plasticity is what allows the brain to develop, adapt, and learn. A remarkable portion of the cellular machinery in neural tissue is devoted to producing and regulating plastic changes. However, just as plasticity is essential, it can also be costly. Indeed, we have shown recently that physiological forms of experience-dependent plasticity that mimic what happens during a normal waking day, such as exploring new objects or practicing a new task, result in a net increase in synaptic strength.1–4 Since stronger synapses consume more energy, occupy more space, and require more cellular supplies, they pose an increasing burden on neural circuits.5,6 We have proposed that this may be why sleep is universally needed: to renormalize synaptic strength and reset the baseline for plasticity.5,7
The best established marker of sleep need and intensity is NREM slow wave activity (SWA), the EEG power in the 0.5-4.5 Hz range during NREM sleep. In humans, SWA is high at the beginning of the night, even higher after sleep deprivation, and decreases in the course of sleep.8–10 Extended wake also results in widespread changes in the wake EEG, with increases in SWA and theta (5-9 Hz) activity.11–19 Wake 2-8 Hz EEG activity and subsequent NREM SWA have a similar topographic distribution, with predominance in frontal cortical areas,10,14,19,20 suggesting that they may reflect similar processes.
Sleep need as measured by SWA is in part regulated at the local level. More specifically, many studies have shown that NREM SWA may reflect not only the prior “use” of specific neuronal circuits, but also the occurrence of changes in synaptic strength in those networks, suggesting that experience-dependent plasticity, more so than mere neuronal activity, is linked to sleep need.5,21 For instance, in humans learning a visuomotor task leads to increased NREM SWA over the cortical regions involved in the task, even if the training lasts for less than an hour and is performed in the morning, suggesting a locally increased need for synaptic homeostasis.22–24 Recently, we have started to investigate in animals the consequences of challenging plasticity mechanisms beyond the normal wake duration. We found that when rats stayed awake longer than usual exploring novel objects and/or training in a reaching task, some cortical neurons went off-line, interrupting and resuming their discharge patterns as they normally do only in sleep.25 These OFF periods were local, were associated with a local EEG slow/theta (2-6 Hz) wave, increased in frequency with the duration of exploratory activities and, when they happened in an area relevant for behavior, they led to performance errors. Intriguingly, during local OFF periods, rats appeared fully awake and the global EEG recorded from the scalp continued to show low voltage fast activity typical of wake.25
Does experience-dependent plasticity extended beyond the normal wake duration lead to a local increase in wake theta activity in humans? And if so, is this increase more prominent in the specific brain regions challenged by plasticity, and is subsequent sleep more intense over the same areas? To address these questions, we measured the specific effects on local cortical circuits induced by a direct manipulation of the content of waking activity in a group of healthy participants. Specifically, we performed resting high-density (hd) EEG recordings during two extended wake periods during which participants practiced either a language task that mainly involves left fronto-temporal areas, or a visuomotor task that mostly relies on occipito-parietal networks.
MATERIALS AND METHODS
Participants
Sixteen healthy participants (age = 19-26 years, mean ± SD: 22 ± 2.7 years, 7 females; all right-handed nonsmokers) were recruited from the University of Wisconsin-Madison campus. All participants had sleep duration of 7-8 h/night, consistent bed/rise times, no daytime nap habit, no excessive daytime sleepiness (total scores in the Epworth Sleepiness Scale ' 10) and no history of sleep, medical, or psychiatric disorders as assessed by a clinical interview and by an 8-h night sleep recording with polysomnography and hd-EEG (baseline sleep night). Each participant signed an IRB approved informed consent form.
Tasks and Experimental Design
All 16 participants completed 2 experiments separated by ≥ 2 weeks, the order of which was randomly assigned and counterbalanced across subjects. In each experiment, participants were asked to stay awake ≥ 24 h (max 36 h), during which they were engaged in a language task or a visuomotor task (Figure 1). The language task (audiobook listening [AB]) and the visuomotor task (driving simulator [DS]) were selected to engage as much as possible plasticity mechanisms involving different cortical circuits (although overlap is inevitable), which could then be distinguished with hd-EEG. Neuroimaging studies have shown that speech listening mostly activates a left fronto-temporal network,26,27 while driving mostly activates a bilateral occipito-parietal network.28,29 To enhance compliance subjects participated in pairs and selected the material for both tasks. Audiobooks' topics included literature, science, religion, and travel, but excluded strong emotional and non-language components (e.g., music). Oral conversation was encouraged during the AB task, while reading and other activities involving visuomotor components were restricted. By contrast, in the DS task participants played driving simulation computer games using the 4 direction keys on the computer keyboard. Any audio component was removed from the game, and talking and listening activities were strictly limited. In pilot experiments, we established that the tasks were well tolerated and interesting enough to engage the participants for up to 24 hours.
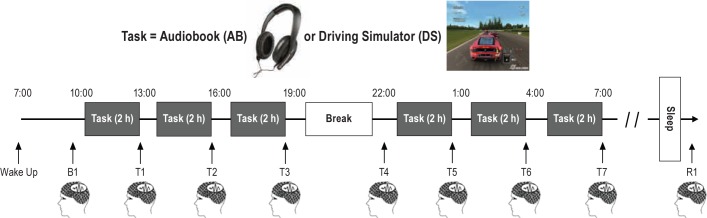
Figure 1.
Experimental design. In each experiment participants woke up at ∼07:00 and underwent a baseline testing session (B1) at 10:00, followed by six 2-h tasks (AB or DS) interleaved by 1-h EEG recording sessions (T1–T7, total wake time: 24 h). A final testing session (R1) was scheduled 30 min after participants woke up from ∼ 8 h of recovery sleep. In most experiments participants remained awake for 24 h and were allowed to sleep in the morning (starting at ∼ 08:30). In 9 experiments, participants were awake for 36 h, and went to sleep in the evening (see Methods for details).
Participants were asked to maintain their regular sleep-wake schedule for at least one week before each experiment, and compliance was verified by sleep diaries and wrist-worn actimeters (Actiwatch 64, MiniMitter). Alcohol and caffeine-containing beverages were prohibited starting the night before and throughout each experiment. The night before the experiment, participants were asked to go to bed at their usual bedtime, wake up at ∼07:00, and arrive at the lab at 08:30 to prepare for hd-EEG recordings (256 electrodes, Electrical Geodesics Inc.). Participants completed a baseline EEG recording session at 10:00, followed by six 2-h bouts of experimental tasks (either AB or DS) interleaved by EEG recording sessions, for ≥ 24 h of continuous wake (Figure 1). The stated goal was to stay awake for up to 36 h. In 9 of the 32 experiments (3 AB, 6 DS), subjects went to sleep in the evening (at ∼ 20:30) after 36 h of wake. In the other cases (13 AB, 10 DS), subjects chose to go to sleep starting at ∼ 08:30, after 24 h of wake (Figure 1). In all cases (whether recovery sleep occurred during the day or at night), participants were woken up after ∼ 8 h of sleep, and after 30 min (to reduce the influence of sleep inertia30) completed a final testing session (R1). R1, therefore, occurred either at ∼ 17:00 or at ∼ 06:00. In the 9 experiments with 36 h of wake, participants performed 4 additional 2-h bouts of tasks interleaved with EEG recordings, but the data from these additional wake sessions are not discussed in this paper.
Each EEG session consisted of two 2-min eyes-open periods, interleaved by two 2-min eyes-closed periods (order counterbalanced across participants). When signs of drowsiness were detected (closure of eyelids, slow eye movements, eye blinks) the experimenter spoke to the participants and alerted them. At the beginning and at the end of each session, sleepiness was evaluated using self-rating questionnaires (Stanford Sleepiness Scale31) and a 10-min psychomotor vigilance task (PVT32,33). Participants performed the PVT (adapted version34 for ∼10 min while fixating a cross placed at the center of a computer screen). Their task was to respond as quickly as possible in order to stop a millisecond counter that started to scroll at randomly selected intervals between 2 and 12 sec. “Lapses” were defined as responses > 500 msec.32 To ensure signal quality during EEG recordings, electrode impedance was checked before each recording session and kept below 50 KΩ. A 15-min break was scheduled at the end of each testing session, and a 1-h dinner break was scheduled at 20:00. Two experimenters took turns attending the participants to prevent them from falling asleep and to ensure adherence to the protocol throughout the entire duration of the experiment.
EEG Analyses
Wake EEG
Analysis of wake EEG was based on the eyes-open sessions. Four min of spontaneous EEG for each testing session were collected at 500 Hz, first-order high-pass filtered (Kaiser type FIR, 0.5 Hz) and low-pass filtered (Kaiser type FIR, 58 Hz). EEG epochs containing signs of sleep (K-complexes or sleep spindles) were excluded from the analysis. An automatic outlier detection tool based on amplitude threshold criteria was used to detect bad channels and confirmed by visual inspection. Rejected channels were then interpolated using spherical splines (NetStation, Electrical Geodesic Inc.). Independent component analysis (ICA)35 was used to remove ocular, muscle, and electrocardiograph artifacts using EEGLAB routines. Only ICA components with specific activity patterns and component maps characteristic of artifactual activity36 were removed (see Hulse et al.37 for examples). Residual slow ocular artifacts were removed using an ocular artifact removal tool (Netstation). After excluding electrodes located on the neck/face region, the signal for each channel was down-sampled to 128 Hz using the Matlab resample routine and re-referenced to the average of the remaining good channels (175-185 channels per recording). For each EEG derivation, power spectral density estimates were computed by fast Fourier transform (FFT) in 4-s Hamming window (applying the pwelch function in the Matlab signal processing toolbox). Spectral power estimates were thus performed with a 0.25 Hz bin resolution.
Analysis of Individual Theta Waves
EEG signals for each derivation were re-referenced to the average of the 2 mastoids and high-pass filtered at 2.5 Hz using the eegfilt FIR filtering option available in EEGLAB. Filter parameters were selected in order to achieve minimal wave shape and amplitude distortion. For each channel, individual half-waves were detected on artifact-free 4-s epochs sampled at 500 Hz. Half-waves were defined as negative deflections between 2 consecutive zero crossings. According to a detection procedure similar to that previously used in our laboratory,38 the position of the negative and positive peak was determined based on the zero crossings of the signal first derivative after applying a 10-msec moving average filter. To avoid possible confounding effects due to spurious low amplitude deflection of the signal, all 5-9 Hz detections were subdivided into 5 equal percentiles based on their negative peak amplitude (the distribution was determined by pooling all 5-9 Hz detections across sessions (B1 to R1) for each subject and channel separately); for each session, only the detections that exceeded the top 20% amplitude threshold were then considered theta waves and selected for further analysis. We focused on the negative peak of the detected waves because a previous study in rats showed that during wake, deep extracellular positive potentials spanning the range of slow and theta activity (2-6 Hz) are associated with periods of neuronal silence (OFF periods25); other studies showed that during sleep deep extracellular positive potentials in the slow activity range, which are associated with OFF periods, revert at the cortical surface, appearing in the EEG as negative potentials.39,40
Sleep EEG
EEG sleep recordings were performed during one baseline night (BSL; recorded a week before the first prolonged wakefulness period occurred) and after 24 or 36 h of AB and DS (recovery sleep). EEG signals were sampled at 500 Hz and acquired referenced to the Cz electrode. Impedances were kept < 50 KΩ at the beginning of each recording. Sleep staging was done on a 30-sec epoch basis according to current established criteria,41 using 6 referential EEG channels (F3-A2, F4-A1, C3-A2, C4-A1, O1-A2, and O2-A1), right and left EOG, and submental EMG signals. After excluding the electrodes located on the neck/face region and those with impedance > 50 KΩ (measured at the end of the recording), EEG signals from the remaining derivations (175-185 channels per recording) were down-sampled to 128 Hz using the Matlab resample routine, average referenced, and filtered (1 to 40 Hz). Spectral density analysis with a 0.16 Hz bin resolution was performed (Welch's averaged modified periodogram with a Hamming window, averages of five 6-sec epochs). NREM sleep epochs exceeding a threshold based on the mean power values in the 0.8 to 4.48 Hz and 20 to 30 Hz frequency bands in at least one channel were excluded from the analysis.
Statistics
Statistical nonparametric mapping (SnPM)42 was used to assess topographical differences in hd-EEG recordings. For all other comparisons analyses of variance (ANOVAs) were used when appropriate. To test contrasts, post hoc two-tailed t-tests were used (P < 0.05).
RESULTS
Effects of Prolonged Wake on Sleepiness, Vigilance, and Global Wake EEG Power
As expected, in both experiments (AB and DS) participants became less vigilant with time spent awake. Specifically, subjective sleepiness as well as mean reaction time and number of lapses in the PVT started increasing after 18 h spent awake, remained high or increased after 24 h of wake (T7), and dropped after recovery sleep (R1, Figure 2). This time course is consistent with the results of a previous study that found that PVT lapses were near-linearly related to the cumulative duration of wakefulness over ∼16 h.43
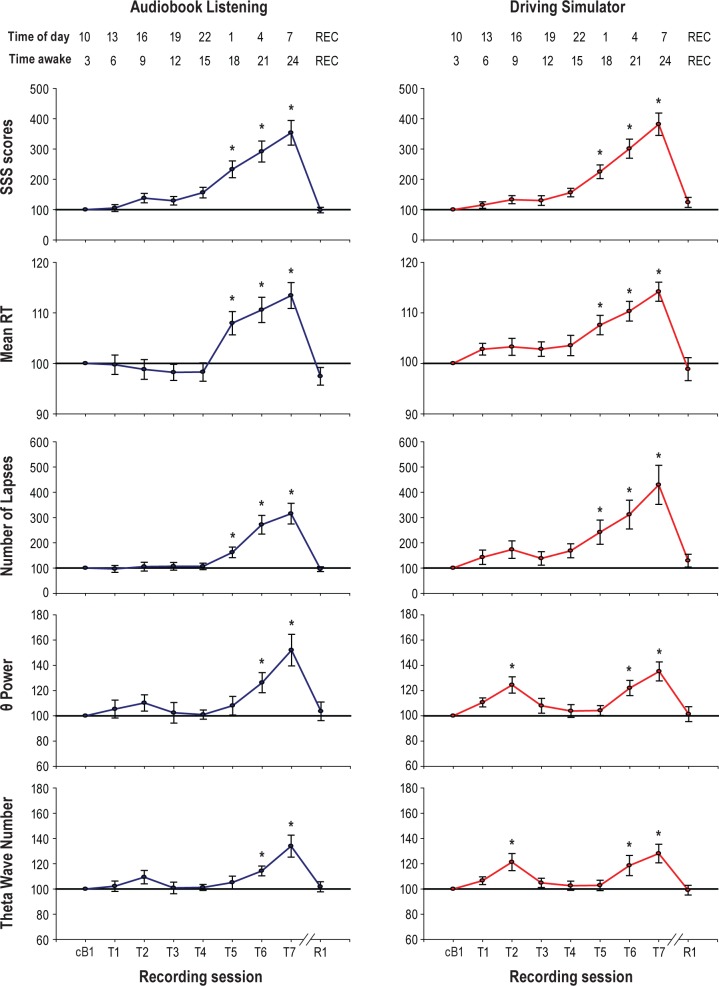
Figure 2.
Changes in vigilance and EEG theta power during prolonged wake. From top to bottom, subjective sleepiness (Stanford Sleepiness Scale, SSS scores), PVT mean reaction time (RT), number of PVT lapses, wake EEG power in the theta range and number of theta waves (average of 185 EEG derivations). Since exploratory analyses demonstrated that baseline values for AB and DS were similar (paired t-test; t = 0.44, P = 0.6), they were combined to calculate a new “common” average baseline (cB1). Each trace depicts mean ± SEM (n = 16) of each session expressed relative to cB1 (= 100). One-way repeated measure ANOVA with factor “Session.” SSS scores: F8,120 = 162.72, P < 0.00001 (AB); F8,120 = 70.94, P < 0.00001 (DS). Mean RT: F8,120 = 33.62, P < 0.00001 (AB); F8,120 = 15.29, P < 0.00001 (DS). Number of Lapses: F8,120 = 36.82, P < 0.00001 (AB), F8,120 = 24.28, P < 0.00001 (DS). Theta power: F8,120 = 5.71, P < 0.00001 (AB); F8,120 = 6.93, P < 0.00001 (DS). Wave number: F8,120 = 8.12, P < 0.00001 (AB and DS). Post hoc 2-tailed paired t-tests compared each testing session with cB1. Asterisks show significant change from cB1 (P < 0.005, Bonferroni corrected). REC: time after recovery sleep.
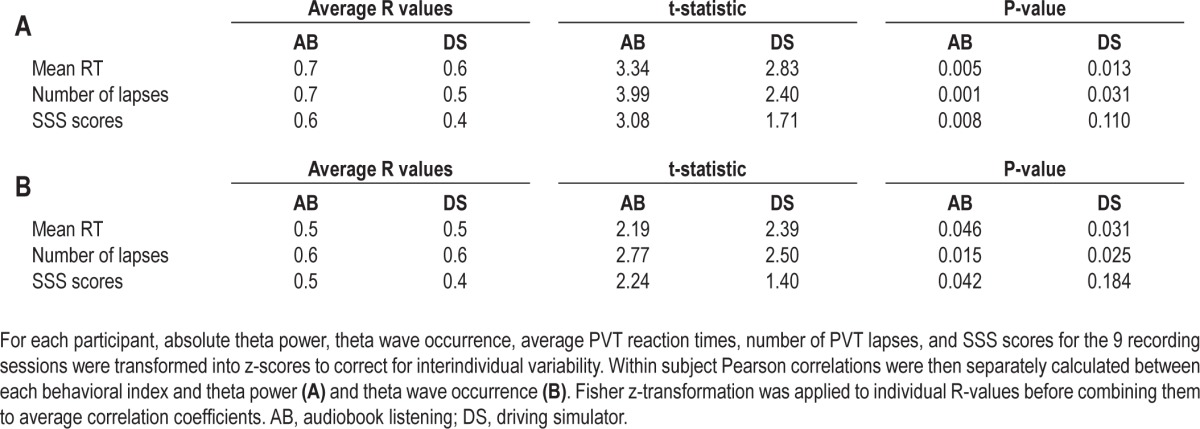
We first assessed changes in the wake EEG spectrum after 24 h of wake as measured by averaging across all channels. Consistent with previous studies,12,16,44 in both experiments the largest increase occurred in the theta band (5-9 Hz; Figure 3A, B). Beta activity (13-20 Hz) also increased, but to a lesser extent compared to theta activity, while EEG power in the SWA and alpha (9-13 Hz) range did not change (Figure 3A, B). Period-amplitude analyses were then applied to investigate whether changes in theta power were paralleled by changes in key parameters of individual theta waves. The number of theta waves measured by averaging across all channels significantly increased in both conditions, while theta wave amplitude significantly increased in AB but showed only a trend in DS (Figure 3C, D). A time course analysis also found that changes in the theta band reflected wake duration, starting to increase after 21 h of wake, peaking 3 h later, and renormalizing after recovery sleep (Figure 2). Correlation analyses showed that in both experiments the temporal evolution of the increase in theta power was paralleled by increase in PVT reaction time and occurrence of PVT lapses (Table 1A). Subjective sleepiness also showed a progressive increase proportional to theta power, but only for the AB condition (Table 1A). The time course of theta wave occurrence paralleled that of the EEG theta power, with significant increases after 21 and 24 h of wake and renormalization after recovery sleep (Figure 2). As for theta power, changes in the number of theta waves also paralleled changes in objective and subjective sleepiness (Table 1B).
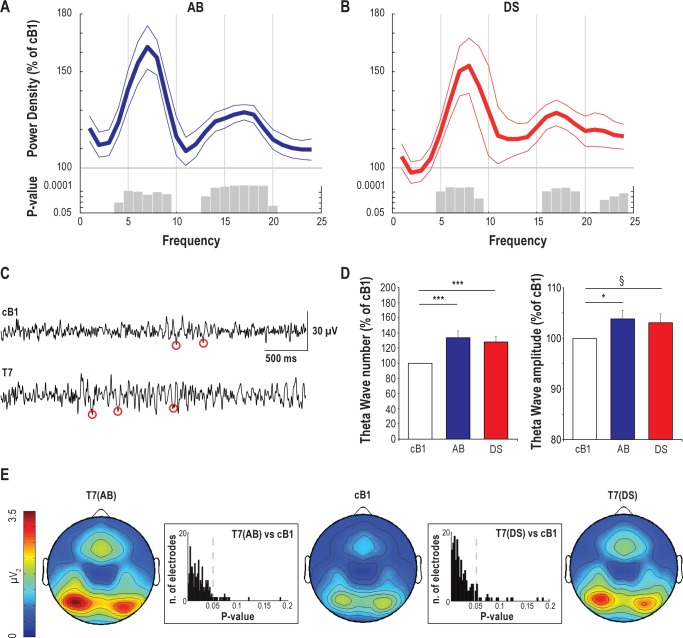
Figure 3.
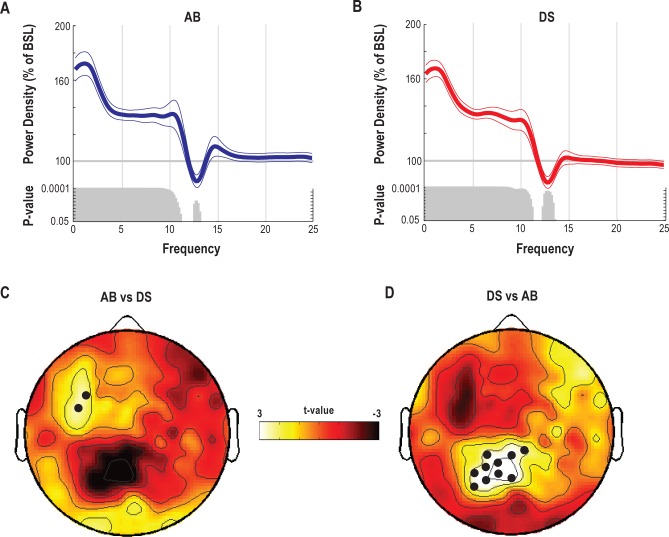
Global effects of prolonged wake on EEG power spectra, theta power distribution, and occurrence of theta waves. (A,B) Relative EEG power spectra during T7 relative to baseline (average of 185 channels, mean ± SEM, n = 16). Since exploratory analyses demonstrated that baseline values for AB and DS were similar (paired t-test; t = 0.44, P = 0.6), they were combined to calculate a new “common” average baseline (cB1). Gray bars indicate significant bins (paired t-test; P < 0.05). (C) Representative EEG records from one subject depicting 4-s of EEG raw signal during cB1 and T7 (channel F3). Red circles indicate the negative peaks of theta waves detected in each EEG trace. (D) Changes in number and negative peak amplitude of theta waves between cB1 (100%) and T7 for the 2 experiments (average of 185 channels, mean ± SEM, n = 16). ***P < 0.005; *P < 0.05; §P = 0.08. (E) Topographic distribution of absolute theta power (average spectral density in the theta range) after 24 h of wake (T7) for both experiments and during cB1. Values (mean of 16 participants) were plotted at the corresponding position on the planar projection of the scalp surface and interpolated (biharmonic spline) between electrodes. Boxes represent the P-value distribution for the theta power topographic statistical comparison at T7 vs. cB1 for AB (left) and DS (right).
Table 1.
Correlations between changes in waking EEG theta power (A) and theta wave occurrence (B) and behavioral indexes of sleepiness
We then performed a topographic analysis to assess whether changes in theta activity were global. As in a previous study,40 we operationally defined as global those changes that were detected in more than 50% of scalp EEG sensors. Changes in theta activity after 24 h of wake involved most of the scalp, and were especially large over bilateral occipital and frontal midline areas. Specifically, SnPM analysis showed increased (P < 0.05) theta power over 170 and 131 out of 185 electrodes for AB and DS, respectively (Figure 3E). The increase in theta wave number was also global, involving 134 and 125 out of 185 EEG derivations for AB and DS, respectively (SnPM corrected; P < 0.05; data not shown). By contrast, the change in theta wave amplitude involved a more limited set of electrodes (43 of 185 for AB and 13 of 185 for DS), only trending toward significance (SnPM corrected; P < 0.06; data not shown).
Topographic EEG Changes Are Task-Specific
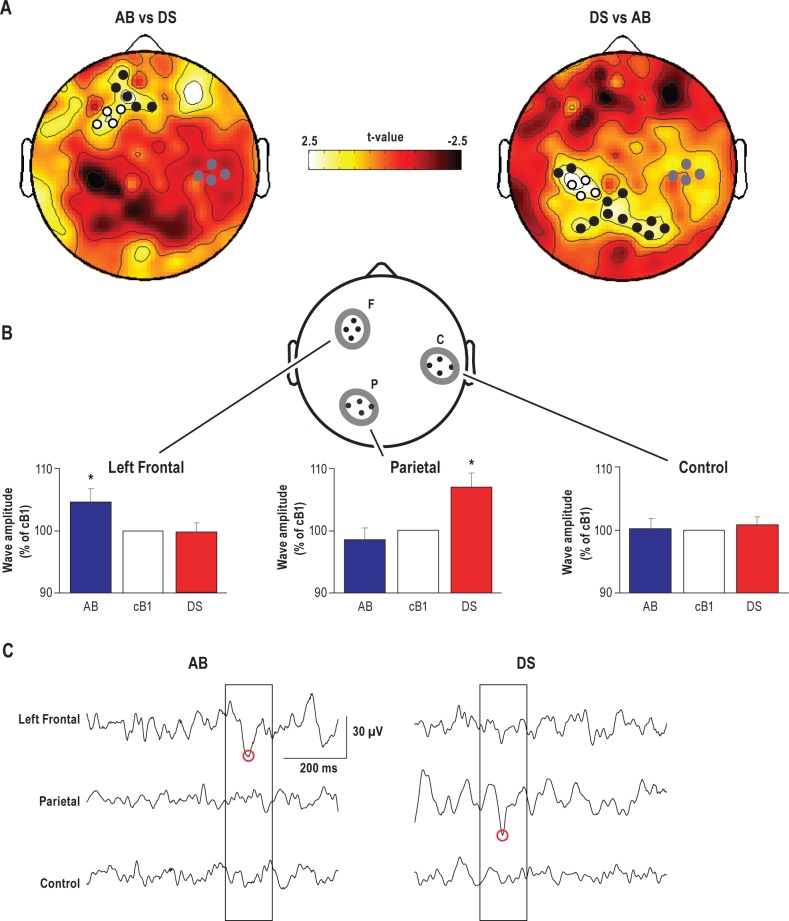
Having established a global effect of prolonged wake on the wake theta activity, we assessed whether each experimental condition left a specific “EEG signature” by contrasting the respective topographic EEG changes (AB vs. DS). We found evidence for region-specific EEG effects after both 21 h (T6) and 24 h (T7) of wake. At T6, we found that, compared to the rest of the scalp, theta power disproportionately increased (SnPM test) over a cluster of electrodes (n = 9) over the left frontal cortex in the AB condition and over a parietal cluster of electrodes (n = 16) in the DS condition (Figure 4A). The average magnitude of these task-related theta power changes calculated over the electrodes included in the 2 significant clusters was 8.5% ± 1%. Specifically, in the AB condition, theta power was 9.5% higher relative to the DS condition over the left frontal cortex, while in the DS condition it was 7.9% higher relative to the AB condition over the parietal cortex.
Figure 4.
Local task-dependent effects of prolonged wake on EEG theta power and theta wave occurrence. (A) Topographic distribution of t-values from paired t-tests contrasting relative power changes from cB1 to T6 session between AB and DS in the theta frequency band (5-9 Hz). Here, negative t-values indicate changes in the opposite direction. Black and white dots show channels with significant increase in spectral power (P < 0.05, SnPM using a supra-threshold cluster analysis). White dots show channels included in the left frontal and parietal regions, while gray dots show channels included in the control region for the theta wave amplitude analysis. (B) Theta wave negative peak amplitude (mean ± SEM, n = 16) in the task-related regions (F: left frontal; P: parietal) and a control region (C) during T6 relative to cB1. One-way ANOVA with repeated-measure in each cluster demonstrated significant condition effect in the task-related clusters F (F2,30 = 3.67, P = 0.03) and P (F2,30 = 3.84, P = 0.03), but not in the control cluster C (F2,30 = 0.03, P = 0.97). Post hoc Student-Newman-Keuls Range tests analyzing the difference between conditions revealed that AB led to a significant increase over the F cluster and DS induced a similar increase over the P cluster (* significantly different from each of the other 2 conditions). Values for each ROI were averaged across the 4 electrodes. Note that the electrodes depicted in panel B are simply a schematic representation of the three ROIs. The exact location of the included electrodes is shown in panel A, white and gray dots. (C) Representative examples of local theta waves (boxed). Red circles indicate the negative peaks of theta waves detected in each EEG trace.
Within these 2 significant clusters, we then selected the electrode reaching the highest t-value to identify 2 regions of interest (ROIs) localized over the left frontal and parietal areas (F and P, Figure 4B). To allow for a between-condition comparison based on the same number of channels, 3 neighboring electrodes included in the significant cluster for theta power and with the minimal geodesic distance from the electrode reaching the highest t-values were selected for each ROI. A third ROI, located over the right hemisphere and equidistant from the 2 ROIs, was used as a control region (C, Figure 4B). For each scalp EEG electrode included in the 3 ROIs, amplitude (negative peak) and number of theta waves were calculated during baseline and at T6. These values were then averaged across the 4 electrodes within each ROI and evaluated for statistical comparison. One-way ANOVAs with repeated measurements in each ROI cluster showed a significant difference between conditions in the F cluster (F2,30 = 3.67, P = 0.037) and P cluster (F2,30 = 3.84, P = 0.032), while no significant condition effect was found in C (F2,30 = 0.03, P = 0.968). Post hoc Student-Newman-Keuls Range tests showed a significant increase in the amplitude of theta waves over a left frontal cluster in the AB experiment and over a parietal cluster in the DS condition, while no significant changes due to the 2 tasks were found in the control region (Figure 4B). Moreover, at T6 the number of theta waves increased over the left frontal ROI in the AB experiment, and over the parietal ROI in the DS experiment (∼20% increase, left frontal ROI, AB; ∼10% increase, parietal ROI, DS; paired t-tests, P < 0.05; data not shown). The regional effects seen at T6 were also present at T7, as detailed in Figure S1 (supplemental material), the only difference being that the increase in the amplitude of theta waves over a left frontal cluster in the AB experiment trended but did not reach significance relative to the DS condition.
The regional changes in theta waves described above do not necessarily mean that these waves occurred only in one area but not in another; in fact, it could be that they were detected at the same time over all 3 ROIs, but were larger in one of them. To distinguish between these 2 possibilities, we included all 12 channels (4 in each ROI); once a theta wave was detected in at least one channel in one ROI (seed), we checked whether the same event was simultaneously occurring in at least one channel included in the other 2 ROIs (target). To allow for a time lag between distal cortical regions, the overlap between target and seed events was calculated over a time window covering the entire seed event duration (i.e., from the zero-crossing preceding the negative peak to the zero-crossing following the positive peak; Figure 4C). If there was no overlap, the seed event was considered “local.” At T6, we found that > 90% of all theta events were local in all 3 ROIs, including the control region (left frontal ROI, AB = 91.05 ± 1.25, DS = 91.67 ± 1.07; parietal ROI, AB = 91.14 ± 1.40, DS = 92.98 ± 1.17; control ROI, AB = 90.72 ± 1.30, DS = 92.02 ± 1.16). Thus, not only sleep slow waves and spindles are mainly local, as we recently found,40,45 but also wake theta waves, as shown here. Crucially, we also found that all task-dependent effects previously described (Figure 4) were confirmed when period-amplitude analysis was applied only to these local events (data not shown). By contrast, theta wave amplitude did not show task-dependent regional difference when only the ∼ 10% of waves that showed overlap were analyzed separately (paired t-tests, P > 0.05; data not shown), suggesting that task-specific EEG changes at T6 are mostly driven by local events.
Finally, we aimed at determining whether the detected events were occurring as isolated waves or as bursts of theta waves, which are known to occur after prolonged wakefulness.46,47 For each participant we calculated the time interval between consecutive theta wave detections in each of the 4 channels included in the 3 ROIs (F, P, and C), both at the beginning (cB1) and at the end (T7) of the prolonged wakefulness period for the 2 conditions. If the end of a wave (zero crossing following the positive peak) was contiguous (delay of 2 msec due to the 500 Hz sampling rate) to the beginning of the next detected wave (zero crossing preceding the negative peak), the 2 waves were considered as part of a burst. All other detected waves were considered isolated waves. We then calculated the proportion of these 2 types of events expressed as percentage of the total number of detected events and averaged the obtained value across the electrodes within a given cluster (F, P, or C). We found that the great majority of detected events were isolated waves (left frontal ROI, cB1 = 94% ± 1%, AB T7 = 91% ± 2%, DS T7 = 91% ± 2%; parietal ROI, cB1 = 93% ± 1%, AB T7 = 90% ± 2%, DS T7 = 92% ± 2%; control ROI, cB1 = 91% ± 2%, AB T7 = 90% ± 2%, DS T7 = 89% ± 2%).
NREM Sleep EEG Changes Are Task-Specific and Correlate with Wake EEG Changes
As shown in Table 2, after both experiments participants slept longer and deeper than during baseline, with more time spent in the deepest stages of slow wave sleep (N3) and REM sleep. During baseline, waking after sleep onset was quite high (49 ± 6 min), decreasing sleep efficiency (85% ± 1%) and increasing REM sleep latency (109 ± 10 min), possibly due to incomplete adaptation to the experimental setting. Conversely, few participants showed sleep onset REM sleep after AB (n = 5) and DS (n = 1), resulting in short group average latency to REM sleep (43 ± 12 min for AB and 57 ± 9 min for DS). Since all these participants had their recovery sleep during the day, circadian influences on REM sleep may have played a role.
Table 2.
Sleep Parameters during baseline and recovery sleep
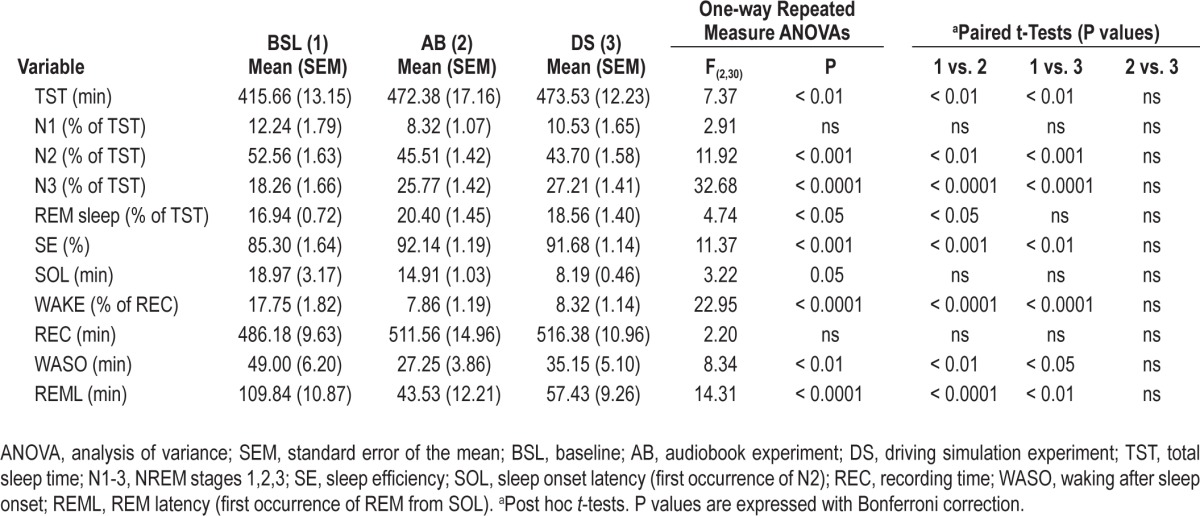
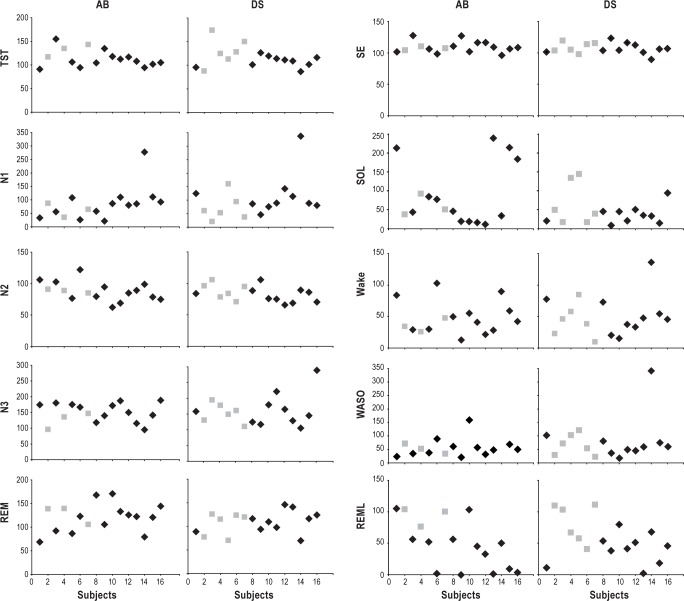
Changes in sleep parameters were very similar after AB and DS, with no significant differences between the 2 conditions (Table 2), despite the fact that recovery sleep occurred at night in 6 of the DS experiments but in only 3 of the 16 AB experiments. Due to the limited number of participants with night sleep in the AB experiment, participants with recovery sleep at night could not be directly compared to participants with day sleep. However, visual inspection of Figure 5 shows a large overlap between these 2 subgroups.
Figure 5.
Sleep parameters during recovery sleep for each of the 16 participants. TST, total sleep time; N1-3, NREM stages 1, 2, 3; REM, REM sleep; SE, sleep efficiency; SOL, sleep onset latency; WASO, wake after sleep onset; REML, latency to first REM sleep episode. All values are % relative to BSL (= 100). In gray are displayed the participants whose recovery sleep was recorded at night, after 36 h of wake. AB, audiobook listening; DS, driving simulator.
As expected the EEG spectra of recovery sleep showed an overall increase (averaged across 185 channels) in a broad range of low frequencies (1-11 Hz) including SWA, and a decrease in the spindle range (12-14 Hz; Figure 6A, B). To test for task-specific effects, we again performed a topographic analysis contrasting the power spectral density of the 2 recovery sleep nights (after AB vs. after DS) in the 1-11 Hz frequency range. As for the wake theta EEG power, region-specific EEG changes were evident during sleep. Specifically, the 1-11 Hz EEG, power disproportionately increased over left frontal derivations in the AB condition and over parietal derivations in the DS condition (Figure 6C, D). Of note, in the course of recovery sleep these regional EEG changes became more evident and extended over a larger cluster of electrodes, reaching a maximum in the third NREM sleep episode when, for example, 8 derivations in the left frontal region showed significant increases in the 1-11 Hz range in the AB experiment (data not shown).
Figure 6.
Effects of prolonged wake on NREM EEG power spectra during recovery sleep. (A,B) Relative EEG power spectra for the AB and DS experiments averaged across 185 channels (mean ± SEM, n = 16) during NREM recovery sleep relative to BSL. Gray bars indicate significant bins (2-tailed paired t-test; P < 0.05). (C,D) Topographic distribution of t-values from paired t-tests contrasting relative power changes between the AB and DS recovery nights in the 1-11 Hz frequency band. Negative values indicate changes in the opposite direction. Channels showing significant increase in spectral power in AB relative to DS are indicated by black dots (P < 0.05 SnPM supra-threshold cluster analysis; note that in C the cluster size does not exceed the minimum threshold cluster size for significance (i.e., n = 2). Thus, the dots for C correspond to P < 0.05, uncorrected).
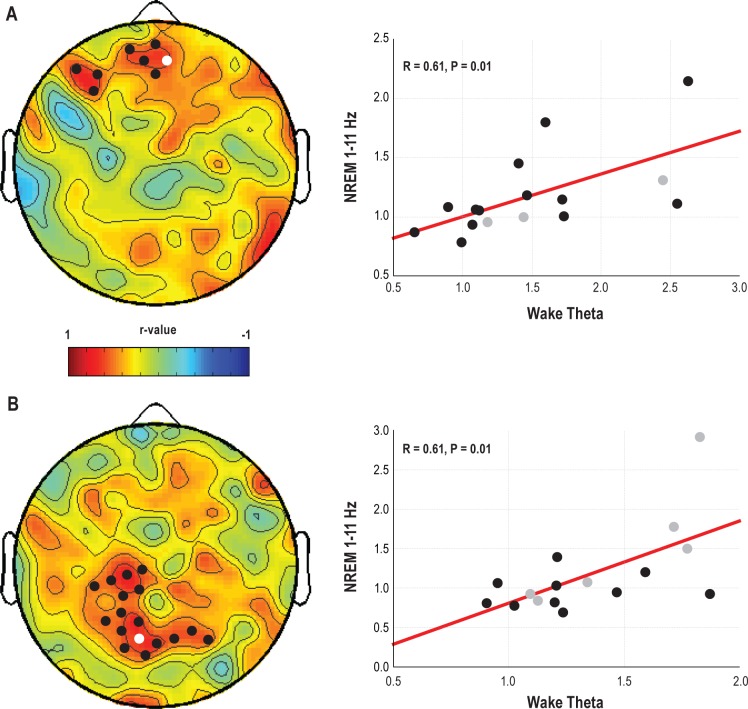
Finally, we tested whether changes in wake theta activity were predictive of the observed EEG changes during recovery sleep. To do so, for each experimental condition we correlated the topographical change in wake theta power at T7 with the topographical change in the NREM sleep 1-11 Hz range during the recovery night. The regional, task-specific increase in theta power during prolonged wake was followed by an increase in the low frequencies during subsequent recovery NREM sleep over the same regions, with high positive correlations especially over frontal derivations for the AB experiment (r = 0.61; P = 0.01) and over parietal derivations for the DS experiment (r = 0.61; P = 0.01; Figure 7).
Figure 7.
Correlation between changes in theta power during prolonged wake and changes in low frequencies during recovery sleep as compared to baseline sleep. Left panels, topographic distribution of r-values from Pearson correlation between changes in wake theta power (T7 relative to cB1) and changes in whole night NREM 1-11Hz power (recovery sleep relative to BSL) for the AB (A) and DS (B) experiments. Black dots represent derivations with significant correlations (P < 0.05). Right panels, scatterplots for a representative channel included in the AB and DS significant clusters (colored in white in the topographies). In gray those participants whose recovery sleep was recorded at night, after 36 h of wake.
DISCUSSION
In the present study we employed resting hd-EEG recordings during prolonged wake associated with extensive practice of either a language task or a visuomotor task, and during subsequent recovery sleep. To our knowledge, this is the first study in which participants were kept awake twice for the same extended period of time, but the “quality” of wake was manipulated to induce two types of physiological, experience-dependent plasticity as different as possible from each other. Indeed, the two tasks, audiobook listening (AB) and driving simulation (DS), were chosen to target functionally and anatomically distinct cortical circuits, mainly left fronto-temporal areas for AB,26,27 and occipito-parietal networks for DS.28,29
As expected, both conditions resulted in a marked (∼50%), global increase in resting wake EEG theta power (5-9 Hz) at the end of 24 h of wake, accompanied by behavioral signs of sleepiness. Several other groups reported also an increase in resting EEG alpha power after prolonged wakefulness.11,48 Here we did not find such an increase after 24 h of wake, possibly due to the strong circadian influences affecting the alpha range early in the morning.15
However, we also found that wake theta power and the amplitude of theta waves showed regional task-dependent changes, being further enhanced (∼8% above the global change) over the left frontal derivations in the AB experiment, and over posterior parietal regions in the DS condition. We also found that local changes in the wake theta power were correlated with similar local changes in a broad range of EEG low frequencies (1-11 Hz) during recovery NREM sleep. Thus, extended experience-dependent plasticity of specific brain circuits during wake results in local wake rest EEG changes over the same regions, and is associated with similar changes in the recovery sleep EEG. Of note, while sleep SWA shows the most consistent changes in response to wake quantity and quality, most studies including the current one found increases in a broad range of low frequencies during recovery sleep (∼0.5-11 Hz)8–10
Classically, theta (5-9 Hz) activity has been described as the wake EEG marker of sleep need,12,14,49 which is associated with the homeostatic component of the two-process model of sleep regulation.50,51 Thus, theta activity increases with time spent awake and it is followed by a proportional increase in low frequencies in subsequent sleep. Our results showing a global increase in theta activity during prolonged wake, combined with an increase in EEG low frequencies during subsequent recovery sleep, support this view. Although this last point was not central in our study, it is worth noting that most of our participants had their recovery sleep recorded in the morning. This, together with the fact that in DS as compared to AB, twice as many participants had their recovery sleep recorded after 36 hours, may explain the degree of interindividual variability shown in Figure 7. Specifically, in the DS condition few individuals exhibited a decrease in recovery NREM EEG low frequency power despite an increased theta power during wakefulness. This suggests that a more rigorous approach is needed in future studies investigating such relationship.
More importantly, our results show that the homeostatic regulation of wake theta activity is as local as NREM sleep SWA, i.e., it can reflect to the same extent local changes triggered by neural activity in specific neural circuits. It is well established that sleep slow waves can be regulated at a local level based on prior waking activity.52–56 Many recent studies, however, also show that changes in NREM SWA reflect the occurrence of changes in synaptic strength in local networks, consistent with the idea that neuronal plasticity and sleep need are linked.5,21,57 Thus in humans, repetitive cortical transcranial magnetic stimulation (rTMS) results in a local potentiation of cortical responses, followed by a local increase in sleep SWA, after a 5-Hz potentiation protocol but not after sham stimulation.58,59 Similarly, paired-associative stimulation (PAS) protocols in which either cortical potentiation or depression could be induced resulted in local NREM SWA increases and decreases, respectively.60,61 Finally, in both humans and rats, learning a motor task increases NREM SWA specifically in the trained cortical area, and more so than merely performing a previously learned task.22,62 Overall, therefore, our current findings not only confirm that wake plasticity is a major determinant of sleep need as measured by NREM SWA and other low frequencies, but also suggest that by using hd-EEG, a “trace” of this need can be detected in the wake EEG of the activated circuits.
Importantly, the regional changes in theta power that we observed were in most cases (∼90%) due to the presence of waves that could be detected only in one cortical area but not in another, rather than to the presence of global waves with different amplitude across regions. In other words, local changes in wake theta activity were due to local theta waves. What cellular mechanism may underlie the occurrence of these local theta waves? A recent study with intracortical multi-array recordings may offer a clue. We found that when rats stayed awake longer than usual to explore or learn, some cortical neurons went off-line, interrupting and resuming their discharge patterns as they normally do only in sleep.25 These OFF periods were local, i.e., at different times they could occur in one cortical area and not another, increased in frequency with the duration of exploratory activities and declined after recovery sleep, and when they happened in an area relevant for behavior they led to performance errors. Crucially, OFF periods were associated with a local EEG slow/theta wave, although rats appeared fully awake and the global scalp EEG continued to show low voltage fast activity typical of wake. Thus, although direct evidence is missing, the local increase in theta power detected with hd-EEG recordings in humans may reflect the same phenomenon described in rats, namely a group of neurons in a restricted area of the brain going off line, perhaps due to increased neuronal instability and activity-dependent synaptic “overload.”25,63 In this respect, intracortical unit recordings combined with hd-EEG and training in specific tasks will be helpful in testing this possibility in humans.40
The local changes in wake theta power were small relative to the global changes (∼ 8% vs. 50% increase). It should be noted that, although participants were highly engaged by the tasks at regular intervals across the entire experimental period, total practice time accounted for ∼ 50% of the 24-h period. Moreover, although AB and DS were selected to activate specific networks, it is likely that both tasks also activated overlapping circuits throughout the brain. Finally, it is likely that EEG recordings, being especially sensitive to synchronous activity, may reflect predominantly transient global changes in cortical excitability associated with fluctuations in the activity of arousal/neuromodulatory systems,64 possibly overshadowing local changes. This is also suggested by the somewhat weaker task-dependent effects observed at T7—where widespread increases in wake theta activity were more prominent relative to T6. In summary, our results add to increasing evidence that sleep deprivation may lead to a covert form of dissociated, “sleep-wake” behavioral state,65,66 with sleep-like periods emerging in an otherwise awake brain over specific cortical circuits as a function of previous waking activity.25,64,67–69 Thus, wakefulness and sleep could be seen as part of a continuum in which the transition between one state and the other reflects the number and spatial extent of these local events. Specifically, prolonged wakefulness associated with low levels of arousal-promoting neuromodulators may be characterized by progressively increasing local sleep-like events until a “critical mass” is reached, driving the system to transition to a full-fledged sleep state. These local sleep-like events, occurring in an otherwise awake individual, may be responsible for the cognitive and behavioral consequences associated with prolonged wakefulness, therefore raising intriguing questions for future studies. For instance, what is the precise association between local theta events and specific cognitive performance failures? In rats, the occurrence of an OFF period recorded from frontal cortical areas within several hundreds of ms before the reaching attempt was often associated with failure to successfully grasp a sugar pellet,25 suggesting that performance deficits observed during acute total sleep deprivation or chronic sleep restriction could indeed be linked to the occurrence of local “sleep-like” events.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Tononi has consulted for Philips Respironics and together with Dr. Brady Riedner, has been involved in a research study in humans supported by Philips Respironics. This study is not related to the work presented in the current manuscript. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Andreas Buchmann, Daniela Dentico, Brad Hulse, and Eric Landsness for helping with the experiment; Jeffrey Guokas for help with data preprocessing, and Yuval Nir and Vladyslav Vyazovskiy for insightful discussion. The authors are particularly grateful to Dr. Ruth Benca and the polysomnographic technologists from Wisconsin Sleep for their contribution to subject screening and sleep scoring. Dr. Tononi was supported by NINDS grant R01NS055185 and NIMH grant 1P20MH-077967-01A1.
SUPPLEMENTAL MATERIAL
(A) Topographic distribution of t-values from paired t-tests contrasting relative power changes from cB1 to T7 session between AB and DS in the theta frequency band (5-9 Hz). Black and white dots show channels with significant increase in spectral power (P < 0.05, SnPM using a supra-threshold cluster analysis). White dots show channels included in the left frontal and parietal regions while gray dots show channels included in the control region for the theta wave amplitude analysis. (B) Theta wave negative peak amplitude (mean ± SEM, n = 16) in the task-related regions (F, left frontal; P, parietal) and a control region (C) during T7 relative to cB1. One-way ANOVA with repeated-measure in each cluster demonstrated significant condition effect in the task-related clusters F (F2,30 = 3.87, P = 0.03) and P (F2,30 = 4.91, P = 0.01), but not in the control cluster C (F2,30 = 0.03, P = 0.97). Post hoc Student-Newman-Keuls Range tests analyzing the difference between conditions revealed that in the F cluster AB led to a significant increase relative to baseline but not relative to the DS condition (#, AB > cB1, AB = DS, DS = cB1), while in the P cluster the increase in DS was significantly different from both cB1 and AB (*). Values for each ROI were averaged across the 4 neighboring electrodes. Note that the electrodes depicted in panel B are simply a schematic representation of the 3 ROIs. The exact location of the included electrodes is shown in panel A, white and gray dots.
REFERENCES
- 1.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–8. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 2.Gilestro GF, Tononi G, Cirelli C. Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science. 2009;324:109–12. doi: 10.1126/science.1166673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu ZW, Faraguna U, Cirelli C, Tononi G, Gao XB. Direct evidence for wake-related increases and sleep-related decreases in synaptic strength in rodent cortex. J Neurosci. 2010;30:8671–5. doi: 10.1523/JNEUROSCI.1409-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bushey D, Tononi G, Cirelli C. Sleep and synaptic homeostasis: structural evidence in Drosophila. Science. 2011;332:1576–81. doi: 10.1126/science.1202839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Mackiewicz M, Shockley KR, Romer MA, et al. Macromolecule biosynthesis - a key function of sleep. Physiol Genomics. 2007;31:441–57. doi: 10.1152/physiolgenomics.00275.2006. [DOI] [PubMed] [Google Scholar]
- 7.Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6:e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borbely AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol. 1981;51:483–95. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- 9.Dijk DJ, Brunner DP, Borbely AA. Time course of EEG power density during long sleep in humans. Am J Physiol. 1990;258:R650–61. doi: 10.1152/ajpregu.1990.258.3.R650. [DOI] [PubMed] [Google Scholar]
- 10.Cajochen C, Foy R, Dijk DJ. Frontal predominance of a relative increase in sleep delta and theta eeg activity after sleep loss in humans. Sleep Res Online. 1999;2:65–9. [PubMed] [Google Scholar]
- 11.Torsvall L, Akerstedt T. Sleepiness on the job: continuously measured EEG changes in train drivers. Electroencephalogr Clin Neurophysiol. 1987;66:502–11. doi: 10.1016/0013-4694(87)90096-4. [DOI] [PubMed] [Google Scholar]
- 12.Cajochen C, Brunner DP, Krauchi K, Graw P, Wirz-Justice A. Power density in theta/alpha frequencies of the waking EEG progressively increases during sustained wakefulness. Sleep. 1995;18:890–4. doi: 10.1093/sleep/18.10.890. [DOI] [PubMed] [Google Scholar]
- 13.Aeschbach D, Matthews JR, Postolache TT, Jackson MA, Giesen HA, Wehr TA. Dynamics of the human EEG during prolonged wakefulness: evidence for frequency-specific circadian and homeostatic influences. Neurosci Lett. 1997;239:121–4. doi: 10.1016/s0304-3940(97)00904-x. [DOI] [PubMed] [Google Scholar]
- 14.Finelli LA, Baumann H, Borbely AA, Achermann P. Dual electroencephalogram markers of human sleep homeostasis: correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience. 2000;101:523–9. doi: 10.1016/s0306-4522(00)00409-7. [DOI] [PubMed] [Google Scholar]
- 15.Aeschbach D, Matthews JR, Postolache TT, Jackson MA, Giesen HA, Wehr TA. Two circadian rhythms in the human electroencephalogram during wakefulness. Am J Physiol. 1999;277:R1771–9. doi: 10.1152/ajpregu.1999.277.6.R1771. [DOI] [PubMed] [Google Scholar]
- 16.Cajochen C, Khalsa SB, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol. 1999;277:R640–9. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- 17.Cajochen C, Wyatt JK, Czeisler CA, Dijk DJ. Separation of circadian and wake duration-dependent modulation of EEG activation during wakefulness. Neuroscience. 2002;114:1047–60. doi: 10.1016/s0306-4522(02)00209-9. [DOI] [PubMed] [Google Scholar]
- 18.Retey JV, Adam M, Gottselig JM, et al. Adenosinergic mechanisms contribute to individual differences in sleep deprivation-induced changes in neurobehavioral function and brain rhythmic activity. J Neurosci. 2006;26:10472–9. doi: 10.1523/JNEUROSCI.1538-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tinguely G, Finelli LA, Landolt HP, Borbely AA, Achermann P. Functional EEG topography in sleep and waking: state-dependent and state-independent features. Neuroimage. 2006;32:283–92. doi: 10.1016/j.neuroimage.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 20.De Gennaro L, Marzano C, Veniero D, et al. Neurophysiological correlates of sleepiness: a combined TMS and EEG study. Neuroimage. 2007;36:1277–87. doi: 10.1016/j.neuroimage.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–50. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 23.Landsness EC, Crupi D, Hulse BK, et al. Sleep-dependent improvement in visuomotor learning: a causal role for slow waves. Sleep. 2009;32:1273–84. doi: 10.1093/sleep/32.10.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maatta S, Landsness E, Sarasso S, et al. The effects of morning training on night sleep: a behavioral and EEG study. Brain Res Bull. 2010;82:118–23. doi: 10.1016/j.brainresbull.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vyazovskiy VV, Olcese U, Hanlon EC, Nir Y, Cirelli C, Tononi G. Local sleep in awake rats. Nature. 2011;472:443–7. doi: 10.1038/nature10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drager B, Jansen A, Bruchmann S, et al. How does the brain accommodate to increased task difficulty in word finding? A functional MRI study. Neuroimage. 2004;23:1152–60. doi: 10.1016/j.neuroimage.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Mazoyer BM, Tzourio N, Frak V, et al. The cortical representation of speech. J Cogn Neurosci. 1993;5:467–79. doi: 10.1162/jocn.1993.5.4.467. [DOI] [PubMed] [Google Scholar]
- 28.Walter H, Vetter SC, Grothe J, Wunderlich AP, Hahn S, Spitzer M. The neural correlates of driving. Neuroreport. 2001;12:1763–7. doi: 10.1097/00001756-200106130-00049. [DOI] [PubMed] [Google Scholar]
- 29.Mader M, Bresges A, Topal R, Busse A, Forsting M, Gizewski ER. Simulated car driving in fMRI--Cerebral activation patterns driving an unfamiliar and a familiar route. Neurosci Lett. 2009;464:222–7. doi: 10.1016/j.neulet.2009.08.056. [DOI] [PubMed] [Google Scholar]
- 30.Tassi P, Muzet A. Sleep inertia. Sleep Med Rev. 2000;4:341–53. doi: 10.1053/smrv.2000.0098. [DOI] [PubMed] [Google Scholar]
- 31.Herscovitch J, Broughton R. Sensitivity of the stanford sleepiness scale to the effects of cumulative partial sleep deprivation and recovery oversleeping. Sleep. 1981;4:83–91. doi: 10.1093/sleep/4.1.83. [DOI] [PubMed] [Google Scholar]
- 32.Dinges D, Kribbs NB. Performing while sleepy: effects of experimentally-induced sleepiness. In: Monk TH, editor. Sleep, sleepiness and performance. New York: John Wiley and Sons; 1991. pp. 97–128. [Google Scholar]
- 33.Basner M, Dinges DF. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34:581–91. doi: 10.1093/sleep/34.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Meth Instr Comp. 1985;17:652–5. [Google Scholar]
- 35.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Jung TP, Makeig S, Humphries C, et al. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37:163–78. [PubMed] [Google Scholar]
- 37.Hulse BK, Landsness EC, Sarasso S, et al. A postsleep decline in auditory evoked potential amplitude reflects sleep homeostasis. Clin Neurophysiol. 2011;122:1549–55. doi: 10.1016/j.clinph.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riedner BA, Vyazovskiy VV, Huber R, et al. Sleep homeostasis and cortical synchronization: III. A high-density EEG study of sleep slow waves in humans. Sleep. 2007;30:1643–57. doi: 10.1093/sleep/30.12.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amzica F, Steriade M. Electrophysiological correlates of sleep delta waves. Electroenceph Clin Neurophysiol. 1998;107:69–83. doi: 10.1016/s0013-4694(98)00051-0. [DOI] [PubMed] [Google Scholar]
- 40.Nir Y, Staba RJ, Andrillon T, et al. Regional slow waves and spindles in human sleep. Neuron. 2011;70:153–69. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silber MH, Ancoli-Israel S, Bonnet MH, et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3:121–31. [PubMed] [Google Scholar]
- 42.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 44.Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 45.Andrillon T, Nir Y, Staba RJ, et al. Sleep spindles in humans: insights from intracranial EEG and unit recordings. J Neurosci. 2011;31:17821–34. doi: 10.1523/JNEUROSCI.2604-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guilleminault C, Billiard M, Montplaisir J, Dement WC. Altered states of consciousness in disorders of daytime sleepiness. J Neurol Sci. 1975;26:377–93. doi: 10.1016/0022-510x(75)90209-9. [DOI] [PubMed] [Google Scholar]
- 47.Banks S, Catcheside P, Lack L, Grunstein RR, McEvoy RD. Low levels of alcohol impair driving simulator performance and reduce perception of crash risk in partially sleep deprived subjects. Sleep. 2004;27:1063–7. doi: 10.1093/sleep/27.6.1063. [DOI] [PubMed] [Google Scholar]
- 48.Drapeau C, Carrier J. Fluctuation of waking electroencephalogram and subjective alertness during a 25-hour sleep-deprivation episode in young and middle-aged subjects. Sleep. 2004;27:55–60. doi: 10.1093/sleep/27.1.55. [DOI] [PubMed] [Google Scholar]
- 49.Vyazovskiy VV, Tobler I. Theta-activity in the waking EEG is a marker of sleep propensity in the rat. Brain Res. 2005;1050:64–71. doi: 10.1016/j.brainres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 50.Borbely AA. A two process model of sleep regulation. Human Neurobiology. 1982;1:195–204. [PubMed] [Google Scholar]
- 51.Achermann P, Borbely AA. Mathematical models of sleep regulation. Front Biosci. 2003;8:s683–93. doi: 10.2741/1064. [DOI] [PubMed] [Google Scholar]
- 52.Kattler H, Dijk DJ, Borbely AA. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J Sleep Res. 1994;3:159–64. doi: 10.1111/j.1365-2869.1994.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 53.Krueger JM, Obal F, Jr., Kapas L, Fang J. Brain organization and sleep function. Behav Brain Res. 1995;69:177–85. doi: 10.1016/0166-4328(95)00015-l. [DOI] [PubMed] [Google Scholar]
- 54.Vyazovskiy V, Borbely AA, Tobler I. Unilateral vibrissae stimulation during waking induces interhemispheric EEG asymmetry during subsequent sleep in the rat. J Sleep Res. 2000;9:367–71. doi: 10.1046/j.1365-2869.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- 55.Vyazovskiy VV, Welker E, Fritschy JM, Tobler I. Regional pattern of metabolic activation is reflected in the sleep EEG after sleep deprivation combined with unilateral whisker stimulation in mice. Eur J Neurosci. 2004;20:1363–70. doi: 10.1111/j.1460-9568.2004.03583.x. [DOI] [PubMed] [Google Scholar]
- 56.Krueger JM, Rector DM, Roy S, Van Dongen HP, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci. 2008;9:910–9. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tononi G, Cirelli C. Time to Be SHY? Some comments on sleep and synaptic homeostasis. Neural Plast. 2012;2012:415250. doi: 10.1155/2012/415250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Esser SK, Hill SL, Tononi G. Modeling the effects of transcranial magnetic stimulation on cortical circuits. J Neurophysiol. 2005;94:622–39. doi: 10.1152/jn.01230.2004. [DOI] [PubMed] [Google Scholar]
- 59.Huber R, Esser SK, Ferrarelli F, Massimini M, Peterson MJ, Tononi G. TMS-induced cortical potentiation during wakefulness locally increases slow wave activity during sleep. PLoS ONE. 2007;2:e276. doi: 10.1371/journal.pone.0000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huber R, Maatta S, Esser SK, et al. Measures of cortical plasticity after transcranial paired associative stimulation predict changes in electroencephalogram slow-wave activity during subsequent sleep. J Neurosci. 2008;28:7911–8. doi: 10.1523/JNEUROSCI.1636-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Gennaro L, Fratello F, Marzano C, et al. Cortical plasticity induced by transcranial magnetic stimulation during wakefulness affects electroencephalogram activity during sleep. PLoS ONE. 2008;3:e2483. doi: 10.1371/journal.pone.0002483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanlon EC, Faraguna U, Vyazovskiy VV, Tononi G, Cirelli C. Effects of skilled training on sleep slow wave activity and cortical gene expression in the rat. Sleep. 2009;32:719–29. doi: 10.1093/sleep/32.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hill S, Tononi G. Modeling sleep and wakefulness in the thalamocortical system. J Neurophysiol. 2005;93:1671–98. doi: 10.1152/jn.00915.2004. [DOI] [PubMed] [Google Scholar]
- 64.Doran SM, Van Dongen HP, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001;139:253–67. [PubMed] [Google Scholar]
- 65.Terzaghi M, Sartori I, Tassi L, et al. Evidence of dissociated arousal states during NREM parasomnia from an intracerebral neurophysiological study. Sleep. 2009;32:409–12. doi: 10.1093/sleep/32.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mahowald MW, Bornemann MA, Schenck CH. State dissociation, human behavior, and consciousness. Curr Top Med Chem. 2011;11:2392–402. doi: 10.2174/156802611797470277. [DOI] [PubMed] [Google Scholar]
- 67.Rector DM, Topchiy IA, Carter KM, Rojas MJ. Local functional state differences between rat cortical columns. Brain Res. 2005;1047:45–55. doi: 10.1016/j.brainres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 68.Chee MWL, Tan JC, Zheng H, et al. Lapsing during sleep deprivation is associated with distributed changes in brain activation. J Neurosci. 2008;28:5519–28. doi: 10.1523/JNEUROSCI.0733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petersen CCH, Poulet JFA. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454:881–U36. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Topographic distribution of t-values from paired t-tests contrasting relative power changes from cB1 to T7 session between AB and DS in the theta frequency band (5-9 Hz). Black and white dots show channels with significant increase in spectral power (P < 0.05, SnPM using a supra-threshold cluster analysis). White dots show channels included in the left frontal and parietal regions while gray dots show channels included in the control region for the theta wave amplitude analysis. (B) Theta wave negative peak amplitude (mean ± SEM, n = 16) in the task-related regions (F, left frontal; P, parietal) and a control region (C) during T7 relative to cB1. One-way ANOVA with repeated-measure in each cluster demonstrated significant condition effect in the task-related clusters F (F2,30 = 3.87, P = 0.03) and P (F2,30 = 4.91, P = 0.01), but not in the control cluster C (F2,30 = 0.03, P = 0.97). Post hoc Student-Newman-Keuls Range tests analyzing the difference between conditions revealed that in the F cluster AB led to a significant increase relative to baseline but not relative to the DS condition (#, AB > cB1, AB = DS, DS = cB1), while in the P cluster the increase in DS was significantly different from both cB1 and AB (*). Values for each ROI were averaged across the 4 neighboring electrodes. Note that the electrodes depicted in panel B are simply a schematic representation of the 3 ROIs. The exact location of the included electrodes is shown in panel A, white and gray dots.