Abstract
Study Objectives:
Sleep disturbances cause neurobehavioral performance and daytime functioning impairments. Postpartum women experience high levels of sleep disturbance. Thus, the study objective was to describe and explore the relation between neurobehavioral performance and sleep among women during the early postpartum period.
Design:
Longitudinal field-based study.
Participants:
There were 70 primiparous women and nine nulliparous women in a control group.
Interventions:
None.
Methods and Results:
During their first 12 postpartum weeks, 70 primiparous women wore continuous wrist actigraphy to objectively monitor their sleep. Each morning they self-administered the psychomotor vigilance test (PVT) to index their neurobehavioral performance. Nine nulliparous women in a control group underwent the same protocol for 12 continuous weeks. Postpartum PVT mean reciprocal (1/RT) reaction time did not differ from that of women in the control group at postpartum week 2, but then worsened over time. Postpartum slowest 10% 1/RT PVT reaction time was significantly worse than that of women in the control group at all weeks. Despite improvements in postpartum sleep, neurobehavioral performance continued to worsen from week 2 through the end of the study. Across the first 12 postpartum weeks, PVT measures were more frequently associated with percent sleep compared with total sleep time, highlighting the deleterious consequences of sleep disruption on maternal daytime functioning throughout the early postpartum period.
Conclusions:
Worsened maternal neurobehavioral performance across the first 12 postpartum weeks may have been influenced by the cumulative effects of sleep disturbance. These results can inform future work to identify the particular sleep profiles that could be primary intervention targets to improve daytime functioning among postpartum women, and indicate need for further research on the effectiveness of family leave policies. The time when postpartum women return to control-level daytime functioning is unknown.
Citation:
Insana SP; Williams KB; Montgomery-Downs HE. Sleep disturbance and neurobehavioral performance among postpartum women. SLEEP 2013;36(1):73–81.
Keywords: Accident, family, fragmentation, maternal, psychomotor vigilance, sleep debt, work
INTRODUCTION
Sleep serves several essential life-sustaining functions.1 Disruptions to sleep cause deleterious effects on hormone regulation, general health, decision making, and neurobehavioral performance.2–5 Insufficient sleep has been established as a contributing cause of several high-profile disasters, including the Exxon-Valdez oil spill, the Space Shuttle Challenger disaster, and the Chernobyl and Three Mile Island nuclear power plant accidents.6
Sleep disturbance can include both sleep deprivation (an overall attenuation of total sleep time) and sleep fragmentation (sleep interruptions that disrupt the integrity of the sleep cycle, but preserve total sleep time). Both sleep disturbance types have a dose-response effect; increased grades of experimentally induced sleep deprivation7 and fragmentation8 lead to greater neurobehavioral deficits. The cumulative effect of sleep fragmentation across consecutive nights leads to impairments equivalent to those following one night of total sleep deprivation. This is important because 1 night of total sleep deprivation causes neurobehavioral impairment equivalent to a 0.10% blood-alcohol concentration.9
The societal implications of sleep deprivation and fragmentation have been evaluated among medical residents, shift workers, and commercial drivers.10–12 Postpartum women are overlooked as an at-risk population vulnerable to sleep disturbance.
Nearly 4.3 million women become the mother of a newborn each yr in the United States, a rate currently higher than during the 1950s “baby boom” era.13 Postpartum sleep disturbance is due primarily to infant signaling during nocturnal periods,14 as well as maternal endocrine and other physiologic changes that affect sleep.15,16 Sleep quality worsens progressively throughout pregnancy and is most affected immediately after delivery17 after which it improves steadily.18,19
Despite women's characteristic sleep disturbance during the early postpartum period, after 6 wk many women in the United States drive, return to work, and continue to bear responsibility for the care and safety of their infant. The functional effect of their sleep disturbance has not been quantified. The study objectives were to (1) quantify postpartum neurobehavioral performance impairment using daily psychomotor vigilance testing during postpartum wk 2 through 13; (2) determine when sleep and neurobehavioral performance normalize compared with control patients; and (3) identify whether sleep deprivation or fragmentation accounted for greater neurobehavioral performance impairment.
MATERIALS AND METHODS
Data for these analyses were from a prospective, longitudinal, field-based study of postpartum sleep disturbance; the postpartum sleep values have been previously reported.19 The study was approved by the West Virginia University Office of Research Compliance (Institutional Review Board). Participants were administered informed consent and Health Information Portability and Accountability Act (HIPAA) authorization prior to participation.
Participants were recruited prenatally via community advertisements and were excluded during enrollment on the basis of premature delivery, pregnancy with multiples, infant admission to the neonatal intensive care unit, history of or current treatment for major depressive disorder, or a score ≥ 16 on the Center for Epidemiologic Studies Depression Scale.20 For comparisons made specifically within the current study, a group of nine nulliparous women without a history of major depressive disorder were recruited via community advertisements and word of mouth.
Measures
Actigraphy
Sleep was objectively measured using wrist actigraphy, a valid method for ambulatory measurement and analysis of sleep/wake periods among adults.21 Mini Mitter Actiwatch-64 actigraphs (Philips Respironics, Bend, OR) were worn on the nondominant wrist for continuous sleep assessment and were set to 15-sec recording epochs with the default (medium) wake threshold value = 40. The 15-sec epoch length was used to maximize the actigraphy collection rate and to conceptually coincide with polysomnography-identified wake scoring rules (i.e., 15 sec). Additionally, within the physical activity literature, shorter epoch lengths are recommended for use when physical activity (e.g., sleep fragmentation) is intermittent within a specific behavioral domain (e.g., sleep).22,23 Concurrent personal digital assistant (PDA)-based sleep diaries with both real-time (i.e., time and date stamped) and post hoc entry methods were used to corroborate actigraphy and to identify the beginning and ending of nocturnal sleep onset-to-offset intervals.21 The beginning of the sleep onset-to-offset interval was identified at the beginning of the first 2 min of inactivity following the sleep diary-reported bedtime; the end of the sleep onset-to-offset interval was identified as the end of the last 2 min of inactivity preceding the diary-reported wake time. Nocturnal sleep onset-to-offset intervals were then analyzed via Actiware 5.5 software (Philips Respironics) to calculate sleep time (min calculated as sleep within a nocturnal onset-to-offset interval) and percent sleep (percent of onset-to-offset interval identified as sleep). Notably, the sleep onset-to-offset interval and percent sleep variables did not include sleep onset latency within their respective values.
Psychomotor Vigilance Test
Neurobehavioral performance was measured using the psychomotor vigilance test (PVT).24,25 The PVT was self-administered using customized software (Bruner Consulting Co., Longmont, CO) on a Palm Zire 72 PDA (Palm, Inc., Sunnyvale, CA), similar to the format used in previous studies.26 This PVT is a 5-min reaction time (RT) task27 during which participants press a button in response to a bull's-eye stimulus presented at random interstimulus intervals with a 10-ms sensitivity resolution. Each 5-min PVT administration yields one trial and each trial administration was automatically time and date stamped in real time. Participants were asked to complete the PVT each morning, within 2 h of awakening, prior to consuming any caffeine, and during a time when they expected minimal environmental disturbance. If a distraction occurred, they were asked to terminate the session and begin again. Terminated sessions were not recorded. Due to the unsupervised nature of the field-based PVT administration within our study context, we recognized the possibility of measuring spurious individual RTs. To minimize the effect of outlying RT responses that may have resulted from environmental distraction, a criterion cutoff RT value was established at two standard deviations (SD) above the mean for all response data; this method is discussed elsewhere.28,29 For postpartum data, RT responses ≥ 1,314 ms were excluded, which resulted in exclusion of 1.84% of individual response times across all trials for all participants. For control data, the criterion cutoff value was ≥ 910 ms, which resulted in exclusion of 1.34% of individual response times across all trials for all control participants. Future field-based PVT utilization can be enhanced by recent, sophisticated techniques developed by Basner and Dinges.30
The PVT outcome variables used in this study were chosen to maximize the sensitivity of the 5-min PVT in measuring overall RTs and RTs in the lapse domain (see the chronic partial sleep deprivation condition at the 5-min period31). PVT trial variables included the mean response reciprocal transformation (1/RT), and the slowest 10% 1/RT. Longer RTs represent greater functional impairment.32
Procedure
Postpartum women participated for 12 continuous wk during postpartum wk 2 to 13. Nulliparous women in a control group participated in the same protocol for 12 consecutive wk. Throughout the protocol, participants wore an actigraph, maintained a sleep diary, and self-administered the PVT each morning.
Participants
Analyses were based on data from 70 primiparous postpartum women; seven of these women withdrew from the study during postpartum wk 5, 7 (n = 2), 8, 9, 10, and 11. Data were also lost due to actigraph or PDA malfunction, as well as participant nonadherence to the protocol. Of the possible 799 recording wk from these 70 postpartum women (excluding wk lost due to participants who withdrew from the study), 33 wk (4.1%) of PVT data and 37 wk (4.9%) of sleep data were missing.
The 70 postpartum women were all primiparous, primarily white (94.1%), 26.3 yr old (SD ± 4.1), 82.9% married or living with their partner, had 15.6 yr (SD ± 3.0) of education, and $60,123 (SD ± $35,105) annual household income.
Control data were collected from 10 nulliparous women. One woman in the control group withdrew from the study during her first study wk; thus, the final analyses were calculated among nine women. Among these, there was 1 wk of missing PVT data (0.93%) and 3 wk of missing sleep data (2.8%) from the 108 possible recording wk.
The nine nulliparous women in the control group were primarily white (66.7%), 29.1 yr old (SD ± 5.7), 77.8% were married/living with their partner, had 16.9 yr (SD ± 2.0) of education, and $80,222 (SD ± $60,276) annual household income. There were no significant differences on these demographic measures between women in the postpartum and control groups. The eight women who withdrew from the study did not differ significantly from women who completed the study on ethnicity, age, relationship status, or income; however, women who withdrew from the study had fewer yr of education (mean = 13.1, SD = 3.1) compared with women who completed the study (mean = 16.1, SD = 2.7 [t = −2.87, P = 0.005]).
Statistical Analyses
Each participant's daily PVT mean 1/RT and slowest 10% 1/RT, as well as their actigraphically measured nocturnal onset-to-offset time, sleep time, and percent sleep were averaged within each study wk. All available data were entered into weekly averages. Across the study, 85.8% of weekly PVT and 93.1% of weekly sleep averages contained ≥ 5 days. The numbers of daily postpartum PVT self-administrations (mean = 6.0, SD ± 1.5) and actigraphically measured sleep nights (mean = 6.4, SD ± 1.2) used to calculate each participant's weekly average were not significantly correlated with the respective averaged values for that wk, indicating stable weekly variables. Latency from postpartum women's awakening time to their PVT self-administration (i.e., wake time minus PVT administration time [mean = 92.4, SD ± 81.9 min]) was not significantly correlated with either PVT variables, reducing concern that the timing of PVT self-administration may have been affected by sleep inertia.33 Participants generally performed the PVT within their instructed 120-min window from wake. Nap data were not included in the analyses because the occurrence of napping was negligible. Mothers napped on average 0.40 times to twice per wk, which accounted for 3.13 to 18.5 min of sleep time per wk. This observation is supported by previous research, which indicates that mothers with young infants do not frequently nap.19,34 No participants in the control group napped across the 12-wk study.
Data were analyzed using SPSS version 18.0 (SPSS Inc., Chicago, IL); P < 0.05 was considered statistically significant. Linear mixed models were calculated to examine differences in sleep and PVT variables between postpartum women and women in the control group across the 12-wk study; confidence intervals (CI) were reported. One-way analyses of variance were used to compare sleep and PVT variables among women in the postpartum and control groups at each study wk; Hedges g effect sizes (for unequal sample sizes) were calculated. To augment the Hedges g effect sizes, the control group sample was used to estimate a population-based sample distribution via an unrestricted random sampling bootstrapping technique (i.e., resampling the dataset with replacement to equal the postpartum sample, n = 70)35; we then calculated Cohen's d effect sizes between the postpartum sample and the control group bootstrapped sample on sleep and PVT variables at each study wk. Both Hedges g and Cohen's d effect sizes were interpreted according to conventional criteria (0.2-0.3 is considered small, > 0.3 – 0.8 is medium, and > 0.8 is large).36 Among postpartum women, bivariate correlations were calculated among sleep and performance variables at each postpartum wk; Pearson's r was considered 0 – 0.1 negligible, > 0.1 – 0.3 small, and > 0.3 – 0.5 moderate.36
RESULTS
Nocturnal Sleep Across Time
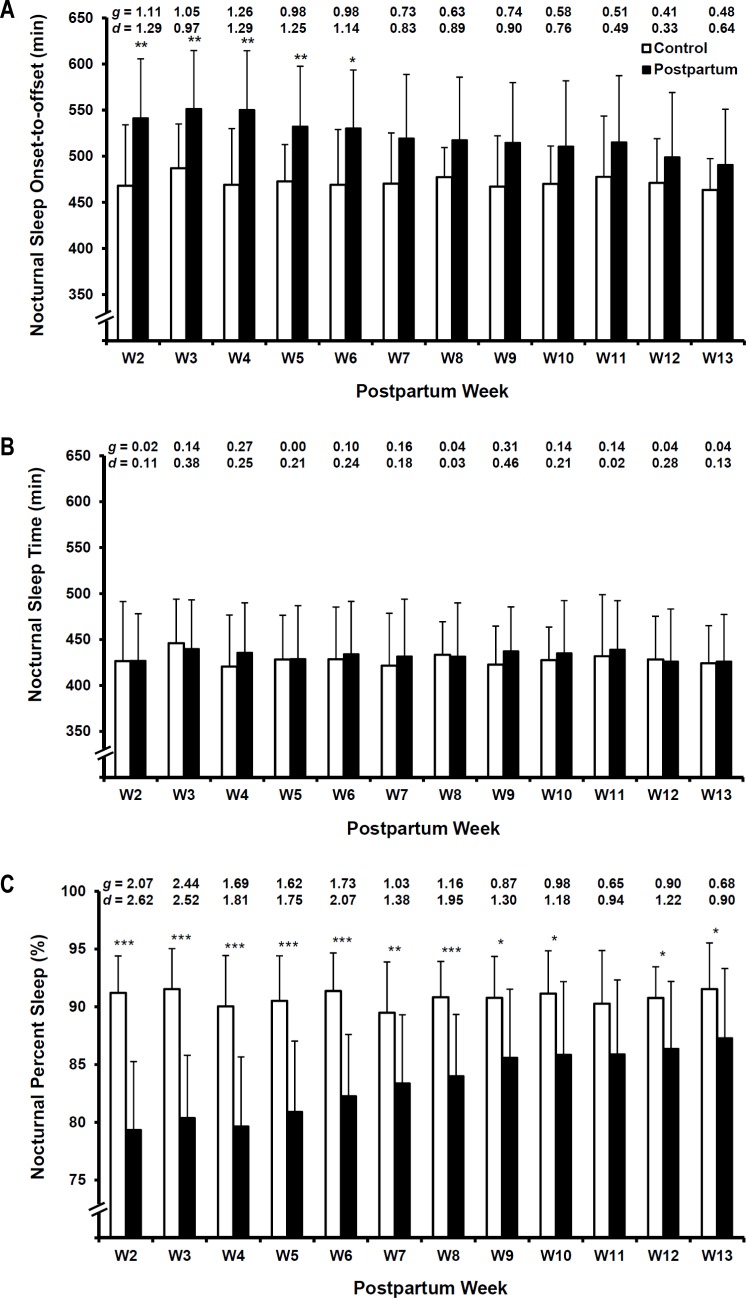
Across the 12-wk study, there was a main effect of parity on sleep onset-to-offset time (F[1, 784.52] = 81.21, P < 0.001, CI: 45.47 – 70.80). Across the study, postpartum women had a linear decrease in sleep onset-to-offset time (F[1, 85.47] = 50.79, P < 0.001, CI: −6.05 – −3.41), whereas onset-to-offset time for women in the control group was unchanged (F[1, 96.04] = 0.29, P = 0.59, CI: −2.33 – 1.33). Compared with the control group, maternal onset-to-offset time was consistently higher across postpartum wk, whereas the magnitude of difference between groups decreased across wk (Figure 1A [wk 2 g = 1.11, wk 13 g = 0.48]).
Figure 1.
Hedges g (g) and Cohen d (d) effect sizes are reported at the top of each figure for comparisons on corresponding study wk. * < 0.05, ** < 0.01, *** < 0.001. (A) Nocturnal sleep onset-to-offset differences between nulliparous control group and postpartum women during postpartum wk 2 to 13. (B) Nocturnal sleep time differences between nulliparous control group and postpartum women during postpartum wk 2 to 13. (C) Nocturnal percent sleep differences between nulliparous control group and postpartum women during postpartum wk 2 to 13.
Across the 12-wk study, there was no main effect of parity on total sleep time (F[1, 772.91] = 0.39, P = 0.53, CI: −6.76 – 13.12). Across the study, nocturnal sleep time was unchanged among both postpartum women (F[1, 80.31] = 0.001, P = 0.98, CI: −0.98 – 1.01), and those in the control group (F[1, 96.03] = 0.19, P = 0.66, CI: −2.04 – 1.29). Postpartum women and those in the control group did not significantly differ in their nocturnal sleep time across postpartum and corresponding control study wk (Figure 1B [g range = 0.00 – 0.31]).
Across the 12-wk study, there was a main effect of parity on percent sleep (F[1, 752.47] = 465.40, P < 0.001, CI: −10.07 – −8.39). Across the study, postpartum women had a linear increase in their percent sleep (F[1, 69.94] = 201.36, P < 0.001, CI: 0.65 – 0.87), but percent sleep among the women in the control group was unchanged (F[1, 10.23] = 0.01, P = 0.91, CI: −0.13 – 0.14). Compared with the control group, maternal percent sleep in the postpartum women was consistently and significantly lower at each postpartum wk, whereas the magnitude of difference decreased across wk (Figure 1C [wk 2 g = 2.07, wk 13 g = 0.68]).
Despite the low frequency of postpartum napping, we conducted post hoc exploratory analyses with a postpartum 24-h sleep variable that combined daytime napping with nocturnal sleep. No 24-h sleep time result diverged from those reported for nocturnal sleep time (see previous paragraphs); thus, only nocturnal sleep time is discussed, for parsimony.
Neurobehavioral Performance Across Time
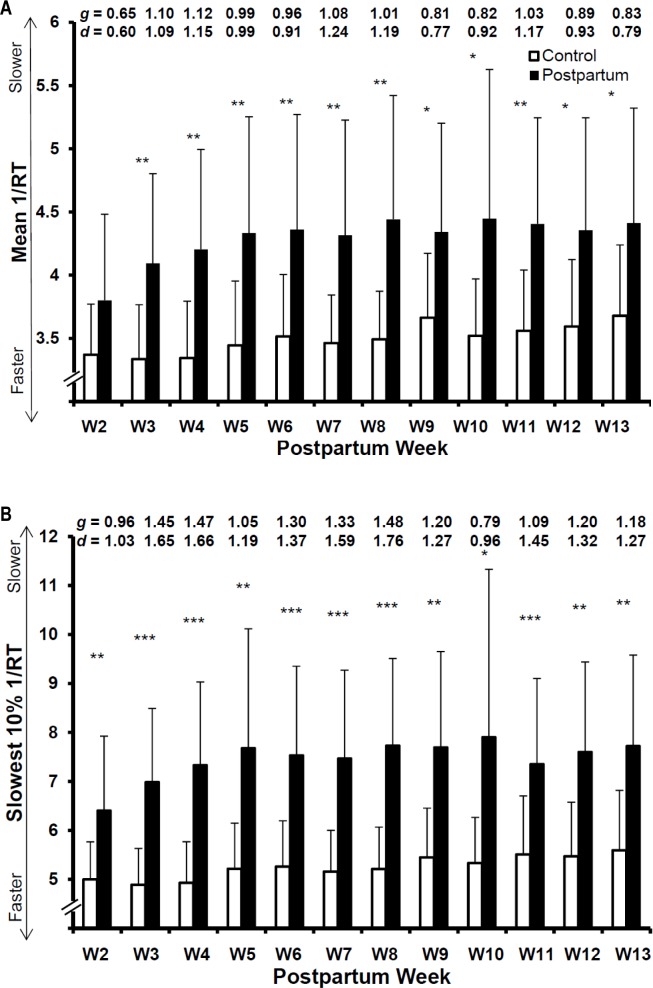
Across the 12-wk study, there was a main effect of parity on PVT mean 1/RT (F[1, 752.81] = 147.83, P < 0.001, CI: 0.48 – 0.67). Among postpartum women, mean 1/RT (F[1, 653.67] = 61.77, P < 0.001, CI: −0.011 – −0.007) worsened across postpartum wk 2 to 13 and was most adequately explained by a quadratic trend. A less pronounced linear profile was also observed among women in the control group for mean 1/RT (F[1, 9.31] = 11.97, P < 0.007, CI: 0.01 – 0.05). Compared with the control group, postpartum PVT mean 1/RTs did not initially differ, after which they were worse than that of the control group during all wk (Figure 2A [g range = 0.64 – 1.28]).
Figure 2.
Mean 1/RT and slowest 10% 1/RT values were transformed (i.e., [-1/[value*100]]) to facilitate direct comparisons. Hedges g (g) and Cohen d (d) effect sizes for corresponding weekly comparisons are reported at the top of each figure. * < 0.05, ** < 0.01, *** < 0.001. (A) Mean response reciprocal transformation (1/RT) differences between nulliparous control group and postpartum women during postpartum wk 2 to 13 on the 5 min psychomotor vigilance test (PVT). (B) Slowest 10% 1/RT differences between nulliparous control group and postpartum women during postpartum wk 2 to 13 on the 5-min PVT.
Across the 12-wk study, there was a main effect of parity on PVT slowest 10% 1/RT (F[1, 784.77] = 157.44, P < 0.001, CI: 1.60 – 2.20). Among postpartum women, slowest 10% 1/RT (F[1, 671.77] = 25.77, P < 0.001, CI: −0.03 – −0.01) worsened across postpartum wk 2 to 13 and was most adequately explained by a quadratic trend. A less pronounced linear profile was also observed among women in the control group for slowest 10% 1/RT (F[1, 9.21] = 6.24, P = 0.03, CI: 0.01 – 0.12). Compared with the control group, postpartum PVT slowest 10% 1/RTs did not initially differ, after which they were worse than that of the control group during all wk (Figure 2B [g range = 1.00 – 1.77]).
The worsening of PVT values observed among women in the control group likely reflected measurement error due to boredom with the PVT task that increased across time. Because this error may also have affected postpartum PVT values, we subtracted the average weekly control values from the corresponding weekly postpartum values in an attempt to account for possible error and calculated analyses on the subtracted postpartum PVT values. Both PVT mean 1/RT (F[1, 654.83] = 51.29, P < 0.001, CI: −0.01 – −0.006) and slowest 10% 1/RT (F[1, 671.90] = 24.90, P < 0.001, CI: −0.03 – −0.01) still worsened according to a quadratic trend across postpartum wk 2 to 13; thus, only the original postpartum PVT values are discussed, for parsimony.
Associations between Nocturnal Postpartum Sleep and Neurobehavioral Performance
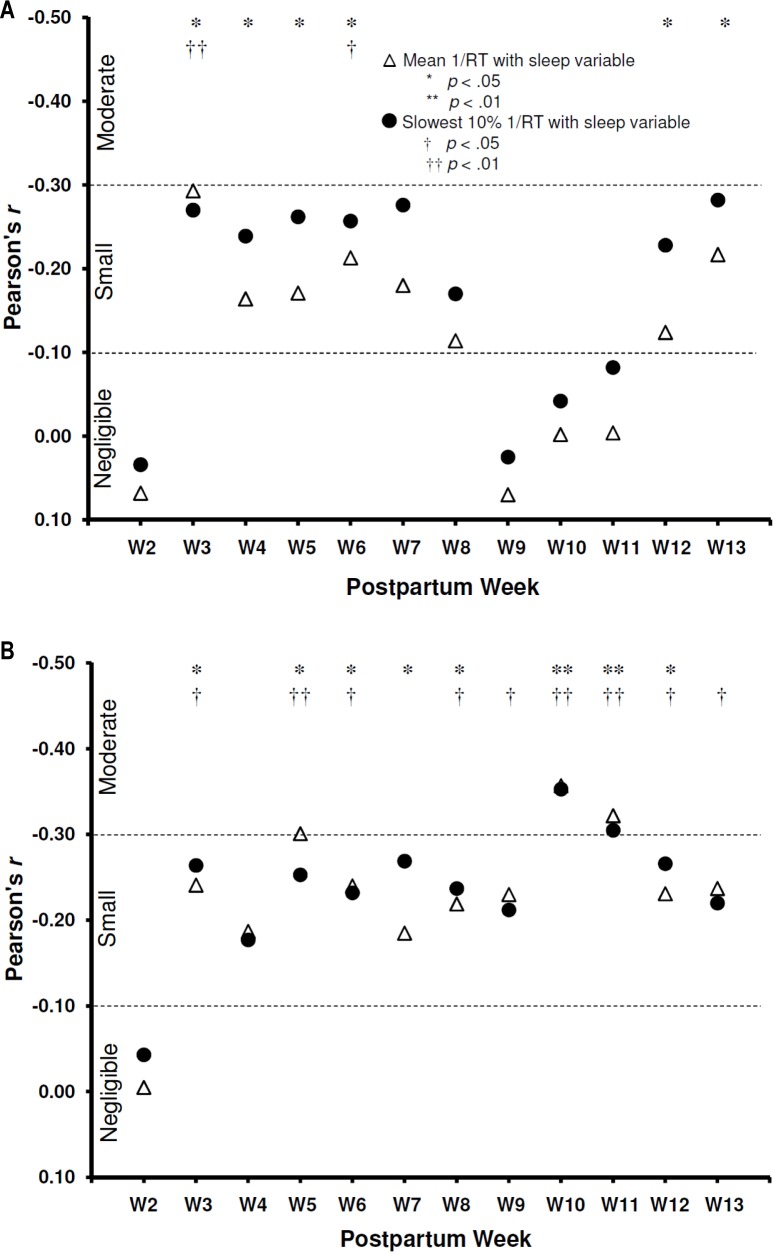
PVT mean 1/RT (Figure 3A) and slowest 10% 1/RT (Figure 3B) values were weakly to moderately associated with total sleep time (r range = −0.002 – −0.29) and percent sleep (r range = −0.005 – −0.36) during most postpartum wk.
Figure 3.
Dashed lines separate conventional criteria for Pearson r interpretations.36 (A) Associations between nocturnal sleep time and the 5-min psychomotor vigilance test (PVT) variables during postpartum wk 2 to 13. (B) Associations between nocturnal percent sleep and the 5-min PVT variables during postpartum wk 2 to 13.
DISCUSSION
Neurobehavioral performance worsened from postpartum wk 2 until the end of the 12-wk longitudinal study, despite improvements in postpartum sleep. Performance may be more coupled with sleep fragmentation than sleep time. Compared with the control group, postpartum nocturnal time in bed was higher and it remained relatively stable. Postpartum nocturnal sleep time was similar to previous reports37,38 and it did not differ from that of women in the control group. These profiles can be explained by the lower percent sleep experienced among postpartum women—which improved across the study. Additionally, postpartum neurobehavioral performance worsened and remained worse than control values throughout the study.
This postpartum neurobehavioral performance profile is concerning because PVT performance has high ecological validity; it is consistent with the attention and rapid response skills that govern everyday tasks such as driving.32 Throughout the 12-wk study, postpartum women had consistently worse neurobehavioral performance values than women in the control group. Thus, postpartum women may experience significant challenges and impairments on everyday tasks, several months after childbirth and long after most women return to work. Their performance continued to worsen until the end of the study so we do not know when, or whether, it may return to control levels.
Neurobehavioral performance worsened despite improved percent sleep and stable sleep time. The cumulative effect of sleep disruption is the likely cause of this seemingly paradoxical interaction. Among a sample of healthy adults, Cohen and colleagues39 demonstrated that despite an appearance of full recovery from sleep loss, chronic sleep debt accumulated and led to faster subsequent deteriorations in neurobehavioral performance. We suggest that, although maternal sleep fragmentation decreased across the early postpartum period, the effects of sleep fragmentation accumulated over this time, were maintained, and manifested when challenged with the neurobehavioral performance task.
Correlations between neurobehavioral performance and sleep values were small. Overall, performance values were more strongly associated with percent sleep than with total sleep time. The difference in associations between performance variables with sleep time and percent sleep may be a function of different sleep profiles having differential influences on various sleep outcomes.40,41 These results support future work that could be designed to identify whether particular sleep profiles (e.g., improved percent sleep verses increased total sleep time) should be the primary targets of interventions to improve daytime functioning among postpartum women.
Methodological Considerations
This longitudinal, field-based exploration of neurobehavioral performance among postpartum women was designed to bolster ecological validity at the possible expense of some experimental control. Thus, participants were unsupervised during PVT self-administration and may have encountered environmental distraction that inflated their performance values even though they were instructed to terminate the session and begin again if such a distraction occurred. The use of a PVT outlier cutoff criterion and calculation of within-wk averages were used to minimize this threat. Values reported from this unsupervised field-based PVT may not adequately compare to laboratory-based values seen within the larger literature; nevertheless, neurobehavioral performance was substantially worse among postpartum women compared with women in the control group.
Because the PVT is devoid of practice effects26 and women in the control group experienced their typical daily routine—which was expected to remain relatively constant compared to postpartum women—the worsening of PVT values in the control group across the 12-wk study, despite unchanged sleep, was interpreted as error that resulted from boredom experienced with the PVT task. Despite our attempt to adjust for this error, postpartum women may have experienced a greater magnitude of this error (i.e., boredom) across the longitudinal study in comparison with those in the control group.
The current study was a secondary data analysis of a study of normative maternal postpartum sleep, from which postpartum data were collected. The control group was recruited specifically for the current study. Although the small control group sample size may seem less than optimal, the threat of type II error was buffered by the detection of significant differences and large effect sizes, each indicating large effects. Furthermore, sleep among the control sample is considered representative among the larger population of nulliparous women via comparison with the larger sleep literature. For instance, control sample values across the current 12-wk study (Figure 1) were similar to those from a sample of young adults (between the ages of 18 to 32 yr and who did not differ by sex) who had a time in bed of 474.5 min, a sleep time of 420.8 min, and a sleep efficiency of 88.8%.42
Implications
This is the first longitudinal report of postpartum performance impairment. The data demonstrate that postpartum women are an at-risk population vulnerable to the deleterious performance effects of sleep disturbance. Postpartum women are significantly and increasingly impaired through the third postpartum month. The postpartum period is a normative life experience for families and should not be considered a `sleep disordered' time. However, the expectations that our society has for postpartum women to function may not be reasonable. These data have implications for both policy and practice.
Researchers and clinicians should consider the cumulative effects of sleep disturbance on postpartum women's neurobehavioral performance when investigating and developing intervention strategies to improve postpartum sleep. Policy makers, researchers, and advocates for women's health in the United States should consider postpartum performance impairment in the context of the United States Family Medical Leave policies.43
Finally, three critical areas deserve specific exploration. Most important, empiric determination of when postpartum women return to normal levels of neurobehavioral performance should be investigated. Second, the influence of social environment including support, demographics, and family sleeping locations and practices on postpartum functioning should be better described. Finally, new fathers' sleep and neurobehavioral performance are impaired during the early postpartum period,44 but the extent of and change in impairment across the postpartum period have yet to be determined.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the research participants and their families. Data collection and processing were carried out with assistance from Megan Clegg-Kraynok, PhD; Timothy Dohm, BA; Sierra Glowacki, BA; Holly Hunsburger; Laura Mancini, BS; Amanda McBean, MS; Eleanor Santy, BA; and Michael Winsor, MS. The authros thank Valerie Crabtree, PhD; and Lisa Meltzer, PhD for their thoughtful reviews of drafts of this work. This work was partially supported by National Institutes of Health Grant R21 HD053836 to Dr. Montgomery-Downs.
REFERENCES
- 1.Hobson JA. Sleep is of the brain, by the brain and for the brain. Nature. 2005;437:1253. doi: 10.1038/nature04283. [DOI] [PubMed] [Google Scholar]
- 2.Copinschi G. Metabolic and endocrine effects of sleep deprivation. Essent Psychopharmacol. 2005;6:341–7. [PubMed] [Google Scholar]
- 3.Alvarez GG, Ayas NT. The impact of daily sleep duration on health: a review of the literature. Prog Cardiovasc Nurs. 2004;19:56–9. doi: 10.1111/j.0889-7204.2004.02422.x. [DOI] [PubMed] [Google Scholar]
- 4.Harrison Y, Horne JA. The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl. 2000;6:236–49. doi: 10.1037//1076-898x.6.3.236. [DOI] [PubMed] [Google Scholar]
- 5.Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29:320–39. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh JK, Dement WC, Dinges DF. Sleep medicine, public policy, and public health. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia, PA: Saunders; 2005. pp. 648–56. [Google Scholar]
- 7.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effect on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet MH. Performance and sleepiness as a function of frequency and placement of sleep disruption. Psychophysiology. 1986;23:263–71. doi: 10.1111/j.1469-8986.1986.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 9.Dawson D, Reid K. Fatigue, alcohol and performance impairment. Nature. 1997;388:235–7. doi: 10.1038/40775. [DOI] [PubMed] [Google Scholar]
- 10.Lockley SW, Landrigan CP, Barger LK, Czeisler CA. When policy meets physiology: the challenge of reducing resident work hours. Clin Orthop Relat Res. 2006;449:116–27. doi: 10.1097/01.blo.0000224057.32367.84. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz JRL, Roth T. Shift work sleep disorder: burden of illness and approaches to management. Drugs. 2006;66:2357–70. doi: 10.2165/00003495-200666180-00007. [DOI] [PubMed] [Google Scholar]
- 12.Stoohs R. Commercial and public transportation impact. In: Kushida CA, editor. Sleep deprivation: clinical issues, pharmacology, and sleep loss effects. New York, NY: Marcel Dekker; 2005. pp. 273–8. [Google Scholar]
- 13.Hamilton BE, Martin JA, Ventrua SJ. Births: preliminary data for 2007. National Vital Statistics Reports. 2009;57:12. Available online at: http://www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_12.pdf. [PubMed] [Google Scholar]
- 14.Nishihara K, Horiuchi S, Eto H, Uchida S. Mothers' wakefulness at night in the post-partum period is related to their infants' circadian sleep-wake rhythm. Psychiatry Clin Neurosci. 2000;54:305–6. doi: 10.1046/j.1440-1819.2000.00689.x. [DOI] [PubMed] [Google Scholar]
- 15.Manber R, Armitage R. Sex, steroids, and sleep: a review. Sleep. 1999;22:540–55. [PubMed] [Google Scholar]
- 16.Santiago JR, Nolledo MS, Kinzler W, Santiago TV. Sleep and sleep disorders in pregnancy. Ann Intern Med. 2001;134:396–408. doi: 10.7326/0003-4819-134-5-200103060-00012. [DOI] [PubMed] [Google Scholar]
- 17.Ross LE, Murray BJ, Steiner M. Sleep and perinatal mood disorders: a critical review. J Psychiatry Neurosci. 2005;30:247–56. [PMC free article] [PubMed] [Google Scholar]
- 18.Kang MJ, Matsumoto K, Shinkoda H, Mishima M, Seo YJ. Longitudinal study for sleep-wake behaviours of mothers from pre-partum to post-partum using actigraph and sleep logs. Psychiatry Clin Neurosci. 2002;56:251–2. doi: 10.1046/j.1440-1819.2002.00992.x. [DOI] [PubMed] [Google Scholar]
- 19.Mongtomery-Downs HE, Insana SP, Clegg-Kraynok M, Mancini LM. Normative longitudinal maternal sleep: first four postpartum months. Am J Obstet Gynecol. 2010;203:465.e1–7. doi: 10.1016/j.ajog.2010.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radloff LA. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 21.Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep Med Rev. 2002;6:113–24. doi: 10.1053/smrv.2001.0182. [DOI] [PubMed] [Google Scholar]
- 22.Edwardson CL, Gorely T. Epoch length and its effect on physical activity intensity. Med Sci Sports Exerc. 2010;42:928–34. doi: 10.1249/MSS.0b013e3181c301f5. [DOI] [PubMed] [Google Scholar]
- 23.Oliver M, Schofield GM, Badland HM, Shepherd J. Utility of accelerometer thresholds for classifying sitting in office workers. Prev Med. 2010;51:357–60. doi: 10.1016/j.ypmed.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Dinges DF, Powell JW. Micorcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods Instrum Comput. 1985;17:652–5. [Google Scholar]
- 25.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann NY Acad Sci. 2008;1129:305–22. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 26.Thorne DR, Johnson DE, Redmond DP, Sing HC, Belenky G. The Walter Reed palm-held psychomotor vigilance test. Behav Res Methods. 2005;37:111–8. doi: 10.3758/bf03206404. [DOI] [PubMed] [Google Scholar]
- 27.Loh S, Lamond N, Dorrian J, Roach G, Dawson D. The validity of psychomotor vigilance tasks of less than 10-minute duration. Behav Res Methods Instrum Comput. 2004;36:339–46. doi: 10.3758/bf03195580. [DOI] [PubMed] [Google Scholar]
- 28.Radcliff R. Methods for dealing with reaction time outliers. Psychol Bull. 1993;114:510–32. doi: 10.1037/0033-2909.114.3.510. [DOI] [PubMed] [Google Scholar]
- 29.Anscombe FE. Rejection of outliers. Technometrics. 1960;2:123–47. [Google Scholar]
- 30.Basner M, Dinges DF. An adaptive-duration version of the PVT accurately tracks changes in psychomotor vigilance induced by sleep restriction. Sleep. 2012;35:193–202. doi: 10.5665/sleep.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basner M, Dinges DF. Maximizing sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34:581–91. doi: 10.1093/sleep/34.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorrian J, Rogers NL, Dinges DF. Psychomotor vigilance performance: neurocognitive assay sensitive to sleep loss. In: Kushida CA, editor. Sleep deprivation: clinical issues, pharmacology, and sleep loss effects (Lung Biology in Health and Disease) New York: Marcel Dekker; 1993. pp. 39–70. [Google Scholar]
- 33.Naitoh P, Kelly T, Babkoff H. Sleep inertia, best time not to wake up? Chronobiol Int. 1993;10:109–18. doi: 10.1080/07420529309059699. [DOI] [PubMed] [Google Scholar]
- 34.Cottrell L, Karraker KH. Correlates of nap taking in mothers of young infants. J Sleep Res. 2002;11:209–12. doi: 10.1046/j.1365-2869.2002.00305.x. [DOI] [PubMed] [Google Scholar]
- 35.Mooney CZ, Duval RD. Bootstrapping: a nonparametric approach to statistical inference. Newbury Park, CA: Sage; 1993. [Google Scholar]
- 36.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 37.Lee KA, Zaffke ME, McEnany G. Parity and sleep patterns during and after pregnancy. Obstet Gynecol. 2000;95:17–8. doi: 10.1016/s0029-7844(99)00486-x. [DOI] [PubMed] [Google Scholar]
- 38.Signal LT, Gander PH, Sangalli MR, Travier N, Firestone RT, Tuohy JF. Sleep duration and quality in healthy nulliparous and multiparous women across pregnancy and post-partum. Aust NZ J Obstet Gynaecol. 2007;47:16–22. doi: 10.1111/j.1479-828X.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 39.Cohen DA, Wang W, Wyatt JK, Kronauer RE, Dijk D, Czeisler CA, et al. Uncovering residual effects of chronic sleep loss on human performance. Sci Transl Med. 2010;2:14ra3. doi: 10.1126/scitranslmed.3000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bei B, Milgrom J, Ericksen J, Trinder J. Subjective perception of sleep, but not its objective quality is associated with immediate postpartum mood disturbances in healthy women. Sleep. 2010;33:531–8. doi: 10.1093/sleep/33.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franzen PL, Siegle GJ, Buysse DJ. Relationships between affect, vigilance, and sleepiness following sleep deprivation. J Sleep Res. 2008;17:34–41. doi: 10.1111/j.1365-2869.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoon IY, Kripke DF, Youngstedt SD, Elliott JA. Actigraphy suggests age-related differences in napping and nocturnal sleep. J Sleep Res. 2003;12:87–93. doi: 10.1046/j.1365-2869.2003.00345.x. [DOI] [PubMed] [Google Scholar]
- 43.United States Department of Labor, Family and Medical Leave Act. Available online at: http://www.dol.gov/esa/whd/fmla/
- 44.Insana SP, Montgomery-Downs HE. Sleep and sleepiness among first-time postpartum parents: A field- and laboratory-based multimethod assessment. Dev Psychobiol. 2012 May 2; doi: 10.1002/dev.21040. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]