Abstract
Prolyl oligopeptidase (PREP) is a serine protease that hydrolyzes peptides shorter than 30-mer, and it has been connected with multiple physiological and pathological conditions. PREP has been mostly studied in the brain, but significant PREP activities have been measured in peripheral tissues. Moreover, increased PREP activities have been found in tumors. In this study, the authors studied the immunohistochemical distribution of PREP protein in human peripheral tissues and in ovarian and colorectal tumors. PREP was found to be widely distributed in human peripheral tissues and specifically in certain cells. The most intense PREP expression was seen in the testis, ovaries, liver, and some parts of the skin. At the cellular level, high PREP levels were seen as a rule in secreting epithelial cells and cells involved in reproduction. Increased PREP expression was seen in most of the tumors studied. PREP expression was higher in malignant than benign tumors, and in ovarian epithelial cancers, there was a trend for increased PREP staining with increased malignancy grade. Results suggest that PREP may be associated with secretory processes as well as in reproduction. A more abundant expression of PREP in malignant than benign tumors suggests that PREP may be associated with expansion and metastasis of tumors.
Keywords: serine protease, peripheral tissues, secreting cells, tumor, metastasis
Prolyl oligopeptidase (PREP, POP; EC 3.4.21.26) belongs to the family S9 of the serine carboxypeptidase (SC) clan (Rawlings and Barrett 1994). The traditional function of PREP is the hydrolysis of the -Pro-Xaa- bond of peptides shorter than 30-mer, where Xaa is any amino acid other than proline (Walter 1976). Several bioactive neuropeptides that are involved cognition, such as substance P, thyrotropin-releasing hormone, bradykinin, and neurotensin, are known to be PREP substrates in vitro (for reviews, see Garcia-Horsman et al. 2007; Männistö et al. 2007). Furthermore, changes in PREP enzyme activity or expression of the PREP protein in tissues have been seen during aging (Agirregoitia et al. 2003a; Rossner et al. 2005) and in various diseases such as Parkinson and Alzheimer diseases (Pittaway et al. 1984; Mantle et al. 1996). These findings have served as a rationale for the development of PREP inhibitors, but the results on neuropeptide levels in vivo are controversial (Männistö et al. 2007). Apart from the neuropeptide cleavage, PREP has been suggested to be involved in regulation of inositol 1,4,5-triphosphate (IP3) signaling (Williams and Harwood 2000; Schulz et al. 2002; Williams et al. 2002), α-synuclein aggregation (Brandt et al. 2008), and functions of the growth-associated protein, GAP-43 (Di Daniel et al. 2009).
PREP is widely distributed in organisms ranging from bacterial and archaeal species to human (Venäläinen et al. 2004). In human and rat, PREP enzyme activities have been found in most tissues, peaking in the brain (Daly et al. 1985; Fuse et al. 1990; Goossens et al. 1996; Irazusta et al. 2002; Agirregoitia et al. 2003b). Very low PREP-like activities have been measured in various body fluids (Goossens et al. 1996). Similar to its enzymatic activity, PREP protein and PREP coding mRNA are widely distributed in the bodies of rats and mice (Bellemere et al. 2004; Myöhänen et al. 2007; Myöhänen et al. 2008a; Myöhänen et al. 2008b), but the PREP protein is distinctly localized in certain types of cells and aspects of tissues. PREP is mainly a cytosolic enzyme (Dresdner et al. 1982; Myöhänen et al. 2008a), but a membrane-bound form of PREP has been found in the membranes of the Golgi apparatus and rough endoplasmic reticulum and also, to some extent, in the plasma membrane (O’Leary and O’Connor. 1995; Myöhänen et al. 2008a; Tenorio-Laranga et al. 2008).
The PREP protein is localized in the nuclei of proliferating cells in mouse tissues (Myöhänen et al. 2008b) and developing neuronal tissues of the rat (Hannula et al. 2010). Moreover, PREP enzyme activities (Agirregoitia et al. 2003b; Agirregoitia et al. 2007), mRNA (Agirregoitia et al. 2010), and protein amounts (Moreno-Baylach et al. 2008; Hannula et al. 2010) are increased during the early days of embryogenesis. Changes in PREP activity and expression during proliferation and differentiation have also been shown in mouse cells (Ishino et al. 1998) and in Sarcophaga peregrina (flesh fly; Ohtsuki et al. 1994). In addition, Goossens et al. (1996) demonstrated that PREP enzyme activity is significantly increased in cancerous tissues. Similar findings were made by Liu et al. (2008) and Larrinaga et al. (2010) in several types of benign and malignant tumors. Moreover, in mRNA high-throughput screening, the PREP mRNA was found to be higher in cancerous tissues compared to normal tissue (GeneSapiens; Kilpinen et al. 2008). Taken together, these studies suggest an association of PREP with cell proliferation and tissue growth.
We are not aware of any studies on the distribution of PREP protein in human tissues, but PREP activities (Goossens et al. 1996; Mantle et al. 1996; Irazusta et al. 2002) and gene expression have been measured using high-throughput screening (GeneSapiens; Kilpinen et al. 2008) in several human tissues. The present study was undertaken to clarify the distribution of PREP protein in peripheral human tissues using a specific PREP antibody and immunohistochemistry. To the best of our knowledge, this has not been studied before. Moreover, to elucidate if PREP protein is increased in tumors, similar to mRNA and enzyme activity, the distribution of PREP protein was studied in certain cancers.
Materials and Methods
Tissue Samples
Normal human tissue samples were collected for this study prospectively from surgical specimens sent to the Department of Pathology at the Turku University Central Hospital for diagnostic purposes (Gardberg et al. 2010). Informed consent was obtained before surgery. Within 1 hr of surgical resection, macroscopically normal tissue areas were sampled. Tissues were fixed in formalin, dehydrated, paraffin embedded, and sectioned.
Ovarian and colorectal tumor tissue arrays was collected from tissue archives. The tissue arrays were prepared in house from formalin-fixed, paraffin-embedded tissue material archived at the Department of Pathology, Turku University Central Hospital. The ovarian array contained serous cystadenomas (n=13), serous borderline tumors (n=15), serous cystadenocarcinomas (n=22), mucinous cystadenomas (n=12), mucinous cystadenocarcinomas (n=18), endometroid carcinomas (n=23), and normal ovarian specimens (n =2). The colorectal cancer array consisted of 48 adenocarcinomas and 4 normal colonic samples. Tumor specimens and normal tissue specimens were from different individuals. The use of all tissue specimens was approved by the Hospital District Ethics Committee; therefore, the studies have been performed in accordance with the ethical standards laid down in the 1975 Declaration of Helsinki.
Chemicals
Chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise specified in the text.
Preparation of Polyclonal PREP Antibody
The polyclonal PREP antibody was prepared as described earlier (Venäläinen et al. 2006). Briefly, purified Escherichia coli–expressed recombinant human PREP (for details of expression and purification, see Venäläinen et al. 2006) was used to generate antibodies against PREP in a hen. Egg yolks were collected, and IgYs were isolated by the water dilution method (Kokko et al. 1994). PREP-specific IgY was then purified by affinity chromatography using a HiTrap NHS column coupled with purified PREP as reported (Venäläinen et al. 2002). Antibody specificity in human tissues has been characterized earlier by Myöhänen et al. (2007).
Immunohistochemistry
Immunostaining was performed using the peroxidase method, by LabVision autostainer device (LabVision/Thermo Fisher Scientific, Cheshire, UK). For antigen retrieval, the slides were treated with citrate buffer (pH 6.0). Endogenous peroxidase was blocked with 5% H2O2. After 30-min incubation in 10% normal goat serum (NGS; Product S-1000, Vector laboratories, Burlingame, CA) at 37C, the sections were incubated overnight at 4C in PREP antibody (1:500 dilution in 1% NGS). After being washed with PBS, the sections were treated with peroxidase-conjugated secondary antibody at room temperature for 2 hr (1:500 dilution in 1% NGS; rabbit anti-chicken peroxidise conjugated; Product 31720, Thermo Fisher Scientific). Diaminobenzidine was used as a chromogene, and Mayer’s Hematoxylin was used to counterstain the nuclei of the samples.
At least two different samples per tissues were used, and the stainings were repeated at least three times with similar results.
Semiquantitative Analysis
After the photomicrographs of immunostained sections were captured, the images were analyzed using Bio-Rad QuantityOne 4.5.1 software (Bio-Rad Laboratories, Hercules, CA) as described earlier (Myöhänen et al. 2007). In optical density (OD) analysis, organs were delineated with the freehand tool of the software. The background values of each organ (obtained by control staining without primary antibody) were subtracted from raw data values of the same organ.
Individual tissues and cell types were evaluated by scoring the PREP staining intensity by grading from 0 (no staining) to +++ (high staining). Threshold OD values for this scoring were < 6.5 (low, +), 6.5–9.0 (moderate, ++), and > 9.0 (high, +++). Background value was on average 3.5, which was considered negative staining. Similar grading and thresholds were used for tumors. Although the expression level of PREP was higher in the tumors than in corresponding healthy tissue, some areas, such as Leydig cells of testis, showed similarly high OD values with epithelial tumors.
Results
Distribution of PREP Protein in Human Peripheral Tissues
Gastrointestinal tract
In the esophagus, the highest PREP immunoreactivity was seen in the epithelial cell layer, with more immunoreactivity in the outermost cell layers than in the deep proliferating layers (Table 1). Only low amounts of PREP protein was present in the smooth muscle cells.
Table 1.
Distribution of Prolyl Oligopeptidase (PREP) mRNA and Protein in Normal Peripheral Human Tissues and Cells
| Tissue/Cell Types | PREP Coding mRNA Levelsa | PREP Protein Amounts |
|---|---|---|
| Esophagus | ||
| Epithelial cells | +++ | |
| Smooth muscle cells | + | |
| Stomach | ++ | |
| Parietal cells | +++ | |
| Peptic cells | +++ | |
| Duodenum, intestine, colon | ++ | |
| Enterocytes | +++ | |
| Spleen | ++ | |
| Red pulp | + | |
| Red pulp sinuses | +++ | |
| Lymph nodes | ||
| Cortex | + | |
| Follicles | ++ | |
| Bone marrow | ++ | |
| Monocytes | ++ | |
| Basophils | ++ | |
| Skeletal muscle | +++ | |
| Muscle fibers | + | |
| Skin | ||
| Stratum granulosum | +++ | |
| Basal cells | ++ | |
| Stratum spinosum | ++ | |
| External root sheath of the hair follicles | +++ | |
| Walls of cells of the sebaceous glands | +++ | |
| Liver | ++ | |
| Hepatocytes | +++ | |
| Kupffer cells | ++ | |
| Pancreas | ++ | |
| Centroacinar cells of the glandular acini | ++ | |
| Lungs | + | |
| Alveolar macrophages | +++ | |
| Pneumocytes | ++ | |
| Kidney | + | |
| Epithelial cells of the tubules | ++ | |
| Glomerulus | + | |
| Adrenal gland | + | |
| Cortical zona fasciculata and zona reticularis | +++ | |
| Medulla | + | |
| Urinary bladder | ++ | |
| Epithelial cells (dome cells) | +++ | |
| Parotid gland | ++ | |
| Epithelial cells of the excretory ducts | + | |
| Thyroid gland | ||
| Epithelial cells of the thyroid follicles | ++ | |
| Parathyroid gland | ||
| Principal cells | ++ | |
| Prostate gland | ++ | |
| Secretory epithelial cells of the glands | +++ | |
| Supporting fibrous stroma | + | |
| Testis | ++ | |
| Seminiferous tubules | +++ | |
| Leydig cells | +++ | |
| Primary spermatocytes | +++ | |
| Spermatogonia | ++ | |
| Spermatids | +++ | |
| Placenta | ||
| Mesenchymal cytotrophoblasts | ++ | |
| Intermediate trophoblasts | +++ | |
| Uterus | ++ | |
| Epithelial cells in the opening of the endometrial gland of uterus | +++ | |
| Epithelial cells of the endometrium | + | |
| Epithelial cells of the stroma | + | |
| Ovaries | ++ | |
| Oocytes | +++ | |
| Zona pellucida | +++ | |
| Primordial follicles | +++ | |
| Zona granulosa | + | |
| Theca folliculi | ++ | |
| Breast | ++ | |
| Epithelial cells of the breast ducts | +++ | |
| Macrophages | +++ |
Threshold of optical density values for PREP immunoreactivity were < 6.5 (low PREP staining, +), 6.5–9.0 (moderate PREP staining, ++), and > 9.0 (high PREP staining, +++). Background value was on average 3.5, which was considered as negative staining.
PREP coding mRNA levels are obtained from BioGPS (Su et al. 2002) and GeneSapines (Kilpinen et al. 2008) databases.
In the duodenum, intestine, and colon, high PREP immunoreactivity was seen in the enterocytes facing the lumen (Fig. 1A).
Figure 1.
The distribution of PREP in human peripheral tissues. Representative photomicrograph depicts the distri-bution of PREP (brown color) in the colon (Panel A), where PREP is seen especially in enterocytes facing the lumen (black arrow). In the lymph nodes, only low amounts of PREP were seen in the cortex (Ctx), but moderately in the follicles (F; Panel B). A rather intense PREP staining was visible in the epidermis (black arrow), sebaceous glands (SG), and hair follicles (H) of the skin (Panels C and D). In the adrenal gland, a high PREP immunoreactivity was present in the cortex, especially in the zona fasciculata (ZF) and zona reticularis (ZR; Panel E). Endothelial cells of the red pulp sinuses of the spleen (black arrow) had high expression of PREP (small panel in Panel F, magnification from the larger picture; black arrow pointing the area), while other parts of the spleen showed only moderate PREP staining (Panel F). Interestingly, high PREP levels were seen in the epithelial cells in the opening of endometrial glands of the uterus (black arrow and small panel in Panel G). In the testis, PREP was highly present in the primary spermatocytes (black arrow), spermatids (black arrowhead), and Leydig cells (L) and moderately in the spermatogonia (white arrow). PREP was also detected in the cell nuclei of the testis (Panel H). Small panels in Panels B, E, F, and G are magnifications from the black arrow area of the corresponding larger picture. Nuclei are visualized by Mayer’s hematoxylin (blue color). Scale bars are 50 µm in Panels A, C, D, F, G, and H; 100 µm in Panel B; and 200 µm in Panel E.
In the stomach, high PREP staining was seen in the lower layers of the mucosa, particularly in the base of the gastric glands (Table 1). PREP protein was highly present in the parietal and peptic cells but not in the mucous cells. No PREP was seen in the smooth muscle layers of the stomach.
Spleen, lymph nodes, and bone marrow
High expression of PREP protein was present in the endothelial cells of the red pulp sinuses (Fig. 1F). The red pulp had otherwise only a low PREP immunoreactivity and no staining was seen in the white pulp (Table 1).
Low amounts of PREP protein were present in the cortex of the lymph nodes, but PREP was moderately present in the follicles. In particular, macrophages in the germinal center of the follicles were highly stained with PREP (Fig. 1B).
Low PREP immunoreactivity was generally seen in the bone marrow and none in the adipocytes. However, monocytes and basophils showed moderate PREP immunoreactivity (Table 1).
Skeletal muscle and skin
Practically no immunoreactive PREP was present in the skeletal muscle except in some muscle fibers where low staining was visible (Table 1).
In the skin, PREP was highly expressed in the epidermis, sebaceous glands, and hair follicles (Fig. 1C). In the epidermis, high PREP immunoreactivity was seen in the stratum granulosum, and PREP was moderately present in the basal cells and stratum spinosum. A high PREP expression was seen in the walls of the cells in the sebaceous glands and a moderate staining in the external root sheath of the hair follicles (Fig. 1D).
Liver and pancreas
In the liver, PREP was highly stained in the hepatocytes (Table 1), particularly close to the portal tracts. Kupffer cells also expressed moderate amounts of PREP protein.
Moderate PREP protein amounts were detected in the glandular acini of the exocrine pancreas, in centroacinar cells (Table 1).
Lungs
The highest staining in the lungs was present in the alveolar macrophages, and pneumocytes showed moderate PREP immunoreactivity. Only low amounts of PREP protein was seen in the alveoles and none in the alveolar endothelial cells (Table 1).
Kidney, adrenal gland, and urinary bladder
Moderate to high levels of PREP protein were detected in the kidney cortex. The most intensively stained cells were the epithelial cells of the walls of the tubules, where the staining was moderate (Table 1). There was only a low amount of PREP present in the glomerulus and even less in the kidney medulla.
In the adrenal gland, the highest amounts of PREP protein were present in the cortex, and staining was high both in the zona fasciculata and zona reticularis (Fig. 1E). Low protein levels were present in the medulla, and the zona glomerulosa lacked PREP immunoreactivity.
High PREP immunoreactivity was seen on the surface of epithelial cells in the urinary bladder wall, especially in dome cells. In the connective tissue or smooth muscle layers, practically no PREP protein was seen (Table 1).
Parotid gland, thyroid gland, and parathyroid gland
PREP was rather weakly present in the parotid gland, and low PREP staining was seen in epithelial cells of the walls of excretory ducts (Table 1). Unlike most of the other organs, practically no PREP was seen in the secreting cells of the parotid gland.
In the thyroid gland, moderate PREP immunoreactivity was seen in the epithelial cells at the walls of thyroid follicles but not inside the follicles. Moderate PREP amounts were seen in the principal cells of parathyroid gland (Table 1).
Prostate and testis
In the prostate gland, high expression of PREP protein was seen in the secreting epithelial cells but much less in the supporting fibrous stroma (Table 1).
High amounts of PREP were seen in the seminiferous tubules and Leydig cells of the testis (Fig. 1H). PREP was highly present in the primary spermatocytes, spermatids, and moderately in the spermatogonia. PREP was not seen in the spermatozoa (Fig. 1H). In the spermatocytes, PREP was detected also in the nucleus.
Placenta, uterus, ovaries, and breast
In the early placenta, PREP was moderately present in the mesenchymal cytotrophoblasts and in high amounts in the intermediate trophoblasts (Table 1).
High PREP immunoreactivity was seen in the epithelial cells in the opening of endometrial glands of the uterus (Fig. 1G). Other epithelial cells and stromal cells of the endometrium showed only low PREP immunostaining.
High PREP immunostaining was seen in the oocytes, zona pellucida, and primordial follicles of the ovaries (Table 1). Moderate PREP immunoreactivity was seen in the theca folliculi but low staining in the zona granulosa and other areas of the ovaries (Table 1).
Rather intense PREP immunostaining was seen in the epithelial cells of the breast ducts. In the fibrous interlobular tissue, some macrophages were intensely stained, while other areas of the tissue showed no PREP immunoreactivity.
Distribution of PREP Protein in Cancerous Tissues
To assess the potential changes in PREP expression levels and distribution during neoplastic transformation, we carried out in silico analysis of PREP mRNA expression in various cancers with the GeneSapiens software (Kilpinen et al. 2008). The analysis indicated increased PREP mRNA transcripts in ovarian and colorectal cancers compared with normal tissue. On the basis of this information, we performed immunostaining for a set of epithelial ovarian neoplasias (benign, borderline, and malignant) and colorectal cancer specimens.
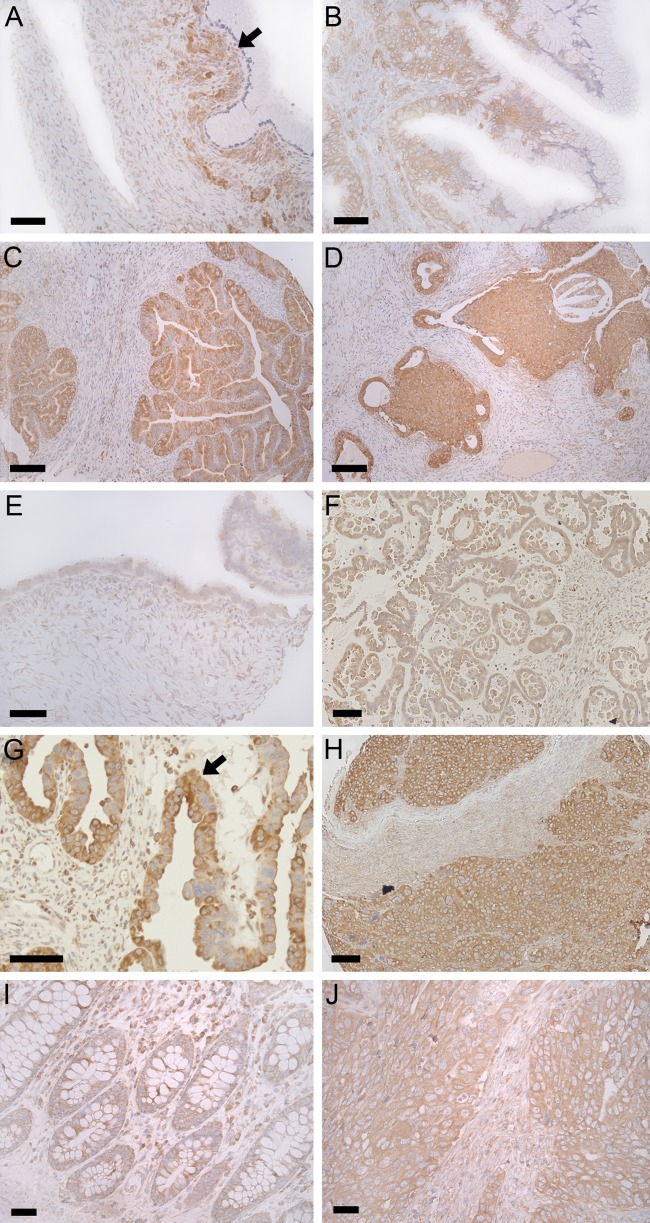
We compared the PREP staining in ovarian epithelial tumors of different subtypes (Fig. 2A–H, Table 2). All three analyzed cancer types were consistently positive for PREP staining, with the exception of one grade 1 mucinous carcinoma. In general, the staining intensity of carcinomas was stronger than that of benign tumors (Fig. 2), and the difference in OD values were, on average, 12%. Moreover, in carcinomas, the PREP staining was increased by an average of 27% compared with corresponding healthy tissue. This trend was seen in both mucinous (Fig. 2A–D) and serous tumors (Fig. 2E–H). Interestingly, serous borderline tumors had a similar staining intensity as cystadenomas. Moreover, the staining intensity of PREP had a tendency to increase with increased malignancy grade in serous carcinomas (Table 2).
Figure 2.
The distribution of PREP protein in ovarian and colorectal tumors. Increased PREP (brown color) expression is seen in mucinous cystadenoma, especially in epithelial cell layer (black arrow; Panel A). Compared to mucinous cystadenoma, PREP immunoreactivity is increased in mucinous adenocarcinoma (Panels B–D), but there is practically no correlation to grading (Panel C is grade 1; Panel D grade 3). In early serous cystadenoma (Panel E), only moderate PREP expression is seen. PREP immunoreactivity is abundant in serous borderline tumors (Panel F), but the grading is not different from serous cystadenoma (Table 2). In malignant epithelial cells (black arrow; Panel G), the expression of PREP is abundant. In severe serous carcinoma (grade 3; Panel H), there is a substantial amount of PREP protein present. Compared with normal colon tissue (Panel I), PREP immunoreactivity is clearly increased in mucinous-type colorectal cancer (Panel J). Nuclei are visualized by Mayer’s hematoxylin (blue color). Scale bars are 50 µm in all Panels except Panels C and D where it is 100 µM.
Table 2.
Prolyl Oligopeptidase (PREP) Immunoreactivity of Ovarian Epithelial Tumors
| Intensity |
||||||
|---|---|---|---|---|---|---|
| Tumor Type | No. of Tumors | 0 | + | ++ | +++ | Weighted Average of PREP Staining |
| Serous carcinoma | ||||||
| Grade 1 | 3 | 0 | 2 | 1 | 0 | 1.3 |
| Grade 2 | 3 | 0 | 1 | 2 | 0 | 1.6 |
| Grade 3 | 22 | 0 | 5 | 11 | 6 | 2.0 |
| Serous borderline | 15 | 1 | 12 | 2 | 0 | 1.1 |
| Serous cystadenoma | 13 | 2 | 9 | 2 | 0 | 1 |
| Mucinous carcinoma | ||||||
| Grade 1 | 1a | 0 | 1 | 0 | 0 | 1 |
| Grade 2 | 6 | 0 | 1 | 2 | 3 | 2.3 |
| Grade 3 | 11 | 1 | 3 | 6 | 1 | 1.6 |
| Mucinous cystadenoma | 12 | 7 | 4 | 1 | 0 | 0.5 |
| Endometrioid cystadenocarcinoma | ||||||
| Grade 1 | 9 | 0 | 4 | 5 | 0 | 1.6 |
| Grade 2 | 10 | 0 | 2 | 5 | 3 | 2.1 |
| Grade 3 | 4 | 0 | 1 | 2 | 1 | 2 |
0, no PREP staining; +, low PREP staining (1 in weighted average); ++, moderate PREP staining (2 in weighted average); +++, high PREP staining (3 in weighted average). Optical density threshold as in Table 1.
Only one analyzed sample.
Colorectal Cancer
Out of 48 colorectal cancer specimens, all except one mucinous type tumor were positive for PREP immunostaining (Table 2, Fig. 2I–J). In 40 cancer cases, the staining intensity was higher than that in the normal colon, and the staining intensity of 7 tumors was similar to a normal colonic mucosa (Fig. 2I–J). Although the expression of PREP was approximately 26% higher in the tumor than in the healthy tissue, the staining intensity did not correlate with the tumor grade.
Discussion
To our knowledge, this is the first study to systematically examine the distribution of PREP protein in human tissues. Previous PREP distribution studies with human tissues have been based on enzyme activity measurements (Goossens et al. 1996; Mantle et al. 1996; Irazusta et al. 2002) and two mRNA databases (BioGPS [Su et al. 2002] and GeneSapiens [Kilpinen et al. 2008]). Moreover, the previous studies of PREP distribution in cancerous tissues were based on enzyme activity measurements (Goossenset al. 1996; Larrinaga et al. 2010). We have now shown that PREP protein is widely present in most of the peripheral tissues, and it is specifically present in certain cell types. Moreover, PREP protein expression is increased in tumors, and malignant tumors show higher PREP expression than benign ones.
Similar to rodents, PREP protein, enzymatic activity, and PREP coding mRNA did not correlate in all the tissues (Myöhänen et al. 2009). The greatest discrepancies between PREP coding mRNA and protein levels were seen in the skeletal muscle, where others showed high (GeneSapiens; Kilpinen et al. 2008) or moderate (BioGPS; Su et al. 2002) mRNA levels whereas we observed only very low amounts of PREP protein. Also, in the parotid gland, there was a moderate PREP mRNA level but only a low amount of PREP protein. The testis and renal cortex had the highest PREP expression, while high PREP protein levels were seen only in certain cells of the testis, and only low levels of PREP protein were seen in the renal cortex. Low PREP activities were found in the stomach, urinary bladder, and ovaries (Goossens et al. 1996), whereas there were moderate to high amounts of both PREP mRNA (BioGPS [Su et al. 2002] and GeneSapiens [Kilpinen et al. 2008]) and protein in these organs (this study). These discrepancies in normal peripheral tissues may be due to the fact there are several factors, such as an endogenous inhibitor and polyamines (Yoshimoto et al. 1982; Yamakawa et al. 1994), estradiol-17β and progesterone (Ohta et al. 1992), that can affect PREP activity and possibly even protein expression. Differences between the expression profiles of PREP determined by mRNA, immunohistochemistry, and enzyme activity in our work and the work by others clearly point to a strict endogenous regulation of PREP; for example, via an endogenous inhibitor and/or protein trafficking. However, epithelial cells showed high PREP mRNA (the above databases), protein (this study), and enzyme activity levels (Goossens et al. 1996). Moreover, in tumors, PREP protein amounts, enzyme activities (Goossens et al. 1996; Larrinaga et al. 2010), and PREP mRNA (GeneSapiens; Kilpinen et al. 2008) were uniformly increased.
Interestingly, the most intense PREP protein expression was seen in different kinds of secreting epithelial cells, such as (1) parietal and peptic cells in the stomach, (2) epithelial cells in the breast ducts, (3) the prostate gland, (4) the kidney’s tubule walls, (5) the opening of the endometrial gland of the uterus, (6) the exocrine pancreas, and (7) the zona fasciculata of the adrenal gland cortex. These findings support a general association of PREP with secretory processes, at least in spatial terms, as has been proposed by Schulz et al. (2005) and us (Myöhänen et al. 2008a), possibly via microtubules or the Golgi-endoplasmic reticulum network. However, additional detailed studies of the role of PREP in protein secretion are required to draw more specific conclusions.
In earlier studies, PREP has been suggested to participate in mouse sperm motility (Kimura et al. 2002), and we have detected high PREP amounts in the mouse testis (Myöhänen et al. 2008b). In humans, we also detected high amounts of PREP in several cell types involved in spermatogenesis, particularly in the early phase. Moreover, PREP was seen in the nuclei of primary spermatocytes and spermatids, favoring an association of PREP with cell differentiation and early development (Ohtsuki et al. 1997; Agirregoitia et al. 2010; Hannula et al. 2010). Also oocytes and primary follicles contained PREP protein, and sex hormones, such as estradiol-17β and progesterone (Ohta et al. 1992), can modify PREP activity. The abundance of PREP in several cells in the reproductive systems of both males and females points to an association of PREP with reproduction. There is certainly a need for functional studies to clarify the role of PREP in reproduction.
Generally, PREP protein was increased in tumors compared with normal tissues. When benign tumors were compared with corresponding healthy tissues, the difference was not particularly high (approximately 15%), but the difference between malignant tumors and healthy tissues was marked (approximately 27%). Similar findings have been made when measuring PREP enzyme activities (Goossens et al. 1996; Larrinaga et al. 2010) and for PREP coding mRNA (GeneSapiens; Kilpinen et al. 2008). Interestingly, in the study of Larrinaga et al. (2010), there was no difference in a PREP activity between colon adenocarcinoma and normal mucosa. However, we saw a 26% increase in PREP OD, suggesting increased PREP protein levels in these tumor samples. The discrepancy between PREP activity and protein levels in carcinomas is in favor of unknown non-hydrolytic functions that have been suggested earlier by Brandt et al. (2008), Di Daniel et al. (2009), and Myöhänen et al. (2011). In ovarian serous carcinomas, increased PREP expression and increased malignancy grade had some correlation (Table 2). Moreover, in ovarian epithelial tumors, with the exception of serous borderline tumors, PREP protein was more abundantly present in malignant than benign tumors, suggesting that PREP may be associated with metastasis. This is supported by our recent finding, where PREP was shown to increase angiogenesis via the release of a tetrapeptide Ac-SDKP (Myöhänen et al. 2011). Angiogenesis is an important step in tumor growth and invasion, and PREP activity and Ac-SDKP release correlate in malignant tumors (Liu et al. 2008). However, a role of PREP in tumor metastasis should be studied more carefully using experimental metastasis assays.
It has been also suggested that PREP could be involved in cell proliferation via DNA processing (see above). However, in contrast to the findings in the mouse, PREP was only rarely present in human proliferating cells and not at all in the nuclei, except in the testis (see above). Also in tumors, PREP was mostly present in the cytosol. These findings do not support the view that altered DNA synthesis would contribute to the participation of PREP on tumor growth.
In conclusion, we present that (1) PREP protein has a wide but cell-specific distribution in human peripheral tissues; (2) PREP is preferentially present in secreting cells, showing generally a spatial association with secretory processes; and (3) a high PREP expression in cells of the male and female reproductive system. Moreover, (4) PREP expression was higher in tumors than in normal tissues, and (5) malignant tumors showed more intense PREP expression than benign tumors.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: These studies were supported by grants from Finnish Cultural Foundation, Orion-Pharmos Foundation, Academy of Finland (No. 138127/2010) and Jane and Aatos Erkko Foundation to Timo T. Myöhänen, by the grants of the Academy of Finland (No. 1131915/2008 and No. 117881/2006) to Pekka T. Männistö, and by the Finnish Cancer Society grant to Olli Carpén.
References
- Agirregoitia N, Bizet P, Agirregoitia E, Boutelet I, Peralta L, Vaudry H, Jegou S. 2010. Prolyl endopeptidase mRNA expression in the central nervous system during rat development. J Chem Neuroanat. 40:53–62 [DOI] [PubMed] [Google Scholar]
- Agirregoitia N, Casis L, Gil J, Ruiz F, Irazusta J. 2007. Ontogeny of prolyl endopeptidase and pyroglutamyl peptidase I in rat tissues. Regul Pept. 139:52–58 [DOI] [PubMed] [Google Scholar]
- Agirregoitia N, Gil J, Ruiz F, Irazusta J, Casis L. 2003a. Effect of aging on rat tissue peptidase activities. J Gerontol A Biol Sci Med Sci. 58:B792–7 [DOI] [PubMed] [Google Scholar]
- Agirregoitia N, Irazusta A, Ruiz F, Irazusta J, Gil J. 2003b. Ontogeny of soluble and particulate prolyl endopeptidase activity in several areas of the rat brain and in the pituitary gland. Dev Neurosci. 25:316–323 [DOI] [PubMed] [Google Scholar]
- Bellemere G, Vaudry H, Mounien L, Boutelet I, Jegou S. 2004. Localization of the mRNA encoding prolyl endopeptidase in the rat brain and pituitary. J Comp Neurol. 471:128–143 [DOI] [PubMed] [Google Scholar]
- Brandt I, Gerard M, Sergeant K, Devreese B, Baekelandt V, Augustyns K, Scharpe S, Engelborghs Y, Lambeir AM. 2008. Prolyl oligopeptidase stimulates the aggregation of alpha-synuclein. Peptides. 29:1472–1478 [DOI] [PubMed] [Google Scholar]
- Daly DJ, Maskrey P, Pennington RJ. 1985. Characterization of proline endopeptidase from skeletal muscle. Int J Biochem. 17:521–524 [DOI] [PubMed] [Google Scholar]
- Di Daniel E, Glover CP, Grot E, Chan MK, Sanderson TH, White JH, Ellis CL, Gallagher KT, Uney J, Thomas J, et al. 2009. Prolyl oligopeptidase binds to GAP-43 and functions without its peptidase activity. Mol Cell Neurosci. 41:373–382 [DOI] [PubMed] [Google Scholar]
- Dresdner K, Barker LA, Orlowski M, Wilk S. 1982. Subcellular distribution of prolyl endopeptidase and cation-sensitive neutral endopeptidase in rabbit brain. J Neurochem. 38:1151–1154 [DOI] [PubMed] [Google Scholar]
- Fuse Y, Polk DH, Lam RW, Reviczky AL, Fisher DA. 1990. Distribution and ontogeny of thyrotropin-releasing hormone degrading enzymes in rats. Am J Physiol. 259:E787–E791 [DOI] [PubMed] [Google Scholar]
- Garcia-Horsman JA, Männistö PT, Venäläinen JI. 2007. On the role of prolyl oligopeptidase in health and disease. Neuropeptides. 41:1–24 [DOI] [PubMed] [Google Scholar]
- Gardberg M, Talvinen K, Kaipio K, Iljin K, Kampf C, Uhlen M, Carpen O. 2010. Characterization of Diaphanous-related formin FMNL2 in human tissues. BMC Cell Biol. 11:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens F, De Meester I, Vanhoof G, Scharpe S. 1996. Distribution of prolyl oligopeptidase in human peripheral tissues and body fluids. Eur J Clin Chem Clin Biochem. 34:17–22 [DOI] [PubMed] [Google Scholar]
- Hannula MJ, Mannisto PT, Myöhänen TT. 2010. Sequential expression, activity and nuclear localization of prolyl oligopeptidase protein in the developing rat brain. Dev Neurosci. 33:38–47 [DOI] [PubMed] [Google Scholar]
- Irazusta J, Larrinaga G, Gonzalez-Maeso J, Gil J, Meana JJ, Casis L. 2002. Distribution of prolyl endopeptidase activities in rat and human brain. Neurochem Int. 40:337–345 [DOI] [PubMed] [Google Scholar]
- Ishino T, Ohtsuki S, Homma K, Natori S. 1998. cDNA cloning of mouse prolyl endopeptidase and its involvement in DNA synthesis by Swiss 3T3 cells. J Biochem (Tokyo). 123:540–545 [DOI] [PubMed] [Google Scholar]
- Kilpinen S, Autio R, Ojala K, Iljin K, Bucher E, Sara H, Pisto T, Saarela M, Skotheim RI, Bjorkman M, et al. 2008. Systematic bioinformatic analysis of expression levels of 17,330 human genes across 9,783 samples from 175 types of healthy and pathological tissues. Genome Biol. 9:R139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Matsui H, Takahashi T. 2002. Expression and localization of prolyl oligopeptidase in mouse testis and its possible involvement in sperm motility. Zoolog Sci. 19:93–102 [DOI] [PubMed] [Google Scholar]
- Kokko M, Kuronen I, Kärenlampi S. 1994. Rapid production of antibodies in chicken and isolation from eggs. In: Celis JE, editor. Cellular Biology, 2nd ed. San Diego (CA): Academic Press; p. 282–288 [Google Scholar]
- Larrinaga G, Perez I, Blanco L, Lopez JI, Andres L, Etxezarraga C, Santaolalla F, Zabala A, Varona A, Irazusta J. 2010. Increased prolyl endopeptidase activity in human neoplasia. Regul Pept. 163:102–106 [DOI] [PubMed] [Google Scholar]
- Liu JM, Kusinski M, Ilic V, Bignon J, Hajem N, Komorowski J, Kuzdak K, Stepien H, Wdzieczak-Bakala J. 2008. Overexpression of the angiogenic tetrapeptide AcSDKP in human malignant tumors. Anticancer Res. 28:2813–2817 [PubMed] [Google Scholar]
- Männistö PT, Venäläinen JI, Jalkanen AJ, Garcia-Horsman JA. 2007. Prolyl oligopeptidase: a potential target for the treatment of cognitive disorders. Drugs News Persp. 20:293–305 [DOI] [PubMed] [Google Scholar]
- Mantle D, Falkous G, Ishiura S, Blanchard PJ, Perry EK. 1996. Comparison of proline endopeptidase activity in brain tissue from normal cases and cases with Alzheimer’s disease, Lewy body dementia, Parkinson’s disease and Huntington’s disease. Clin Chim Acta. 249:129–139 [DOI] [PubMed] [Google Scholar]
- Moreno-Baylach MJ, Felipo V, Mannisto PT, Garcia-Horsman JA. 2008. Expression and traffic of cellular prolyl oligopeptidase are regulated during cerebellar granule cell differentiation, maturation, and aging. Neuroscience. 156:580–585 [DOI] [PubMed] [Google Scholar]
- Myöhänen T, Tenorio-Laranga J, Jokinen B, Vazquez-Sanchez R, Moreno-Baylach M, Garcia-Horsman J, Mannisto P. 2011. Prolyl oligopeptidase induces angiogenesis both in vitro and in vivo in a novel regulatory manner. Br J Pharmacol. 163:1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myöhänen TT, Garcia-Horsman JA, Tenorio-Laranga J, Mannisto PT. 2009. Issues about the physiological functions of prolyl oligopeptidase based on its discordant spatial association with substrates and inconsistencies among mRNA, protein levels, and enzymatic activity. J Histochem Cytochem. 57:831–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myöhänen TT, Venäläinen JI, Garcia-Horsman JA, Piltonen M, Männistö PT. 2008a. Cellular and subcellular distribution of rat brain prolyl oligopeptidase and its association with specific neuronal neurotransmitters. J Comp Neurol. 507:1694–1708 [DOI] [PubMed] [Google Scholar]
- Myöhänen TT, Venäläinen JI, Garcia-Horsman JA, Piltonen M, Männistö PT. 2008b. Distribution of prolyl oligopeptidase in the mouse whole-body sections and peripheral tissues. Histochem Cell Biol. 130:993–1003 [DOI] [PubMed] [Google Scholar]
- Myöhänen TT, Venäläinen JI, Tupala E, Garcia-Horsman JA, Miettinen R, Männistö PT. 2007. Distribution of immunoreactive prolyl oligopeptidase in human and rat brain. Neurochem Res. 32:1365–1374 [DOI] [PubMed] [Google Scholar]
- Ohta N, Takahashi T, Mori T, Park MK, Kawashima S, Takahashi K, Kobayashi H. 1992. Hormonal modulation of prolyl endopeptidase and dipeptidyl peptidase IV activities in the mouse uterus and ovary. Acta Endocrinol (Copenh).127:262–266 [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Homma K, Kurata S, Komano H, Natori S. 1994. A prolyl endopeptidase of Sarcophaga peregrina (flesh fly): its purification and suggestion for its participation in the differentiation of the imaginal discs. J Biochem (Tokyo). 115:449–453 [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Homma K, Kurata S, Natori S. 1997. Nuclear localization and involvement in DNA synthesis of Sarcophaga prolyl endopeptidase. J Biochem (Tokyo). 121:1176–1181 [DOI] [PubMed] [Google Scholar]
- O’Leary RM, O’Connor B. 1995. Identification and localisation of a synaptosomal membrane prolyl endopeptidase from bovine brain. Eur J Biochem. 227:277–283 [DOI] [PubMed] [Google Scholar]
- Pittaway KM, Reynolds GP, Emson PC. 1984. Decreased proline endopeptidase activity in the basal ganglia in Huntington’s disease. J Neurochem. 43:878–880 [DOI] [PubMed] [Google Scholar]
- Rawlings ND, Barrett AJ. 1994. Families of serine peptidases. Methods Enzymol. 244:19–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossner S, Schulz I, Zeitschel U, Schliebs R, Bigl V, Demuth HU. 2005. Brain prolyl endopeptidase expression in aging, APP transgenic mice and Alzheimer’s disease. Neurochem Res. 30:695–702 [DOI] [PubMed] [Google Scholar]
- Schulz I, Gerhartz B, Neubauer A, Holloschi A, Heiser U, Hafner M, Demuth HU. 2002. Modulation of inositol 1,4,5–triphosphate concentration by prolyl endopeptidase inhibition. Eur J Biochem. 269:5813–5820 [DOI] [PubMed] [Google Scholar]
- Schulz I, Zeitschel U, Rudolph T, Ruiz-Carrillo D, Rahfeld JU, Gerhartz B, Bigl V, Demuth HU, Rossner S. 2005. Subcellular localization suggests novel functions for prolyl endopeptidase in protein secretion. J Neurochem. 94:970–979 [DOI] [PubMed] [Google Scholar]
- Su AI, Cooke MP, Ching KA, Hakak Y, Walker JR, Wiltshire T, Orth AP, Vega RG, Sapinoso LM, Moqrich A, et al. 2002. Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci U S A. 99:4465–4470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenorio-Laranga J, Venalainen JI, Mannisto PT, Garcia-Horsman JA. 2008. Characterization of membrane-bound prolyl endopeptidase from brain. FEBS J. 275:4415–4427 [DOI] [PubMed] [Google Scholar]
- Venäläinen JI, Garcia-Horsman JA, Forsberg MM, Jalkanen A, Wallen EA, Jarho EM, Christiaans JA, Gynther J, Männistö PT. 2006. Binding kinetics and duration of in vivo action of novel prolyl oligopeptidase inhibitors. Biochem Pharmacol. 71:683–692 [DOI] [PubMed] [Google Scholar]
- Venäläinen JI, Juvonen RO, Forsberg MM, Garcia-Horsman A, Poso A, Wallen EA, Gynther J, Männistö PT. 2002. Substrate-dependent, non-hyperbolic kinetics of pig brain prolyl oligopeptidase and its tight binding inhibition by JTP-4819. Biochem Pharmacol. 64:463–471 [DOI] [PubMed] [Google Scholar]
- Venäläinen JI, Juvonen RO, Männistö PT. 2004. Evolutionary relationships of the prolyl oligopeptidase family enzymes. Eur J Biochem. 271:2705–2715 [DOI] [PubMed] [Google Scholar]
- Walter R. 1976. Partial purification and characterization of post-proline cleaving enzyme: enzymatic inactivation of neurohypophyseal hormones by kidney preparations of various species. Biochim Biophys Acta. 422:138–158 [DOI] [PubMed] [Google Scholar]
- Williams RS, Cheng L, Mudge AW, Harwood AJ. 2002. A common mechanism of action for three mood-stabilizing drugs. Nature. 417:292–295 [DOI] [PubMed] [Google Scholar]
- Williams RS, Harwood AJ. 2000. Lithium therapy and signal transduction. Trends Pharmacol Sci. 21:61–64 [DOI] [PubMed] [Google Scholar]
- Yamakawa N, Shimeno H, Soeda S, Nagamatsu A. 1994. Regulation of prolyl oligopeptidase activity in regenerating rat liver. Biochim Biophys Acta. 1199:279–284 [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Tsukumo K, Takatsuka N, Tsuru D. 1982. An inhibitor for post-proline cleaving enzyme: distribution and partial purification from porcine pancreas. J Pharmacobiodyn. 5:734–740 [DOI] [PubMed] [Google Scholar]