Abstract
The pancreatic islet comprises endocrine, vascular, and neuronal cells. Signaling among these cell types is critical for islet function. The extracellular matrix (ECM) is a key regulator of cell-cell signals, and while some islet ECM components have been identified, much remains unknown regarding its composition. We investigated whether hyaluronan, a common ECM component that may mediate inflammatory events, and molecules that bind hyaluronan such as versican, tumor necrosis factor–stimulated gene 6 (TSG-6), and components of inter-α-trypsin inhibitor (IαI), heavy chains 1 and 2 (ITIH1/ITIH2), and bikunin, are normally produced in the pancreatic islet. Mouse pancreata and isolated islets were obtained for microscopy (with both paraformaldehyde and Carnoy’s fixation) and mRNA. Hyaluronan was present predominantly in the peri-islet ECM, and hyaluronan synthase isoforms 1 and 3 were also expressed in islets. Versican was produced in α cells; TSG-6 in α and β cells; bikunin in α, β, and δ cells; and ITIH1/ITIH2 predominantly in β cells. Our findings demonstrate that hyaluronan, versican, TSG-6, and IαI are normal islet components and that different islet endocrine cell types contribute these ECM components. Thus, dysfunction of either α or β cells likely alters islet ECM composition and could thereby further disrupt islet function.
Keywords: extracellular matrix, hyaluronan, islet, proteoglycan
The pancreatic islet is a complex mini-organ comprising numerous cell types. These include the well-recognized endocrine α, β, δ, and pancreatic polypeptide (PP) cell types, producing predominantly the hormones glucagon, insulin, somatostatin, and pancreatic polypeptide, respectively (Baetens et al. 1979; Halban 2004). The extensive intra-islet capillary network comprises endothelial cells, together with supporting pericytes and fibroblasts (Ballian and Brunicardi 2007; Olsson and Carlsson 2006). Sympathetic and parasympathetic fibers innervate the islet (Ahren 2000) and also require supporting cells such as Schwann cells (Smith 1975). The normal control of glucose metabolism requires exquisite integrated regulation of all of these cell types.
To achieve this, communication between these cells is required. This can be mediated by direct cell-cell contact (Orci et al. 1975) and/or via the extracellular matrix (ECM) (Hammar et al. 2004; Sorokin 2010). The islet ECM has been the focus of some recent studies that have demonstrated the presence and organization of several collagen and laminin isoforms, together with the expression of a variety of cell surface integrins that relay matrix signals into the cell (Hammar et al. 2004; Hughes et al. 2006; Irving-Rodgers et al. 2008; Kaido et al. 2004; Nikolova et al. 2006; Virtanen et al. 2008). Integrin-laminin interactions have been shown to be important in regulating insulin release from β cells, underscoring the importance of ECM components in regulating β-cell function (Parnaud et al. 2006). Despite these studies, much still remains unknown regarding the composition of the islet ECM and the roles of other ECM components such as proteoglycans and hyaluronan in islet structure and function.
Proteoglycans are a diverse family of extracellular and cell surface molecules comprising a core protein covalently linked to one or more glycosaminoglycan (GAG) chains. The heparan sulfate proteoglycan, perlecan, is present in the human (Kahn et al. 1999) and mouse islet (Irving-Rodgers et al. 2008) and is expressed and released from cultured β cells (Potter-Perigo et al. 2003). Cell surface heparan sulfate has been shown to be critical for internalization of the transcription factors BETA2/NeuroD and PDX-1 by cultured β cells (Noguchi et al. 2007; Ueda et al. 2008). Insulin release and islet morphology are both disrupted following degradation of islet heparan sulfate or transgenic disruption of islet heparan sulfate synthesis, respectively (Takahashi et al. 2009). Recent studies have shown a key role for heparan sulfate in β cell survival (Ziolkowski et al. 2012). On the other hand, the presence of other proteoglycans/glycosaminoglycans in the islet has not been reported.
Hyaluronan is a large GAG composed of repeating disaccharides of N-acetyl glucosamine and glucuronic acid and differs from other GAGs in that the disaccharides do not undergo sulfation (Laurent and Fraser 1992). Hyaluronan is a common ECM component that has been implicated in angiogenesis, cell motility, wound healing, cell adhesion, and inflammation (Jiang et al. 2007; Tammi et al. 2002; Toole et al. 2002). Hyaluronan exerts its biological effects by forming complexes with a number of other molecules. It is well established that, in many tissues, high molecular weight hyaluronan exists in the ECM as link protein–stabilized complexes with chondroitin sulfate proteoglycans, such as versican, which are important in regulating cell processes, such as proliferation (Evanko et al. 1999). Hyaluronan chains can also be organized into cross-linked networks via interactions with both tumor necrosis factor–stimulated gene 6 (TSG-6) and inter-α-trypsin inhibitor (IαI) (Baranova et al. 2011; Day and de la Motte 2005; Zhao et al. 1995). The presence of both of these hyaluronan binding proteins is essential for ovulation; transgenic deletion of either TSG-6 or the IαI light chain component bikunin results in female infertility (Fulop et al. 2003; Zhuo et al. 2001). However, while these hyaluronan networks are critical for physiological processes, formation of cross-linked networks of hyaluronan also occurs during pathological processes such as inflammation. Indeed, in the islet, hyaluronan has been detected during insulitis in the nonobese diabetic (NOD) mouse model of type 1 diabetes (Weiss et al. 2000), and hyaluronan content is increased in the whole pancreas from individuals with pancreatic cancer (Fries et al. 1994; Masuda et al. 1989; Theocharis et al. 2000), suggesting a pathogenic role for this molecule. Whether hyaluronan is a component of normal islets has not previously been described. In this study, we sought to determine whether hyaluronan and its binding partners versican, TSG-6, and IαI (comprising IαI heavy chains 1 and 2 [ITIH1 and ITIH2] and the light chain bikunin) are present in normal mouse islets and to determine which islet cells are the source of these ECM molecules.
Materials and Methods
Mouse Pancreas Specimens, Islet Isolation, and Dissociated Cell Preparation
Pancreata from male and female mice (C57BL/6 or F1 C57BL/6 × DBA/2 background; n=8) were used for tissue procurement using paraformaldehyde (PFA) (n=8) or Carnoy’s fixation (n=6); 6 mice were used for islet isolation for whole islet mRNA analysis, and a further 10 mice were used for islet isolation in order to obtain enriched β cell and non–β cell (α/δ cell) samples. Note that, for the latter, islets from three to four mice were pooled prior to generating each enriched cell sample. Animals were euthanized under pentobarbital anesthesia, and pancreata and/or pancreatic islets were harvested, as we have done previously (Hull et al. 2005; Hull et al. 2007). These studies were approved by the Institutional Animal Care and Use Committee of the Veterans Affairs Puget Sound Health Care System.
For cell sorting, isolated islets were pooled from three to four mice and dissociated to single cells using Cell Dissociation Solution (Sigma-Aldrich; St. Louis, MO). Islet cells were sorted on a BD Aria II high speed cell sorter (BD Biosciences; Franklin Lakes, NJ) based on fluorescence at 488 nm (detecting flavin adenine dinucleotide autofluorescence) and forward light scatter (a measure of cell size), resulting in the separation and collection of β cell- and non-β cell (α/δ cell)-enriched populations (Cirulli et al. 1993). Cell sorting was performed on three independent islet cell preparations, which were harvested for mRNA analyses. Effective separation of cell populations was verified by analysis of insulin, glucagon, and somatostatin mRNA levels in each cell population, normalized to levels in β cell- and α/δ cell-enriched populations, respectively. Insulin mRNA levels were 1.00 ± 0.06 versus 0.01 ± 0.006, while glucagon mRNA levels were 0.04 ± 0.007 versus 1.00 ± 0.01 in the β cell- and α/δ cell-enriched populations, and somatostatin mRNA levels were 0.11 ± 0.02 versus 1.00 ± 0.13 in the β cell- and α/δ cell-enriched populations, respectively.
Immunohistochemical Procedures
Pancreas specimens were immersion-fixed in phosphate-buffered PFA (4% w/v) or Carnoy’s fixative (10% acetic acid, 60% methanol, 30% chloroform, all v/v) overnight and were kept in 70% ethanol prior to processing. Specimens were dehydrated in a series of graded ethanol, followed by xylene, paraffin infiltration, and embedding. Five-mm sections were cut.
After deparaffinization, endogenous peroxidases were blocked using H2O2 in methanol and tissue sections rehydrated in a series of graded ethanol. For hyaluronan affinity histochemistry, rehydrated tissue sections were blocked in 1% (w/v) bovine serum albumin (BSA) in phosphate- buffered saline (PBS) for 1 hr at room temperature and incubated overnight at 4C with biotinylated hyaluronan binding protein (in house; 4 μg/ml) (Evanko et al. 1998). After two PBS washes, the tissue sections were incubated with the Vector “Elite” ABC-HP kit (Vector Labs; Burlingame, CA) in a moist chamber for 30 min at room temperature. Detection was performed using the Vector NovaRed substrate (Vector Labs) for 10 min at room temperature. The sections were counterstained with Gill no. 3 hematoxylin. For versican immunohistochemistry, the same protocol was used except the sections were digested with 0.2 U/ml of chondroitinase ABC to expose versican epitopes prior to blocking in 5% nonfat milk (w/v), 1% (v/v) normal goat serum, and 1% (w/v) BSA in PBS. The specific primary antibody used was a rabbit anti-mouse versican polyclonal antibody (6 μg/ml) that recognizes the βGAG domain of versican (catalog no. AB1033; Chemicon/Millipore, Billerica, MA). This was followed by biotinylated goat anti-rabbit immunoglobulin G (IgG) antibody (Jackson ImmunoResearch; West Grove, PA) for 1 hr at room temperature. For TSG-6 immunohistochemistry, the same protocol was used as for versican immunohistochemistry but without chondroitinase pretreatment. Primary antibodies were rabbit anti-mouse TSG-6 (RAM-1; 1:160) (Carrette et al. 2001) or polyclonal goat anti-mouse TSG-6 (8 μg/ml, catalog no. AF2326; R&D Systems, Minneapolis, MN). Both antisera showed the same staining patterns under all conditions examined; data are shown for the RAM-1 antibody only. For ITIH1 and ITIH2, sections were blocked with either Animal Free block (Vector Labs) or CAS Blocker (Invitrogen; Carlsbad, CA). Primary antibodies for ITIH1 and ITIH2 were tested alone and in combination. We determined that the staining pattern for each individual antibody was identical and that a combination of both primary antibodies together (2.5 µg/ml for each, catalog no. sc33944 and sc21978, both raised in goat; Santa Cruz Biotechnology, Santa Cruz, CA) with overnight incubation at 4C worked optimally. The remaining protocol was as for TSG-6 immunohistochemistry, with appropriate biotinylated anti-goat IgG (Vector Labs). For bikunin, Triton X-100 (0.05% v/v) was included in the blocking buffer, primary antibody solution, and wash buffers. Sections were blocked with 5% (v/v) normal donkey serum and 1% BSA (w/v) in PBS. The primary antibody was rabbit anti-mouse bikunin (BIK-N-127; 1:800). The remaining protocol was as for TSG-6 immunohistochemistry.
For Carnoy’s-fixed sections, immunohistochemistry procedures were identical to those for PFA-fixed sections, with the following exceptions. For ITIH1/ITIH2, antigen retrieval (incubation in 1 mM EDTA, pH 8, at 95C for 20 min) was used; Triton X-100 (0.05% v/v) was added to wash buffers, and immunostaining was visualized by donkey anti-goat horseradish peroxidase (HRP) (3.2 μg/ml; Jackson ImmunoResearch) in place of biotinylated antibodies/ABC. Negative controls for staining included the use of hyaluronidase-treated sections for hyaluronan staining or appropriate irrelevant IgG for all other immunohistochemistry.
Double immunofluorescent staining was performed for versican, TSG-6, ITIH1/ITIH2, or bikunin, together with insulin, glucagon, or somatostatin, respectively, in order to determine cellular localization of these molecules. This double staining was performed on PFA-fixed pancreata only, because the alcohol present in Carnoy’s fixative extracts insulin (Scott 1912) as well as other peptide hormones from the pancreas, meaning that Carnoy’s fixative cannot be used for insulin, glucagon, or somatostatin staining. Staining was as described for versican and ITIH1/ITIH2 above, except for the use of Alexa Fluor 488–conjugated IgG (5 μg/ml; Invitrogen) in place of biotin-ABC-Nova Red. For visualization of TSG-6 and bikunin, a tyramide amplification system was used (Life Technologies; Carlsbad, CA). HRP goat anti-rabbit IgG (1:100) and Alexa Fluor 488 tyramide (1:100) were prepared following the manufacturer’s instructions. The amplification reaction was incubated for 10 min. Insulin and glucagon monoclonal antibodies (catalog no. I2018 and G2654, respectively; Sigma-Aldrich) were both diluted 1:1000, and somatostatin monoclonal antibody (MS12; a kind gift from Dr. John Ensinck, University of Washington, Seattle, WA) (Ensinck et al. 2002) was diluted 1:250. For visualization of insulin, glucagon, and somatostatin immunostaining, an appropriate Alexa Fluor 546-conjugated IgG (5 μg/ml; Invitrogen) was used. Appropriate IgGs were used as negative controls for both sets of primary antisera.
RNA Isolation and Real-Time Polymerase Chain Reaction
Islets or dispersed cells were harvested for total RNA isolation using the High Pure RNA isolation kit (Roche Applied Science; Indianapolis, IN) and reverse-transcribed using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems; Foster City, CA). For real-time polymerase chain reaction (PCR), all reagents were supplied by Applied Biosystems, unless otherwise noted. Relative quantitation of hyaluronan synthase isoform 1 (Has1), Has2, Has3, and versican gene expression was performed using TaqMan Gene Expression Assays Mm00468496_m1, Mm00515089_m1, Mm00515092_m1, and Mm01283063_m1, respectively. Briefly, 100 ng cDNA was amplified in 1X TaqMan Gene Expression Master Mix with a 250-nM TaqMan probe in a 20-μl reaction. Amplification of TSG-6, ITIH1, ITIH2, and bikunin was performed using 100 ng cDNA in 1X Power Sybr Green PCR Master Mix and 1-μM primers. Melting curve analysis confirmed that only one product was amplified. Expression was normalized to eukaryotic 18S rRNA Endogenous Control part no. 4333760. All reactions were run using the standard program for 50 cycles on an ABI7900HT thermocycler. All samples were performed in duplicate, and copy number estimates were generated from a standard curve created by using a selected reference cDNA template and TaqMan probe (Shih and Smith 2005). Primers for TSG-6, ITIH1, ITIH2, and bikunin (α-1-microglobulin/bikunin precursor [AMBP]) were designed with NCBI Primer-BLAST and synthesized by Sigma-Aldrich and are as follows: TSG-6F: 5′ATTTGAAGGTGGTCGTCTCG3′, TSG-6R: 5′GTTTC ACAATGGGGTATCCG3′, ITIH1F: 5′CGGCTCGGAGA TTGTGGTGGCT3′, ITIH1R: 5′TCCTCGTCCACTAGG CAGGTTGC3′, ITIH2F: 5′CTTTGCTCCTGAGAACCT GG3′, ITIH2R: 5′ATCGTCTTCATTGCCTCCAC3′, AMBPF: 5′AGGA GGGGGCGACAGAAACAGAG3′, and AMBPR: 5′GTCC GTCTTCTGGTACGCCCCA3′. Data for mRNA expression are provided as mean ± standard error of the mean of the estimated copy number, normalized to 18S rRNA, and differences between enriched primary islet cell populations were analyzed by the t-test (with p<0.05 being considered statistically significant).
Results
ECM Staining in PFA- or Carnoy’s-Fixed, Paraffin-Embedded Normal Mouse Pancreas
Hyaluronan staining was visible in the PFA-fixed pancreas in the peri-islet ECM (Fig. 1A, arrows). However, this peri/intra-islet staining was seen only in a few islets per section and did not encompass the whole islet periphery. The vast majority of islets in all eight PFA-fixed pancreas sections had no visible hyaluronan staining. Because hyaluronan matrices are better visualized in tissue fixed with acid- ethanol-based fixatives rather than PFA or formalin alone (Evanko et al. 2009; Lin et al. 1997), we also performed staining on Carnoy’s-fixed pancreas samples. In contrast to PFA, with Carnoy’s fixation, hyaluronan staining was abundantly present around islets in all sections (Fig. 1B) and was also infrequently present within islets in association with intra-islet capillaries (Fig. 1B, arrows). The latter staining pattern was also rarely observed in the PFA-fixed pancreas (Fig. 1A, arrowhead). Hyaluronan staining around the exocrine lobules was also much more intense with Carnoy’s than with PFA fixation (example denoted in Fig. 1B with arrowheads). Specificity of this staining was shown by hyaluronidase pretreatment in both PFA- (Fig. 1C) and Carnoy’s-fixed pancreas sections (Fig. 1D).
Figure 1.
Representative images showing staining for islet extracellular matrix (ECM) components in paraformaldehyde (PFA)– or Carnoy’s-fixed, paraffin-embedded mouse pancreas samples. Hyaluronan affinity staining of PFA-fixed pancreata resulted in faint staining in the peri-islet ECM of some islets (A, peri-islet staining denoted by arrows). Hyaluronan staining was much more intense with Carnoy’s fixation (B). Hyaluronan was generally observed in peri-islet localization but was also sometimes seen associated with intra-islet capillaries (A, B, arrowheads). Hyaluronidase (H’ase) pretreated, negative control sections are shown for (C) PFA- and (D) Carnoy’s-fixed tissue. Versican immunoreactivity was cytoplasmic and present in scattered islet cells in all cases with both PFA (E) and Carnoy’s fixation (F), with the latter showing more intense staining. Immunoglobulin G (IgG) controls are shown for (G) PFA- and (H) Carnoy’s-fixed tissue. Tumor necrosis factor–stimulated gene 6 (TSG-6) immunoreactivity was also cytoplasmic (I), labeling scattered islet cells (I, arrowheads) in many islets, with diffuse staining also being present. In Carnoy’s-fixed tissue (J), diffuse TSG-6 staining was markedly reduced, while scattered islet cells were still intensely stained. IgG controls are shown for (K) PFA- and (L) Carnoy’s-fixed tissue. Inter-α-trypsin inhibitor heavy chain 1 (ITIH1)/inter-α-trypsin inhibitor heavy chain 2 (ITIH2) immunoreactivity was exclusively present in PFA-fixed tissue as faint, diffuse staining throughout the islet (M), with occasional staining appearing in the peri-islet ECM (M, arrows). In contrast, ITIH1/ITIH2 staining was absent in Carnoy’s-fixed tissue (N); staining was also absent in IgG control (O) PFA- or (P) Carnoy’s-fixed samples. Bikunin immunoreactivity was present as both diffuse staining throughout the islet (Q) and also as scattered islet cells (Q, arrows). With Carnoy’s fixation, only scattered islet cell staining was observed (R). A small amount of staining was observed in the PFA control for bikunin staining (S), while the corresponding Carnoy’s-fixed control was negative (T). Scale bar = 100 µm (all panels correspond with the scale bar in A unless otherwise noted).
Versican immunoreactivity was present in scattered islet cells in seven of eight PFA-fixed pancreata (Fig. 1E). The localization was similar with both fixatives; however, intracellular versican staining was more intense in Carnoy’s-fixed tissue (Fig. 1F). Irrelevant IgG controls are shown for PFA- and Carnoy’s-fixed samples (Fig. 1G, H, respectively). TSG-6 staining in PFA-fixed sections was present as diffuse immunoreactivity throughout the islet, which was more intense than in the surrounding exocrine tissue; this was present in five of eight mice. Intense immunoreactivity in scattered islet cells and/or in cells around the periphery of the islet was also observed in seven of eight animals (Fig. 1I, arrows). Both staining patterns (diffuse vs intense scattered cells) were observed in islets from the same mice. Localization patterns for TSG-6 immunoreactivity were similar with the RAM-1 antibody and with that from R&D Systems (data not shown); thus, staining is shown for the RAM-1 antibody only. In the Carnoy’s-fixed pancreas, intense TSG-6 staining in scattered cells was present, but the diffuse islet staining was much reduced (Fig. 1J). IgG controls are shown for PFA- and Carnoy’s-fixed samples (Fig. 1K, L, respectively). ITIH1/ITIH2 immunoreactivity in the PFA-fixed pancreas was faintly present in islets (Fig. 1M) in a pattern similar to the diffuse staining observed with TSG-6 (Fig. 1I). ITIH1/ITIH2 cytoplasmic immunoreactivity was only observed in this pattern, being present in all islets analyzed. Some ITIH1/ITIH2 immunoreactivity was also detected in the peri-islet ECM (Fig. 1M, arrows), but this was not present in all cases. ITIH1/ITIH2 immunoreactivity was much less intense in the Carnoy’s-fixed pancreas, being virtually undetectable (Fig. 1N). IgG controls are shown for PFA- and Carnoy’s-fixed samples (Fig. 1O, P, respectively). Bikunin immunoreactivity was observed in the PFA-fixed pancreas as diffuse staining throughout the islet (Fig. 1Q), with some scattered cells demonstrating intense staining (Fig. 1Q, arrows). Again, this staining pattern was the only one observed. Faint diffuse staining was also present throughout the islet in the IgG control for bikunin (Fig 1S), suggesting that the low-level staining was at least in part nonspecific, with scattered bikunin-positive cells showing specific staining.
In Carnoy’s-fixed pancreata, bikunin, like TSG-6, intense staining in scattered islet cells was clearly visible, while the diffuse staining was absent (Fig. 1R). IgG controls are shown for PFA- and Carnoy’s-fixed samples (Fig. 1S, T, respectively). Negative controls showed an absence of staining in all cases except for bikunin (Fig. 1S), as described above. Of note, no differences were observed in staining patterns between genders.
Localization of ECM Components in Islet Endocrine Cells
Given that hyaluronan localization in islets was predominantly extracellular (i.e., in the ECM surrounding the islet), while versican, TSG-6, ITIH1/ITIH2, and bikunin immunoreactivity was predominantly intracellular, we next sought to determine which islet endocrine cell types demonstrated immunoreactivity for versican, TSG-6, ITIH1/ITIH2, and bikunin. This co-localization was performed using PFA-fixed material due to the effect of Carnoy’s fixation to extract peptide hormones, thus precluding our ability to perform double staining in Carnoy’s-fixed material.
Immunofluorescence for versican (Fig. 2A), TSG-6 (Fig. 2G), ITIH1/ITIH2 (Fig. 2M), and bikunin (Fig. 2S) was comparable to our findings in Figure 1. For clarity, insulin immunostaining alone is shown in Figure 2B, H, N, and T. Versican immunoreactivity was present in glucagon-producing α cells (Fig. 2D) but was absent from insulin-producing β cells (Fig. 2C) and somatostatin-producing δ cells (Fig. 2E). IgG control for versican/hormone immunostaining is shown in Figure 2F. In contrast, TSG-6 staining was present in β cells (Fig. 2I) and α cells (Fig. 2J) but was not present in δ cells (Fig. 2K; IgG control shown in Fig. 2L). ITIH1/ITIH2 staining was localized to β cells exclusively (Fig. 2O) and was not detected in α or δ cells (Fig. 2P, Q, respectively; IgG control shown in Fig. 2R). Finally, bikunin immunoreactivity was present in β cells (Fig. 2U), α cells (Fig. 2V), and some δ cells (Fig. 2W, arrows). Of note, the IgG control for bikunin (Fig. 2X) showed a complete absence of staining, confirming that islet and particularly β cell immunoreactivity for bikunin is indeed specific.
Figure 2.
Immunofluorescent staining for versican (A, C–E, green), tumor necrosis factor–stimulated gene 6 (TSG-6) (G, I–K, green), inter-α-trypsin inhibitor heavy chain 1 (ITIH1)/inter-α-trypsin inhibitor heavy chain 2 (ITIH2) (M, O–Q, green), and bikunin (S, U–W, green) alone is shown in the left column (A, G, M, S, respectively). For clarity, insulin immunostaining alone is shown in the second column (B, H, N, T). Then, versican, TSG-6, ITIH1/ITIH2, and bikunin immunoreactivity are shown together with insulin (third column: C, I, O, U), glucagon (fourth column: D, J, P, V), or somatostatin (fifth column: E, K, Q, W). Appropriate immunoglobulin G (IgG) controls are shown in F, L, R, and X. Versican staining co-localized with glucagon staining (orange staining in D) but not with insulin (C) or somatostatin (E). TSG-6 staining co-localized with both insulin (I) and glucagon (J) but not somatostatin (K). ITIH1/ITIH2 staining co-localized only with insulin (O) but not glucagon (P) or somatostatin (Q), while bikunin staining co-localized with insulin (U), glucagon (V), and some somatostatin-positive cells (W, arrows). Scale bar = 100 mm (all panels correspond with the scale bar in A unless otherwise noted).
mRNA Levels of ECM Components in Isolated Mouse Islets and Enriched Primary β and α/δ Cell Samples
In order to provide an independent confirmation of the production of these molecules in pancreatic islets, quantitative real-time PCR was performed on total islet cDNA from six mice. In line with our immunohistochemistry data, Has1 and Has3 were expressed in islets, with Has2 being at or below the level of detection (Fig. 3A). Versican mRNA was present in all islet samples at low levels (Fig. 3B), while TSG-6 was quite abundantly expressed (Fig. 3B). ITIH1 and ITIH2 mRNA were both detected (Fig. 3C), and bikunin was also abundantly expressed in normal mouse islets (Fig. 3D).
Figure 3.
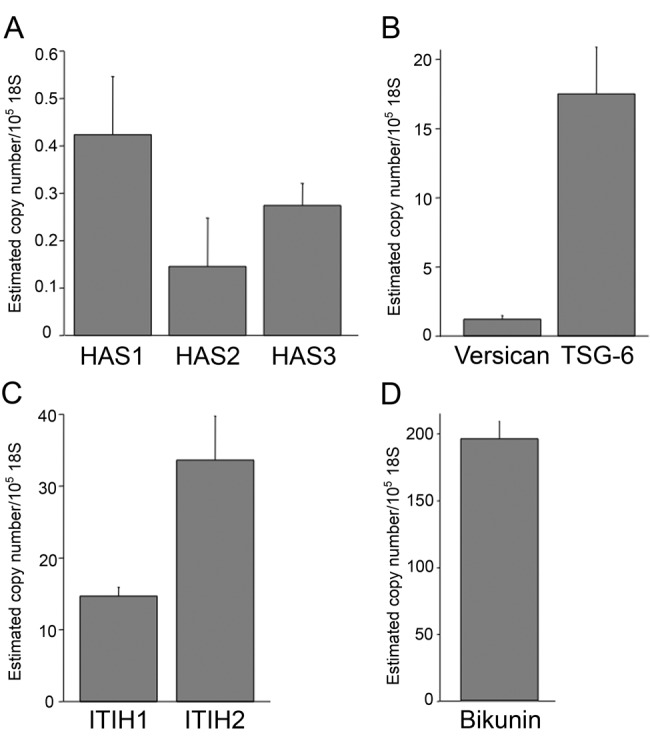
mRNA levels in isolated mouse islets (n=6; data are mean ± standard error of the mean). Hyaluronan synthase isoform 1 (Has1) mRNA was present at low levels in mouse islets, Has2 mRNA was close to or below the limit of detection, and Has3 mRNA was expressed at intermediate levels between the other two isoforms (A). Versican mRNA was present in islets at low levels (B), while tumor necrosis factor–stimulated gene 6 (TSG-6) (B), inter-α-trypsin inhibitor heavy chain 1 (ITIH1) (C), inter-α-trypsin inhibitor heavy chain 2 (ITIH2) (C), and bikunin (D) mRNA were abundant.
In primary β cells, Has1 and Has3 mRNA were present at low levels, while Has2 was absent (Fig. 4A). Has3 was the only isoform for which mRNA was detectable in primary α/δ cells (Fig. 4A). In line with our immunostaining data, versican mRNA levels were higher in primary α/δ cells than in β cells (p<0.05) (Fig. 4B), while TSG-6 mRNA levels were equivalent between the enriched cell populations (Fig. 4C). ITIH1 mRNA was detected and tended to be more abundant in β cells (Fig. 4D), while both ITIH2 and bikunin mRNA appeared to be present in both cell types (Fig. 4D). Of note, there was significant variability among islet cell preparations with respect to the abundance of several of the ECM components, but the relative abundance of each transcript was similar in β cell versus α/δ cell samples from each individual islet cell preparation.
Figure 4.

mRNA levels in samples of primary islet β cells (open bars; n=4) or α/δ cells (solid bars; n=4), defined according to enrichment for insulin or glucagon/somatostatin expression, respectively. Data are mean ± standard error of the mean. Hyaluronan synthase isoform 1 (Has1) mRNA was only detectable at low levels in β cells, Has2 mRNA was undetectable in both cell types, and Has3 mRNA was present at low levels in both cell types (A). Versican mRNA levels were higher in α/δ cells than in β cells (p<0.05) (B), tumor necrosis factor–stimulated gene 6 (TSG-6) was abundant in both cell types (C), inter-α-trypsin inhibitor heavy chain 1 (ITIH1) was detectable with slightly higher levels present in β cells (D; note that this panel is plotted on a log scale), and inter-α-trypsin inhibitor heavy chain 2 (ITIH2) and bikunin mRNA were both abundant in both cell types with a greater abundance in α/δ cells (D).
Discussion
We have shown for the first time that hyaluronan is a normal component of islet ECM and that versican, TSG-6, and components of IαI (ITIH1, ITIH2, and bikunin) are also expressed in normal mouse islets. Some differences were observed in the region of the islet and/or endocrine cell types to which each component is localized. Immunoreactivity and mRNA for versican are present predominantly in α cells, Has1, ITIH1 and ITIH2 are present predominantly in β cells, while Has3 and TSG-6 are present in both α and β cells, and bikunin is present in α and β cells and some δ cells.
Our data clearly show that hyaluronan is a normal and rather abundant component of the peri-islet ECM and is best visualized with Carnoy’s fixation, consistent with reports using other tissues (Evanko et al. 2009; Lin et al. 1997). Because Carnoy’s fixation results in extraction of insulin from islets (Scott 1912), it is rarely used in the pancreas. However, this study demonstrates that it is extremely effective for the visualization of ECM components, especially hyaluronan. Our observation that hyaluronan is present in the normal mouse islet ECM contrasts with another published study where hyaluronan was shown to be absent (Weiss et al. 2000). This discrepancy is most likely due to differences in the fixation approaches used between these two studies. Our finding is in line with those from many other tissues, which suggest hyaluronan is a widespread, perhaps even ubiquitous ECM component (Fraser et al. 1997). Our observation that islets express low levels of the hyaluronan synthetic enzymes Has1 and Has3 further supports this finding. That Has2 mRNA was undetectable in islets was somewhat surprising, given that this is the most abundant Has isoform in several cells including smooth muscle cells (Pienimaki et al. 2001; Sussmann et al. 2004). In islets, Has1 and Has3 mRNA were detected in β cells and α/δ cells, suggesting that hyaluronan in the islet ECM could derive, at least in part, from local synthesis by different islet endocrine cell types. The significance of differential expression of the three Has isoforms is still a matter of some debate in the literature, but a recent study showed that dual knockout of Has1 and Has3 in a mouse model of cutaneous injury response resulted in a proinflammatory milieu including increased neutrophil recruitment (Mack et al. 2011). Thus, constitutive expression of Has1 and Has3 in the islet may promote an anti-inflammatory environment. Based on this and literature from other tissues, we propose that, under normal conditions, hyaluronan acts to stabilize the islet ECM, providing tissue integrity cues and exerting its anti-inflammatory properties to maintain normal islet function (Jiang et al. 2007; Tammi et al. 2002; Toole et al. 2002). Consistent with this, treatment of cultured β cells with exogenous high molecular weight hyaluronan has been shown to increase insulin secretion and content (Li et al. 2006), and hyaluronan treatment decreased oxidative stress and neutrophil activation in a rat model of pancreatitis (Campo et al. 2004), suggesting that hyaluronan may be beneficial for islet/pancreas function.
Stabilization of hyaluronan-containing matrices is achieved in part through interaction with the many hyaluronan binding proteins. Our observation that several of these are also normal products of the mouse islet supports the concept that these molecules may play a role in the maintenance of normal islet homeostasis in an anti-inflammatory capacity. TSG-6 production has typically been described under conditions of inflammation, such as arthritis, where it has been shown to play a protective role (Mahoney et al. 2011; Milner et al. 2006; Szanto et al. 2004). However, our observation that TSG-6 is expressed and produced in normal mouse islets is in line with recent data demonstrating constitutive TSG-6 expression in a number of tissues, including murine bone marrow (Mahoney et al. 2008), human skin (Tan et al. 2011), and human neutrophils (Maina et al. 2009). Interestingly, in neutrophils, TSG-6 is stored in secretory granules and released only in response to a pro-inflammatory signal. Given that islet endocrine cells also contain secretory granules that store peptide hormones for subsequent release, it seems plausible that the same may occur for TSG-6 in the islet. The presence of TSG-6 has been described in islets from NOD mice, where it was co-localized with infiltrating immune cells, while it was absent from normal mouse islets (Kvezereli et al. 2008). The reason for the discrepant findings between the Kvezereli et al. (2008) study and our own is not clear, but it could be due to the different mouse backgrounds used (Balb/c vs C57BL/6 or B6D2, respectively). Our demonstration of TSG-6 immunoreactivity with two independent antisera and detection of TSG-6 mRNA level in both islets and primary endocrine cells makes us confident that our observed production of TSG-6 in normal islets is genuine.
IαI is known to be critical for several biological processes, including ovulation (Zhuo et al. 2001). Interestingly, despite the requirement of intact IαI for normal ovulation, IαI is not synthesized locally but rather enters the follicle from serum following the gonadotropin surge in the mouse (Powers et al. 1995) and is also present in follicular fluid in larger mammals (Clarke et al. 2006; Nagyova et al. 2004). In order to determine whether the islet synthesizes IαI, all three components (bikunin, ITIH1, and ITIH2) must be measured. The presence of IαI, or synthesis of its constituents, has not previously been described in mouse islets, although the presence of IαI light chain (bikunin) has been detected in whole human pancreata (Itoh et al. 1996). We observed ITIH1, ITIH2, and bikunin mRNA expression in whole mouse islets, demonstrating that islets can locally produce all three components of IαI. Bikunin mRNA was also abundantly expressed in enriched primary β cells and α/δ cells, and its immunoreactivity was detected in all three islet endocrine cell types. In contrast, ITIH1 and ITIH2 mRNA levels were detectable in β cells and/or α/δ cells but were much lower in the enriched primary endocrine cell populations than in whole islets. Further, double immunofluorescence demonstrated that ITIH1 and ITIH2 were present exclusively in β cells. Thus, our immunofluorescence and mRNA data differ somewhat here. One explanation for this discrepancy is that ITIH2 may also be present in another islet cell, which we did not evaluate by immunofluorescence (e.g., pancreatic polypeptide cells). Alternatively, the minor cross-contamination of our α/δ cell fraction with β cells may confound our mRNA data in this regard. Interestingly, for ITIH1, ITIH2, and bikunin (and also TSG-6), the mRNA levels in enriched islet cells were noticeably higher than in intact islets. One explanation for this is that intact islets were cultured for at least 24 hr prior to RNA extraction, whereas fluorescence-activated cell–sorted cells were harvested on the day of isolation. Thus, expression of these ECM molecules may be downregulated in islets with prolonged in vitro culture.
Our finding that the chondroitin sulfate proteoglycan versican is localized to α cells but not β cells is consistent with our previous work in cultured immortalized β cells, which show that β cells do not express versican mRNA (unpublished observation) and do not produce large molecular weight chondroitin sulfate proteoglycans (Potter-Perigo et al. 2003). Further, immunoreactivity for the keratan sulfate proteoglycan lumican has been described in α cells from human islet specimens (Ping Lu et al. 2002), while we found no keratanase-sensitive material to be produced by β cells (Potter-Perigo et al. 2003) (unpublished observation). In contrast, we have shown that β cells synthesize predominantly heparan sulfate proteoglycans (Potter-Perigo et al. 2003). These data suggest that islet endocrine cells synthesize distinct populations of proteoglycans. The differentiated phenotype of islet endocrine cell types is associated with a specific gene signature, which our data suggest includes a specific subset of proteoglycans. Heparan sulfate proteoglycans have already been demonstrated to be important for β cell function and survival (Noguchi et al. 2007; Takahashi et al. 2009; Ueda et al. 2008; Ziolkowski et al. 2012). Whether versican is similarly important for α cells is an area of future investigation.
The lack of co-localization of versican, TSG-6, or IαI constituents with hyaluronan in the islet ECM seems at first to be an unexpected finding. However, hyaluronan that is saturated with other binding proteins (such as versican, TSG-6, and IαI) may not readily be detected. Similarly, antibody binding sites for versican, TSG-6, and IαI may be masked upon binding to hyaluronan. However, hyaluronidase pretreatment of pancreas sections did not reveal staining for any of these proteins in the islet ECM (data not shown). Thus, we propose that the local concentrations of these molecules in the ECM are likely to be significantly lower than those seen intracellularly. This is consistent with the concept that they may be stored intracellularly (e.g., in hormone-containing secretory granules), as has been shown for TSG-6 immunolocalization in neutrophils, and provides an explanation for the observation of the predominant intracellular localization of versican, TSG-6, and IαI components in the present study.
Whether altered localization of these molecules occurs in models of islet dysfunction is the focus of future studies. For example, under disease conditions, co-localization of hyaluronan with its binding molecules might occur in an attempt to stabilize the islet ECM. Dysregulation of the islet ECM is known to occur in diabetes. One well-described example of this is the deposition of the β cell peptide islet amyloid polypeptide (also known as amylin) as amyloid in the islet ECM in type 2 diabetes (Cooper et al. 1987; Westermark 1972; Westermark et al. 1987). Heparan sulfate proteoglycans, which are normally present in the islet ECM, are components of these amyloid deposits and are thought to play an important role in their formation (Hull et al. 2004; Hull et al. 2007). Further, islet fibrosis is another example of ECM dysregulation that has been described in several rodent models of diabetes (Homo-Delarche et al. 2006; Nakamura et al. 1995; Tikellis et al. 2004). Both islet fibrosis and particularly islet amyloid deposition have negative consequences for islet function, with fibrosis contributing to islet hypoxia and amyloid deposition being associated with β cell apoptosis and decreased β cell mass (Donath et al. 2008; Jurgens et al. 2011).
In summary, we have for the first time identified the expression and location within the islet of specific components of a family of ECM components. Production of different ECM components by distinct islet cell types illustrates the complexity of this system and may be important in maintaining islet morphology and function. These findings form a basis for future studies that will evaluate the role of these specific ECM components in normal islet development and function as well as mediate or protect against islet dysfunction and destruction in diabetes.
Acknowledgments
We thank Joshua Willard, Michael Peters, Christina Braddock, Breanne Barrow, and Maria Cone for technical assistance and Virginia Green for assistance in preparing the article.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Department of Veterans Affairs, Veterans Affairs Puget Sound Health Care System (Seattle, WA), National Institutes of Health grants DK088082 (RLH) and DK017047 (University of Washington Diabetes Research Center), and the Juvenile Diabetes Research Foundation (nPOD 25–2010–648 [TNW]).
References
- Ahren B. 2000. Autonomic regulation of islet hormone secretion: implications for health and disease. Diabetologia. 43:393–410 [DOI] [PubMed] [Google Scholar]
- Baetens D, Malaisse-Lagae F, Perrelet A, Orci L. 1979. Endocrine pancreas: three-dimensional reconstruction shows two types of islets of Langerhans. Science. 206:1323–1325 [DOI] [PubMed] [Google Scholar]
- Ballian N, Brunicardi FC. 2007. Islet vasculature as a regulator of endocrine pancreas function. World J Surg. 31:705–714 [DOI] [PubMed] [Google Scholar]
- Baranova NS, Nileback E, Haller FM, Briggs DC, Svedhem S, Day AJ, Richter RP. 2011. The inflammation-associated protein TSG-6 cross-links hyaluronan via hyaluronan-induced TSG-6 oligomers. J Biol Chem. 286:25675–25686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo GM, Avenoso A, Campo S, Ferlazzo AM, Calatroni A. 2004. Administration of hyaluronic acid and chondroitin-4-sulfate limits endogenous antioxidant depletion and reduces cell damage in experimental acute pancreatitis. Pancreas. 28:E45–E53 [DOI] [PubMed] [Google Scholar]
- Carrette O, Nemade RV, Day AJ, Brickner A, Larsen WJ. 2001. TSG-6 is concentrated in the extracellular matrix of mouse cumulus oocyte complexes through hyaluronan and inter-alpha-inhibitor binding. Biol Reprod. 65:301–308 [DOI] [PubMed] [Google Scholar]
- Cirulli V, Halban PA, Rouiller DG. 1993. Tumor necrosis factor-alpha modifies adhesion properties of rat islet B cells. J Clin Invest. 91:1868–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HG, Hope SA, Byers S, Rodgers RJ. 2006. Formation of ovarian follicular fluid May be due to the osmotic potential of large glycosaminoglycans and proteoglycans. Reproduction. 132:119–131 [DOI] [PubMed] [Google Scholar]
- Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KB. 1987. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A. 84:8628–8632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day AJ, de la Motte CA. 2005. Hyaluronan cross-linking: a protective mechanism in inflammation? Trends Immunol. 26:637–643 [DOI] [PubMed] [Google Scholar]
- Donath MY, Schumann DM, Faulenbach M, Ellingsgaard H, Perren A, Ehses JA. 2008. Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care. 31(Suppl 2):S161–S164 [DOI] [PubMed] [Google Scholar]
- Ensinck JW, Baskin DG, Vahl TP, Vogel RE, Laschansky EC, Francis BH, Hoffman RC, Krakover JD, Stamm MR, Low MJ, et al. 2002. Thrittene, homologous with somatostatin-28((1–13)), is a novel peptide in mammalian gut and circulation. Endocrinology. 143:2599–2609 [DOI] [PubMed] [Google Scholar]
- Evanko SP, Angello JC, Wight TN. 1999. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscl Thromb Vasc Biol. 19:1004–1013 [DOI] [PubMed] [Google Scholar]
- Evanko SP, Potter-Perigo S, Johnson PY, Wight TN. 2009. Organization of hyaluronan and versican in the extracellular matrix of human fibroblasts treated with the viral mimetic poly I:C. J Histochem Cytochem. 57:1041–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanko SP, Raines EW, Ross R, Gold LI, Wight TN. 1998. Proteoglycan distribution in lesions of atherosclerosis depends on lesion severity, structural characteristics, and the proximity of platelet-derived growth factor and transforming growth factor-beta. Am J Pathol. 152:533–546 [PMC free article] [PubMed] [Google Scholar]
- Fraser JR, Laurent TC, Laurent UB. 1997. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 242:27–33 [DOI] [PubMed] [Google Scholar]
- Fries H, Elsasser HP, Mahlbacher V, Neumann K, Kern HF. 1994. Localisation of hyaluronate (HA) in primary tumors and nude mouse xenografts of human pancreatic carcinomas using a biotinylated HA-binding protein. Virchows Arch. 424:7–12 [DOI] [PubMed] [Google Scholar]
- Fulop C, Szanto S, Mukhopadhyay D, Bardos T, Kamath RV, Rugg MS, Day AJ, Salustri A, Hascall VC, Glant TT, Mikecz K. 2003. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development. 130:2253–2261 [DOI] [PubMed] [Google Scholar]
- Halban PA. 2004. Cellular sources of new pancreatic beta cells and therapeutic implications for regenerative medicine. Nat Cell Biol. 6:1021–1025 [DOI] [PubMed] [Google Scholar]
- Hammar E, Parnaud G, Bosco D, Perriraz N, Maedler K, Donath M, Rouiller DG, Halban PA. 2004. Extracellular matrix protects pancreatic beta-cells against apoptosis: role of short- and long-term signaling pathways. Diabetes. 53:2034–2041 [DOI] [PubMed] [Google Scholar]
- Homo-Delarche F, Calderari S, Irminger JC, Gangnerau MN, Coulaud J, Rickenbach K, Dolz M, Halban P, Portha B, Serradas P. 2006. Islet inflammation and fibrosis in a spontaneous model of type 2 diabetes, the GK rat. Diabetes. 55:1625–1633 [DOI] [PubMed] [Google Scholar]
- Hughes SJ, Clark A, McShane P, Contractor HH, Gray DW, Johnson PR. 2006. Characterisation of collagen VI within the islet-exocrine interface of the human pancreas: implications for clinical islet isolation? Transplantation. 81:423–426 [DOI] [PubMed] [Google Scholar]
- Hull RL, Shen ZP, Watts MR, Kodama K, Carr DB, Utzschneider KM, Zraika S, Wang F, Kahn SE. 2005. Long-term treatment with rosiglitazone and metformin reduces the extent of, but does not prevent, islet amyloid deposition in mice expressing the gene for human islet amyloid polypeptide. Diabetes. 54:2235–2244 [DOI] [PubMed] [Google Scholar]
- Hull RL, Westermark GT, Westermark P, Kahn SE. 2004. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab. 89:3629–3643 [DOI] [PubMed] [Google Scholar]
- Hull RL, Zraika S, Udayasankar J, Kisilevsky R, Szarek WA, Wight TN, Kahn SE. 2007. Inhibition of glycosaminoglycan synthesis and protein glycosylation with WAS-406 and azaserine result in reduced islet amyloid formation in vitro. Am J Physiol Cell Physiol. 293:C1586–C1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving-Rodgers HF, Ziolkowski AF, Parish CR, Sado Y, Ninomiya Y, Simeonovic CJ, Rodgers RJ. 2008. Molecular composition of the peri-islet basement membrane in NOD mice: a barrier against destructive insulitis. Diabetologia. 51:1680–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Tomita M, Kobayashi T, Uchino H, Maruyama H, Nawa Y. 1996. Expression of inter-alpha-trypsin inhibitor light chain (bikunin) in human pancreas. J Biochem. 120:271–275 [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Noble PW. 2007. Hyaluronan in tissue injury and repair. Annu Rev Cell. Dev Biol. 23:435–461 [DOI] [PubMed] [Google Scholar]
- Jurgens CA, Toukatly MN, Fligner CL, Udayasankar J, Subramanian SL, Zraika S, Aston-Mourney K, Carr DB, Westermark P, Westermark GT, et al. 2011. Beta-cell loss and beta-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am J Pathol. 178:2632–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, Andrikopoulos S, Verchere CB. 1999. Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 48:241–253 [DOI] [PubMed] [Google Scholar]
- Kaido T, Yebra M, Cirulli V, Montgomery AM. 2004. Regulation of human beta-cell adhesion, motility, and insulin secretion by collagen IV and its receptor alpha1beta1. J Biol Chem. 279:53762–53769 [DOI] [PubMed] [Google Scholar]
- Kvezereli M, Michie SA, Yu T, Creusot RJ, Fontaine MJ. 2008. TSG-6 protein expression in the pancreatic islets of NOD mice. J Mol Histol. 39:585–593 [DOI] [PubMed] [Google Scholar]
- Laurent TC, Fraser JR. 1992. Hyaluronan. FASEB J. 6:2397–2404 [PubMed] [Google Scholar]
- Li Y, Nagira T, Tsuchiya T. 2006. The effect of hyaluronic acid on insulin secretion in HIT-T15 cells through the enhancement of gap-junctional intercellular communications. Biomaterials. 27:1437–1443 [DOI] [PubMed] [Google Scholar]
- Lin W, Shuster S, Maibach HI, Stern R. 1997. Patterns of hyaluronan staining are modified by fixation techniques. J Histochem Cytochem. 45:1157–1163 [DOI] [PubMed] [Google Scholar]
- Mack JA, Feldman RJ, Itano N, Kimata K, Lauer M, Hascall VC, Maytin EV. 2011. Enhanced inflammation and accelerated wound closure following tetraphorbol ester application or full-thickness wounding in mice lacking hyaluronan synthases Has1 and Has3. J Invest Dermatol. 132:198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney DJ, Mikecz K, Ali T, Mabilleau G, Benayahu D, Plaas A, Milner CM, Day AJ, Sabokbar A. 2008. TSG-6 regulates bone remodeling through inhibition of osteoblastogenesis and osteoclast activation. J Biol Chem. 283:25952–25962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney DJ, Swales C, Athanasou NA, Bombardieri M, Pitzalis C, Kliskey K, Sharif M, Day AJ, Milner CM, Sabokbar A. 2011. TSG-6 inhibits osteoclast activity via an autocrine mechanism and is functionally synergistic with osteoprotegerin. Arthritis Rheum. 63:1034–1043 [DOI] [PubMed] [Google Scholar]
- Maina V, Cotena A, Doni A, Nebuloni M, Pasqualini F, Milner CM, Day AJ, Mantovani A, Garlanda C. 2009. Coregulation in human leukocytes of the long pentraxin PTX3 and TSG-6. J Leukocyte Biol. 86:123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H, Ozeki T, Takazono I, Tanaka Y. 1989. Composition of glycosaminoglycans in human pancreatic cancer. Biochem Med Metab Biol. 41:193–200 [DOI] [PubMed] [Google Scholar]
- Milner CM, Higman VA, Day AJ. 2006. TSG-6: a pluripotent inflammatory mediator? Biochem Soc Trans. 34:446–450 [DOI] [PubMed] [Google Scholar]
- Nagyova E, Camaioni A, Prochazka R, Salustri A. 2004. Covalent transfer of heavy chains of inter-alpha-trypsin inhibitor family proteins to hyaluronan in in vivo and in vitro expanded porcine oocyte-cumulus complexes. Biol Reprod. 71:1838–1843 [DOI] [PubMed] [Google Scholar]
- Nakamura M, Kitamura H, Konishi S, Nishimura M, Ono J, Ina K, Shimada T, Takaki R. 1995. The endocrine pancreas of spontaneously diabetic db/db mice: microangiopathy as revealed by transmission electron microscopy. Diabetes Res Clin Pract. 30:89–100 [DOI] [PubMed] [Google Scholar]
- Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fassler R, Gu G, Gerber HP, Ferrara N, et al. 2006. The vascular basement membrane: a niche for insulin gene expression and beta cell proliferation. Dev Cell. 10:397–405 [DOI] [PubMed] [Google Scholar]
- Noguchi H, Ueda M, Matsumoto S, Kobayashi N, Hayashi S. 2007. BETA2/NeuroD protein transduction requires cell surface heparan sulfate proteoglycans. Hum Gene Ther. 18:10–17 [DOI] [PubMed] [Google Scholar]
- Olsson R, Carlsson PO. 2006. The pancreatic islet endothelial cell: emerging roles in islet function and disease. Int J Biochem Cell Biol. 38:492–497 [DOI] [PubMed] [Google Scholar]
- Orci L, Malaisse-Lagae F, Ravazzola M, Rouiller D, Renold AE, Perrelet A, Unger R. 1975. A morphological basis for intercellular communication between alpha- and beta-cells in the endocrine pancreas. J Clin Invest. 56:1066–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnaud G, Hammar E, Rouiller DG, Armanet M, Halban PA, Bosco D. 2006. Blockade of beta1 integrin-laminin-5 interaction affects spreading and insulin secretion of rat beta-cells attached on extracellular matrix. Diabetes. 55:1413–1420 [DOI] [PubMed] [Google Scholar]
- Pienimaki JP, Rilla K, Fulop C, Sironen RK, Karvinen S, Pasonen S, Lammi MJ, Tammi R, Hascall VC, Tammi MI. 2001. Epidermal growth factor activates hyaluronan synthase 2 in epidermal keratinocytes and increases pericellular and intracellular hyaluronan. J Biol Chem. 276:20428–20435 [DOI] [PubMed] [Google Scholar]
- Ping Lu Y, Ishiwata T, Asano G. 2002. Lumican expression in alpha cells of islets in pancreas and pancreatic cancer cells. J Pathol. 196:324–330 [DOI] [PubMed] [Google Scholar]
- Potter-Perigo S, Hull RL, Tsoi C, Braun KR, Andrikopoulos S, Teague JC, Verchere CB, Kahn SE, Wight TN. 2003. Proteoglycans synthesized and secreted by pancreatic islet ß-cells bind amylin. Arch Biochem Biophys. 413:182–190 [DOI] [PubMed] [Google Scholar]
- Powers RW, Chen L, Russell PT, Larsen WJ. 1995. Gonadotropin-stimulated regulation of blood-follicle barrier is mediated by nitric oxide. Am J Physiol. 269:E290–E298 [DOI] [PubMed] [Google Scholar]
- Scott EL. 1912. On the influence of intravenous injections of an extract of the pancreas on experimental pancreatic diabetes. Am J Physiol. 29:306–310 [Google Scholar]
- Shih SC, Smith LE. 2005. Quantitative multi-gene transcriptional profiling using real-time PCR with a master template. Exp Mol Pathol. 79:14–22 [DOI] [PubMed] [Google Scholar]
- Smith PH. 1975. Structural modification of Schwann cells in the pancreatic islets of the dog. Am J Anat. 144:513–517 [DOI] [PubMed] [Google Scholar]
- Sorokin L. 2010. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 10:712–723 [DOI] [PubMed] [Google Scholar]
- Sussmann M, Sarbia M, Meyer-Kirchrath J, Nusing RM, Schror K, Fischer JW. 2004. Induction of hyaluronic acid synthase 2 (HAS2) in human vascular smooth muscle cells by vasodilatory prostaglandins. Circ Res. 94:592–600 [DOI] [PubMed] [Google Scholar]
- Szanto S, Bardos T, Gal I, Glant TT, Mikecz K. 2004. Enhanced neutrophil extravasation and rapid progression of proteoglycan-induced arthritis in TSG-6-knockout mice. Arthritis Rheum. 50:3012–3022 [DOI] [PubMed] [Google Scholar]
- Takahashi I, Noguchi N, Nata K, Yamada S, Kaneiwa T, Mizumoto S, Ikeda T, Sugihara K, Asano M, Yoshikawa T, et al. 2009. Important role of heparan sulfate in postnatal islet growth and insulin secretion. Biochem Biophys Res Commun. 383:113–118 [DOI] [PubMed] [Google Scholar]
- Tammi MI, Day AJ, Turley EA. 2002. Hyaluronan and homeostasis: a balancing act. J Biol Chem. 277:4581–4584 [DOI] [PubMed] [Google Scholar]
- Tan KT, McGrouther DA, Day AJ, Milner CM, Bayat A. 2011. Characterization of hyaluronan and TSG-6 in skin scarring: differential distribution in keloid scars, normal scars and unscarred skin. J Eur Acad Derm Venereol. 25:317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theocharis AD, Tsara ME, Papageorgacopoulou N, Karavias DD, Theocharis DA. 2000. Pancreatic carcinoma is characterized by elevated content of hyaluronan and chondroitin sulfate with altered disaccharide composition. Biochim Biophys Acta. 1502:201–206 [DOI] [PubMed] [Google Scholar]
- Tikellis C, Wookey PJ, Candido R, Andrikopoulos S, Thomas MC, Cooper ME. 2004. Improved islet morphology after blockade of the renin-angiotensin system in the ZDF rat. Diabetes. 53:989–997 [DOI] [PubMed] [Google Scholar]
- Toole BP, Wight TN, Tammi MI. 2002. Hyaluronan-cell interactions in cancer and vascular disease. J Biol Chem. 277:4593–4596 [DOI] [PubMed] [Google Scholar]
- Ueda M, Matsumoto S, Hayashi S, Kobayashi N, Noguchi H. 2008. Cell surface heparan sulfate proteoglycans mediate the internalization of PDX-1 protein. Cell Transplant. 17:91–97 [DOI] [PubMed] [Google Scholar]
- Virtanen I, Banerjee M, Palgi J, Korsgren O, Lukinius A, Thornell LE, Kikkawa Y, Sekiguchi K, Hukkanen M, Konttinen YT, Otonkoski T. 2008. Blood vessels of human islets of Langerhans are surrounded by a double basement membrane. Diabetologia. 51:1181–1191 [DOI] [PubMed] [Google Scholar]
- Weiss L, Slavin S, Reich S, Cohen P, Shuster S, Stern R, Kaganovsky E, Okon E, Rubinstein AM, Naor D. 2000. Induction of resistance to diabetes in non-obese diabetic mice by targeting CD44 with a specific monoclonal antibody. Proc Natl Acad Sci U S A. 97:285–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P. 1972. Quantitative studies on amyloid in the islets of Langerhans. Ups J Med Sci. 77:91–94 [DOI] [PubMed] [Google Scholar]
- Westermark P, Wernstedt C, Wilander E, Hayden DW, O’Brien TD, Johnson KH. 1987. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci U S A. 84:3881–3885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Yoneda M, Ohashi Y, Kurono S, Iwata H, Ohnuki Y, Kimata K. 1995. Evidence for the covalent binding of SHAP, heavy chains of inter-alpha-trypsin inhibitor, to hyaluronan. J Biol Chem. 270:26657–26663 [DOI] [PubMed] [Google Scholar]
- Zhuo L, Yoneda M, Zhao M, Yingsung W, Yoshida N, Kitagawa Y, Kawamura K, Suzuki T, Kimata K. 2001. Defect in SHAP-hyaluronan complex causes severe female infertility: a study by inactivation of the bikunin gene in mice. J Biol Chem. 276:7693–7696 [DOI] [PubMed] [Google Scholar]
- Ziolkowski AF, Popp SK, Freeman C, Parish CR, Simeonovic CJ. 2012. Heparan sulfate and heparanase play key roles in mouse beta cell survival and autoimmune diabetes. J Clin Invest. 122:132–141 [DOI] [PMC free article] [PubMed] [Google Scholar]




