Abstract
Bacterial biofilms are ubiquitous in nature, industry, and medicine, and understanding their development and cellular structure is critical in controlling the unwanted consequences of biofilm growth. Here, we report the ultrastructure of a novel bacterial form observed by scanning electron microscopy in the luminal vegetations of catheters from patients with active Staphylococcus aureus bacteremia. This novel structure had the general appearance of a normal staphylococcal cell but up to 10 to 15 times as large. Transmission electron microscopy indicated that these structures appeared as sacs enclosing multiple normal-sized (~0.6 µm) staphylococcal forms. Using in vitro cultivated biofilms, cytochemical studies using fluorescent reagents revealed that these structures were rich in lipids and appeared within 15 min after S. aureus inoculation onto clinically relevant abiotic surfaces. Because they appeared early in biofilm development, these novel bacterial forms may represent an unappreciated mechanism for biofilm surface adherence, and their prominent lipid expression levels could explain the perplexing increased antimicrobial resistance of biofilm-associated bacteria.
Keywords: biofilm, Staphylococcus aureus, ultrastructure, catheter, vegetation, neutral lipid
Biofilms develop as surface-associated microbial communities in liquid environments and are found throughout nature as well as in industrial and medical settings. Biofilms are associated with many chronic infectious diseases such as periodontitis, otitis media, endocarditis, and osteomyelitis; biofilms can be found in the lungs of patients with cystic fibrosis and ventilator-associated pneumonia as well as on implantable medical devices such as joint prostheses, mechanical heart valves, and catheters (Palmer et al. 2007; Hall-Stoodley and Stoodley 2009; Nadell et al. 2009; Cos et al. 2010; Cogan et al. 2011). Unfortunately, biofilm bacteria are often more antibiotic resistant than their planktonic (free-living) counterparts (Monds and O’Toole 2008; Cos et al. 2010; Cogan et al. 2011). Due to the myriad and often deleterious effects of biofilms, much attention has focused on clarifying their development and structure (Palmer et al. 2007; Nadell et al. 2009; Flemming and Wingender 2010; Cogan et al. 2011).
Bacteria within a biofilm are often embedded in a hydrated matrix composed of an extracellular polymeric substance containing proteins, glycoproteins, glycolipids, polysaccharides, and extracellular DNA (Flemming et al. 2007; Stewart and Franklin 2008; Cos et al. 2010; Flemming and Wingender 2010). The composition of this substance varies with the organism and the environmental conditions, giving biofilm bacteria great phenotypic diversity (Flemming et al. 2007; Monds and O’Toole 2008; Stewart and Franklin 2008; Nadell et al. 2009; Flemming and Wingender 2010). Even under similar growth conditions, no two biofilms are exactly alike, making experimental reproducibility problematic. The developmental model is the most popular model of biofilm formation and has several stages: 1) planktonic bacteria reversibly attach to a substratum; 2) bacteria begin to secrete an extracellular matrix, and attachment generally becomes irreversible; 3) the biofilm acquires a three-dimensional shape that may be characterized by pillars of bacteria interspersed with channels that deliver nutrients and facilitate waste disposal; and 4) bacteria (individual cells and cell clumps) disperse from the biofilm and return to the planktonic state or colonize a distal site (Monds and O’Toole 2008; Richards and Melander 2009). Although this model is widely accepted, its relevance remains open to question. Monds and O’Toole (2008) proposed an alternative model based on local ecological adaptation of individual cells to changing microenvironments. The developmental and individualist models are both reasonable, but no model accounts for the novel structures reported in this article.
Because catheter contamination is a primary source of systemic bacterial infection in hospitalized patients (Zhang et al. 2011), we have been studying the ultrastructural morphology of Staphylococcus aureus biofilms on catheter materials, a primary source of systemic infection in hospitalized patients (Walz et al. 2010). Using high-resolution scanning electron microscopy (SEM), we detected unusually large structures that looked like staphylococcal cells or cell clusters but up to 15 times the normal size. After repeated observations in both in vitro and in vivo samples, experiments were carried out to clarify the occurrence and composition of these structures.
Materials and Methods
Bacterial Inoculum
In vitro experiments used two wild-type strains of S. aureus, RN6390 and ATCC 25923. For bacterial inocula, pure cultures were incubated overnight at 37C in tryptic soy broth (TSB), washed, and resuspended to 108 cells/ml using densitometry, with results confirmed by quantitative culture. After inoculation onto silicone elastomer coupons (Invotec International Inc.; Jacksonville, FL) for SEM, or onto positively charged glass slides for fluorescent microscopy, bacteria were cultivated 15 min to 48 hr in a humid chamber at 37C. Incubation media included phosphate-buffered saline (PBS), Hank’s balanced salt solution (HBSS), or 66% TSB with 0.2% glucose (TSB/g). All media and reagents were passed through a 0.45-µm filter to eliminate microscopic debris.
Scanning Electron Microscopy
Silicone coupons were processed for SEM according to the methodology of Erlandsen et al. (2004). Briefly, samples were fixed overnight in a mixture of 2% glutaraldehyde, 2% paraformaldehyde, 4% sucrose, and 0.15% Alcian blue in 0.15 M sodium cacodylate buffer and then washed in cacodylate buffer, postfixed in 1% osmium tetroxide and 1.5% potassium ferricyanide in cacodylate buffer. Samples were then rinsed, and dehydrated through a graded ethanol series, followed by critical point drying with CO2. Samples were coated with 1–2 nm of platinum with an Ion Tech argon ion beam coater (VCR Group, Inc., San Francisco, CA, USA) and viewed with a Hitachi S-4700 field emission scanning electron microscope (Tokyo, Japan) operated at 2.5 kV.
Fluorescent Microscopy
Samples on glass slides were rinsed in HBSS and incubated 5–10 min at room temperature with a mixture of three fluorescent probes (Invitrogen; Grand Island, NY): wheat germ agglutinin conjugated to a red fluorescent dye (Alexa Fluor 594, 10 µg/ml) that binds the S. aureus cell surface carbohydrate N-acetyl-glucosamine (Maira-Litran et al. 2002), a cell membrane-permeant dye that labels DNA blue (Hoechst 33342, 5 µg/ml), and a label for a neutral lipid conjugated to a green fluorophore (HCS LipidTOX Green, 1:500). Samples were mounted in Vectashield HardSet (Vector Labs; Burlingame, CA) and visualized using a Nikon E-800 microscope (Tokyo, Japan) equipped with a spinning disc BD CARV II confocal image adapter (BD Biosciences; Franklin Lakes, NJ). Using a 100× 1.45 NA objective lens, widefield z-stacks were acquired at 0.2-µm intervals with a Cascade 1k EMCCD camera (Photometrics; Tucson, AZ). Maximum intensity projections were derived from deconvolved z-stacks (Huygens Pro; Scientific Volume Imaging, Thousand Oaks, CA) and overlaid onto differential interference contrast images. To confirm the results of the LipidTOX staining (Invitrogen), the neutral lipid was also labeled with Oil Red O (Sigma-Aldrich; St. Louis, MO); here, samples were rinsed with HBSS, fixed in 4% paraformaldehyde, stained with 0.3% Oil Red O in 36% triethyl phosphate, rinsed, and mounted in Vectashield HardSet.
Human Patient Catheters
As part of normal medical care, vascular catheters were removed from 41 patients at the University of Minnesota Medical Center, Fairview, Minneapolis, from July 2009 to October 2010. Forty-one catheters were processed from 32 patients with no documented evidence of systemic infection and from 9 patients with systemic infection defined as isolation of bacteria from the blood culture prior to catheter removal. Catheter segments were placed in fixative within 1 hr after removal and processed for SEM, with selected samples processed for transmission electron microscopy (TEM) (Mauer et al. 1984) and visualized with a JEOL 100CX transmission electron microscope (JEOL, Ltd., Tokyo, Japan). Clinical samples were handled according to the guidelines of the University of Minnesota Institutional Review Board.
Results
Figure 1 presents SEM images of enlarged forms of S. aureus after cultivation on silicone coupons for 15 min to 48 hr. In some instances, these structures appeared as giant bacterial cells, similar in many respects to normal staphylococcal cells (Fig. 1A–G); in other cases, they appeared as chains or clusters of cocci often covered with fibrillar material (Fig. 1H–L). Because bacteria disperse from the biofilm to colonize distal sites, it was not possible to determine how long an enlarged form had been in contact with the surface. Given these data, we conclude that the large cellular-appearing forms were present within 15 min after inoculation onto an abiotic surface. It should be noted that the large forms covered with fibrillar material likely took longer to develop; that is, a number of the fibrillar structures were seen after an 8-hr incubation, although an occasional example was noted after a 2-hr incubation (Fig. 1H). Interestingly, although biofilm structure can often be modulated by providing different nutrients in the growth medium (Flemming and Wingender 2010), the large forms were not nutrition dependent as evidenced by their appearance using media with and without a carbon source, namely, TSB/g, HBSS, and PBS.
Figure 1.

Scanning electron microscopy (SEM) images of Staphylococcus aureus RN6390 (A–G, I, J) and ATCC 25923 (H, K, L) after inoculation onto glass or silicone coupons, showing large bacterial forms, some covered with short, fuzzy fibrillar material (H–L), after incubation for 15 min (A, C, E), 2 hr (H), 8 hr (G, I, J), 16 hr (D), or 48 hr (B, F, K, L) in tryptic soy broth with glucose (B–D, F–K), Hank’s balanced salt solution (A), or phosphate-buffered saline (E). (J, L) Higher magnifications of a portion of the images in I and K, respectively. Scale bar = 3 µm.
Using S. aureus incubated on glass slides for 15 min, 2 hr, 4 hr, and 16 hr, the enlarged forms were characterized using cytochemistry and fluorescent microscopy. Cell density increased rapidly, resulting in densely populated biofilms within 2–4 hr. After 16 hr, biofilms were up to 20 µm in depth and appeared as irregular hills and valleys of cells. The enlarged forms were seen at each time point but did not appear to increase over time and were not numerous (1 in every 50 to 300 high power fields, although an occasional field had 2 or 3 large forms). These samples were labeled with three fluorescent probes: 1) wheat germ agglutinin (conjugated to a red fluorescent dye) that binds an S. aureus cell surface carbohydrate, 2) a cell membrane–permeant Hoechst stain that labels DNA blue, and 3) a label for a neutral lipid conjugated to a green fluorophore. Although the enlarged bacterial forms stained similarly at each time point from 15 min to 16 hr, Figure 2 contains images only at the 15-min time point because bacteria were not densely packed, allowing greater resolution of individual structures. In the more classic appearance, early biofilms appeared as a group of bacteria held together by a matrix, with sparse lipid staining. Although some faint green staining was likely due to cell wall glycolipid interactions, few lipids were associated with the bacterial cluster itself (Fig. 2A). In contrast, the novel structures stained intensely for neutral lipids, with variable staining for extracellular DNA, and scant or no staining for cell surface N-acetyl-glucosamine (Fig. 2B–E). Figure 2D shows a saccular structure with coccal cells at its internal periphery, and this particular structure appeared to be leaking internal contents. Here, cocci immediately outside the structure stained for bacterial DNA and cell surface carbohydrates, while unstained coccal forms were seen at the internal periphery of the “green-staining” saccular structure, suggesting that the lipid material interfered with penetration of these two fluorophores. The samples in Figure 2A–E were stained simultaneously with the three fluorophores. The sample in Figure 2F was stained for cell surface carbohydrates and DNA before the lipid stain. Here, red and blue staining was not evident in areas where bacteria are covered with the green lipid dye, indicating the initial two reagents could not penetrate the lipid material. Because the Hoechst (blue) stain is a small molecule (616 molecular weight [MW]) that readily penetrates cell membranes, this result further indicates that lipid material inhibited penetration of at least some small molecules. Oil Red O (Sigma-Aldrich), another neutral lipid stain, confirmed the specific lipid staining of the large bacterial-like structures (Fig. 3).
Figure 2.
Staphylococcus aureus RN6390 (A–D, F) and ATCC 25923 (E) incubated 15 min in Hank’s balanced salt solution on glass slides. Each row presents a differential interference contrast microscopy image followed by corresponding maximum intensity projections from deconvoluted z-stacks localizing cell surface material (red), DNA (blue), and the neutral lipid (green), followed by an overlay to emphasize the lipid as a hallmark of the large bacteria-like forms. Row A is a typical early biofilm containing cells in a matrix with scant lipids. Rows B–E contain large bacterial forms that stain prominently with the neutral lipid. Row D contains a circular form leaking internal contents; note the inset in the image highlighting the neutral lipid alone where a portion of the adjacent overlay has been placed to more clearly show staining for cell surface binding of wheat germ agglutinin and DNA in the isolated diplococcus on the left of the inset, while two coccal forms remain unstained within the periphery of the circular lipid structure on the left of the inset. Row F contains a sample stained for cell surface carbohydrates and DNA before staining for lipids, with the initial red and blue staining not evident where bacteria are covered with lipids, supporting the concept that the red and blue staining cannot penetrate into cocci covered with the neutral lipid. With the exception of the inset, all images are the same magnification. Scale bar = 5 µm.
Figure 3.

Phase contrast images of Staphylococcus aureus ATCC 25923 (A, B) and RN6390 (C, D) incubated for 15 min in Hank’s balanced salt solution on glass slides and then stained with Oil Red O (for neutral lipids), showing red staining only on the large bacterial structures. (A, C) Diplococcal-appearing forms. (D) A chain of cocci with arrows highlighting what appear to be cross-walls (similar topography to scanning electron microscopy images). These data confirm that the lipid is a unique hallmark of the large bacterial forms. Scale bars = 2 µm.
Although the above in vitro experiments used filtered media and reagents, we cannot eliminate the possibility that low numbers of the novel forms were present in the original inocula and were possibly enriched by their propensity for surface adherence. Assuming the large forms were heavier than normal bacterial cells, an attempt was made to enrich for these forms in samples cultivated in a manner similar to cultivation of the original inocula. Bacteria were incubated overnight in 35 ml of TSB and centrifuged at 100 rpm for 30 sec, and the upper 34.5 ml of spent medium was removed and replaced with fresh medium. This enrichment was repeated daily for 8 days. At days 1, 5, and 8, the 0.5 ml in the bottom of the centrifuged tube was processed for SEM and for fluorescent microscopy as described above. These preparations were observed for at least 8 hr, with no evidence of the large bacterial structures, although we recognize this is not a definitive test because it is difficult to prove that something does not exist.
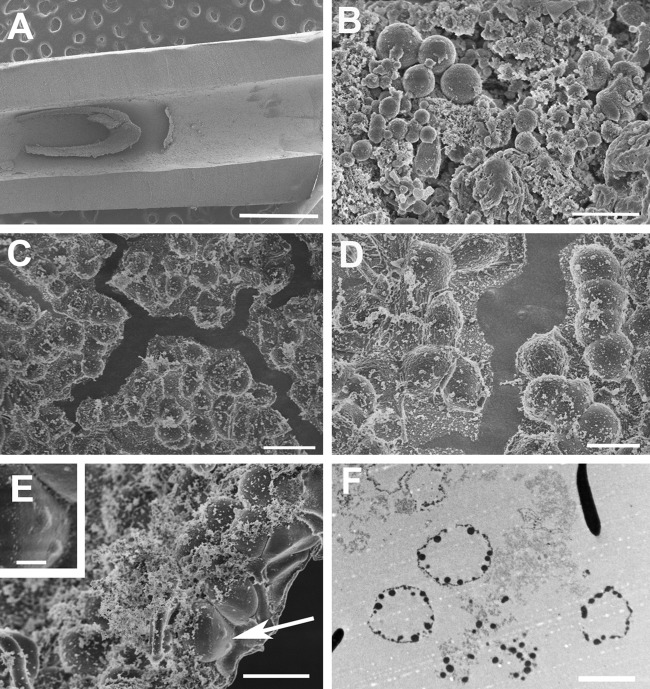
Forty-one catheters were processed from 32 patients with no documented evidence of systemic infection and from 9 patients with systemic infection. There was much phenotypic variability in the SEM ultrastructure, and all catheters had vegetative material containing amorphous material, fibrillar elements, and host cells. Of the nine patients with documented bacteremia (positive blood culture), seven had staphylococcal bacteremia, including five with coagulase-negative staphylococci and two with S. aureus. Normal-sized staphylococcal forms were located in only one catheter associated with coagulase-negative staphylococci, and no bacteria were detected in the remaining four catheters associated with this organism. (As a caveat, due to the inherent sampling problem in high-resolution SEM, failure to find specific structures cannot prove their absence.) Interestingly, enlarged coccal forms were located in the two samples from patients with active S. aureus bacteremia. These novel structures were not seen in any of the other 39 catheters in this study. In the samples from both cases of S. aureus bacteremia, we observed large bacterial-like structures, morphologically similar to those seen in the in vitro experiments, and Figure 4 contains SEM images from one of these catheters. Whereas the enlarged forms were relatively rare in experimental in vitro biofilms, rendering TEM impractical, this in vivo vegetation had discreet areas with a high density of enlarged forms. A high density area was processed for TEM, revealing a collection of sacs with multiple coccal forms at the internal periphery (Fig. 4D), and an SEM photograph of the vegetation edge revealed an enclosed sac with multiple internal structures the size of staphylococcal cells (Fig. 4C). The TEM image obtained from this in vivo vegetation (Fig. 4D) is also consistent with the fluorescent image from an in vitro sample presented in Figure 2D. Thus, the novel bacterial forms noted in in vitro experiments (Fig. 1) were also found in vivo (Fig. 4).
Figure 4.
Ultrastructure of vegetation in a silicone catheter removed (after 2 weeks in vivo) from a patient with active Staphylococcus aureus bacteremia. (A) Low-magnification scanning electron microscopy (SEM) image of catheter vegetation. (B–E) SEM images of vegetation showing large coccal forms, similar to the forms in Figure 1, with B containing coccal forms of varying sizes amid copious amorphous material, and C–E showing areas containing an almost confluent carpet of large coccal forms, with the arrow in E pointing to structures consistent with typically sized S. aureus coccal cells residing under the surface (and seen at higher magnification in the inset). (F) Transmission electron microscopy image of this vegetation showing saccular structures with coccal elements at the internal periphery, consistent with the image in E. Scale bars: A and E (inset) = 1 µm; B = 2 µm; C = 10 µm; D, E, F = 5 µm.
Discussion
Herein, we present evidence of novel bacterial structures in S. aureus biofilms. Because there is currently intense interest in biofilm structure and development, it seems logical to question why these structures have not been reported to date. We suspect our success was due, in large part, to the fact that initial observations were made using high-resolution SEM of single cells or cell clusters. We initially dismissed the enlarged forms as contaminants, likely of fungal origin, even though no sample had detectable contamination after microbial culture on nonselective media. Once we became aware of the large bacterial forms by SEM, it took some effort to localize them under light or fluorescent microscopy, where these structures would likely be dismissed as acellular debris without a prior awareness of the SEM image.
The finding of large bacterial forms early (within 15 min) after inoculation of a planktonic culture (containing no detectable large bacterial forms) onto a surface was surprising but reproducible. This finding suggests a heretofore unappreciated bacterial metabolic capacity that is beyond the scope of this study but may have considerable significance for the ability to prevent unwanted bacterial adherence on abiotic surfaces, for example, implanted medical devices such as catheters. Under SEM, these structures were not uniform in appearance, and some were covered with fibrillar material. Preservation of these structures was likely facilitated by incorporation of a cationic dye into the fixative, which we have previously shown to facilitate preservation of bacterial exopolysaccharides (Erlandsen et al. 2004). In addition, because lipids are presumed to act as a barrier to the penetration of antimicrobial agents (Lambert 2002), the prominent presence of neutral lipids in these novel structures may help explain the increased resistance of biofilms to many antibacterial agents and biocides. There is evidence that the peptidoglycan in the cell wall of Gram-positive bacteria allows passage of molecules in the range of 30,000–57,000 MW, and thus, most antimicrobial agents are not excluded based on size alone (Scherrrer and Gerhardt 1971). However, the neutral lipid covering the large bacterial structures reported herein prevented passage of the small (616 MW) cell wall–permeant Hoechst stain, indicating that this lipid would also inhibit passage of most antimicrobial agents. Therefore, the finding of large lipid-covered bacterial structures early in biofilm development has important implications, not only for bacterial surface adherence but for antimicrobial resistance as well.
The data presented herein suggest a mechanism for development of these novel bacterial forms, whereby S. aureus cells initially form clusters that secrete an extracellular material to encase the cluster. Then, continued secretion of a liquid (or amorphous solid) causes bacteria to stick to the internal periphery of the sac, resulting in a mature form. This scenario is speculative but provides a working hypothesis and is presented in pictorial form in Figure 5. These unique bacterial forms may eventually give us greater insight into biofilm propagation, persistence, and antibiotic resistance. However, the important finding is the existence of lipid-rich, large bacterial structures within in vitro and in vivo S. aureus biofilms. This finding highlights the fact that most studies of bacterial biofilms involve phenomenological observations of cell populations, and more studies at the single-cell level are needed to understand the structure and physiology of bacterial biofilms.
Figure 5.

Proposed development of novel bacterial forms. Scanning electron microscopy images of Staphylococcus aureus RN6390 suspended in Hank’s balanced salt solution and imaged 15 min (A–C) or 2 hr (D) after inoculation onto silicone coupons. (A) Low-magnification images showing tendency of cocci to arrange in clusters, seen at a higher magnification in B. (C, D) Clusters of bacteria become covered with an extracellular matrix, presumably containing the neutral lipid that stains with Oil Red O as well as LipidTOX. (E) Transmission electron microscopy image of catheter vegetation from a patient with active S. aureus bacteremia, showing mature forms that appear as sacs with peripheral distribution of coccal elements; note the comparatively clear appearance of the central portion of the sacs. (F) Diagram of the proposed development of the large bacterial structures where the solid black circle represents the lipid. Scale bar = 2 µm.
Acknowledgments
Parts of this work were carried out in the Characterization Facility, University of Minnesota, which receives partial support from the National Science Foundation through the Materials Research Science and Engineering Center program. Computing resources for deconvolution processing were provided by the University of Minnesota Supercomputing Institute.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by National Institutes of Health grants R01 AI058134 (GMD) and R01 GM095553 (CLW), by funds from the Department of Surgery, University of Minnesota (DJH), and by the University of Minnesota Medical Scientist Training Program grant NIGMS T32-GM008244 (AMTB).
References
- Cogan NG, Gunn JS, Wozniak DJ. 2011. Biofilms and infectious diseases: biology to mathematics and back again. FEMS Microbiol Lett. 322:1–7 [DOI] [PubMed] [Google Scholar]
- Cos P, Tote K, Horemans T, Maes L. 2010. Biofilms: an extra hurdle for effective antimicrobial therapy. Curr Pharm Des. 16:2279–2295 [DOI] [PubMed] [Google Scholar]
- Erlandsen SL, Kristich CJ, Dunny GM, Wells CL. 2004. High-resolution visualization of the microbial glycocalyx with low-voltage scanning electron microscopy: dependence on cationic dyes. J Histochem Cytochem. 52:1427–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming H-C, Neu TR, Wozniak DJ. 2007. The EPS matrix: the house of biofilm cells. J Bacteriol. 198:7945–7947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming H-C, Wingender J. 2010. The biofilm matrix. Nat Rev Microbiol. 8:623–633 [DOI] [PubMed] [Google Scholar]
- Hall-Stoodley L, Stoodley P. 2009. Evolving concepts in biofilm infections. Cell Microbiol. 11:1034–1043 [DOI] [PubMed] [Google Scholar]
- Lambert PA. 2002. Cellular impermeability and uptake of biocides and antibiotics in Gram-positive bacteria and mycobacteria. J Appl Microbiol. 92:46S–54S [PubMed] [Google Scholar]
- Maira-Litran T, Kropec A, Abeygunawardana C, Joyce J, Mark G, 3rd, Goldman DA, Pier GB. 2002. Immunochemical properties of the staphylococcal poly-N-acetylglucosamine surface polysaccharide. Infect Immun. 270:4433–4440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. 1984. Structural-functional relationships in diabetic nephropathy. J Clin Invest. 74:1143–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monds RD, O’Toole GA. 2008. The developmental model of microbial biofilms: ten years of a paradigm up for review. Trends Microbiol. 217:73–87 [DOI] [PubMed] [Google Scholar]
- Nadell CD, Xavier JB, Foster KR. 2009. The sociology of biofilms. FEMS Microbiol Rev. 33:206–224 [DOI] [PubMed] [Google Scholar]
- Palmer J, Flint S, Brooks J. 2007. Bacterial cell attachment, the beginning of a biofilm. J Ind Microbiol Biotechnol. 34:577–578 [DOI] [PubMed] [Google Scholar]
- Richards JJ, Melander C. 2009. Controlling bacterial biofilms. Chembiochem. 10:2287–2294 [DOI] [PubMed] [Google Scholar]
- Scherrer R, Gerhardt P. 1971. Molecular sieving by the Bacillus megaterium cell wall and protoplast. J Bacteriol 107:718–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PS, Franklin MJ. 2008. Physiological heterogeneity in biofilms. Nat Rev Microbiol. 6:199–210 [DOI] [PubMed] [Google Scholar]
- Walz JM, Memtsoudis SG, Heard SO. 2010. Analytic reviews: prevention of central venous catheter blood stream infections. J Intensive Care Med. 25:131–138 [DOI] [PubMed] [Google Scholar]
- Zhang L, Gowardman J, Richard CM. 2011. Impact of microbial attachment on vascular catheter-related infections. Int J Antimicrob Agents. 38:9–15 [DOI] [PubMed] [Google Scholar]