Abstract
Dental pulp is involved in the formation of bone-like tissue in response to external stimuli. However, the origin of osteoblast-like cells constructing this tissue and the mechanism of their induction remain unknown. We therefore evaluated pulp mineralization induced by transplantation of a green fluorescent protein (GFP)–labeled tooth into a GFP-negative hypodermis of host rats. Five days after the transplantation, the upper pulp cavity became necrotic; however, cell-rich hard tissue was observed adjacent to dentin at the root apex. At 10 days, woven bone-like tissue was formed apart from the dentin in the upper pulp. After 20 days, these hard tissues expanded and became histologically similar to bone. GFP immunoreactivity was detected in the hard tissue-forming cells within the root apex as well as in the upper pulp. Furthermore, immunohistochemical observation of α–smooth muscle actin, a marker for undifferentiated cells, showed a positive reaction in cells surrounding this bone-like tissue within the upper pulp but not in those within the root apex. Immunoreactivities of Smad4, Runx2, and Osterix were detected in the hard tissue-forming cells within both areas. These results collectively suggest that the dental pulp contains various types of osteoblast progenitors and that these cells might thus induce bone-like tissue in severely injured pulp.
Keywords: tooth transplantation, pulp mineralization, osteoblast progenitor, α–smooth muscle actin, GFP-transgenic rat
Mineralization in the dental pulp is an important phenomenon as a reparative process to external stimuli. Therefore, the formation of mineralized tissue within the pulp cavity has been widely examined in both clinical and animal studies. These studies ordinarily focused on the formation of reparative dentin and the dentin bridge, although several other reports described the induction of bone-like tissues in the pulp cavity following various stimulations such as tooth transplantation (Hosoya et al. 2003) or replantation (Zhao et al. 2007; Mutoh et al. 2011), traumatic injury (Robertson et al. 1997), and laser irradiation (Tate et al. 2006). These bone-like tissues are quite similar to normal bone regarding their histological appearance and matrix properties; however, the origin of the osteoblast-like cells that appear in the dental pulp is still controversial. Several possibilities regarding this origin can be proposed to explain their involvement in pulp regeneration. One is that bone marrow–derived mesenchymal cells participate in osteoblast-like cell differentiation. Such undifferentiated cells might be supplied via the bloodstream within the injured pulp and then differentiate into osteoblast-like cells there. A second possibility is that cells in the dental pulp itself differentiate into osteoblast-like cells. Previous reports have indicated that the dental pulp of human permanent (Gronthos et al. 2000) and deciduous (Miura et al. 2003) teeth harbors multipotent stem cells. These stem cells are found at the periphery of blood vessels in the central region of the pulp and have multilineage potential (Iohara et al. 2008; Karaoz et al. 2011). In fact, earlier we showed that cells in the subodontoblast cell-rich layer have high mineralization ability and form a bone-like matrix after subcutaneous transplantation (Hosoya et al. 2012). In addition to these possibilities, the periodontal ligament (PDL) is also thought to be a source of osteoblast-like cells. PDL cells have high alkaline phosphatase activity (Giannopoulou and Cimasoni 1996) and produce mineralized nodules (Mukai et al. 1993) and bone matrix proteins (Nohutcu et al. 1997) under osteoinductive culture conditions, and they also migrate extensively after transplantation (Hosoya et al. 2008).
Undifferentiated cells express a number of marker proteins during the process of their differentiation. α–Smooth muscle actin (α-SMA) is known to localize in mesenchymal stem and precursor cells (Kinner et al. 2002; Yamada et al. 2005). This protein has also been found in various tissues during tissue repair and regeneration (van Beurden et al. 2005). In addition, the polycomb protein Bmi-1 is also localized to some stem and precursor cells in various tissues, including bone and tooth (Li et al. 2011; Zhang et al. 2010). On the other hand, the differentiation process of osteoblasts is regulated by cytokines and their associated signaling molecules. After bone morphogenetic proteins bind to their receptors, the R-Smads/Smad4 complex is translocated to the nucleus (Derynck et al. 1998; Shi and Massague 2003). Thereafter, runt-related transcription factor 2 (Runx2) and Osterix (Katagiri and Takahashi 2002) are expressed in osteogenic cells and induce the expression of bone matrix proteins such as osteopontin (Lian et al. 2004).
Previously, we reported that two different types of cell-rich hard tissues are induced in the pulp cavity of rat molars after tooth transplantation into subcutaneous tissue. These hard tissues were shown to be immunonegative for dentin sialoprotein, a protein highly specific to dentin (D’Souza et al. 1992; Bronckers et al. 1993; Hosoya, Nakamura, Akahane, et al. 2006) and reparative dentin (D’Souza et al. 1995), suggesting that their matrix property resembled that of bone (Hosoya et al. 2003). Therefore, in the present study, we subcutaneously transplanted green fluorescent protein (GFP)–labeled rat molars into the hypodermis of normal host rats. Our findings revealed that the formation of two bone-like tissues was initiated at the root apex and root canal orifice within the pulp cavity and that these cells originated from cells in the dental pulp. In addition, to analyze the differentiation processes of these undifferentiated progenitor cells, we examined these cells immunohistochemically by using antibodies specific for α-SMA and Bmi-1 as markers for undifferentiated stem cells, as well as those specific for osteoblastic differentiation markers Smad4, Runx2, Osterix, and osteopontin.
Materials and Methods
Transplantation
All experiments were performed according to guidelines set forth by the Matsumoto Dental University Committee on Intramural Animal Use. Thirty-two Lewis wild-type or GFP-transgenic rats, 4 weeks of age and weighing 100 to 110 g, were used in this experiment. After the animals had been anesthetized by an intraperitoneal injection of pentobarbital (40 mg/kg), the upper first molars of Lewis transgenic rats that ubiquitously expressed enhanced GFP (Hakamata et al. 2001; Inoue et al. 2005) were extracted with a pair of dental tweezers. The GFP-positive teeth were immediately rinsed in phosphate-buffered saline (PBS, pH 7.4) and then transplanted into a preformed subcutaneous pouch in the abdominal region of wild-type recipient rats. At 5, 10, 20, and 30 days after the transplantation, the transplanted teeth were excised along with portions of the surrounding tissue. Ten teeth were collected from five rats at each stage. Conversely, to clarify the cell migration from the host rat, 10 molars of wild-type rats were also transplanted into the hypodermis of GFP-transgenic rats, harvested at 20 days after the transplantation, and examined.
To avoid the influence of cells in the PDL, we incubated 10 teeth from GFP-transgenic rats for 1 hr at 37C prior to the transplantation in α–minimum essential medium (α-MEM; Sigma-Aldrich, St. Louis, MO) containing 2 mg/ml collagenase (Wako Pure Chemical Industries, Ltd.; Osaka, Japan), 0.25% trypsin (Invitrogen Japan K.K.; Tokyo, Japan), 10% fetal bovine serum (FBS; Biological Industries Ltd., Haemek, Israel), and 100 µg/ml kanamycin sulfate. This procedure completely removes PDL tissue from an extracted tooth (Hiraga et al. 2009). The teeth were then transplanted into the hypodermis of wild-type rats and fixed at 20 days after transplantation.
Tissue Preparation
The specimens were fixed with 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4) at 4C for 24 hr and then demineralized with 10% ethylenediamine tetraacetic acid (EDTA, pH 7.4) at 4C for 2 weeks. After having been embedded in paraffin, the samples were sectioned sagittally at a thickness of 4 µm. Some sections were stained with hematoxylin and eosin. As a control, the maxillae of two untreated rats were processed in the same way.
Immunohistochemistry
Serial sections were processed for immunohistochemistry using the following antibodies: mouse monoclonal antibodies against human α-SMA, human Smad4 (Santa Cruz Biotechnology; Santa Cruz, CA), human Bmi-1 (R&D Systems; Minneapolis, MN), and human Runx2 (MBL Co. Ltd.; Tokyo, Japan), as well as and rabbit polyclonal antibodies against mouse Osterix (Abcam; Cambridge, UK), mouse osteopontin (Nakamura et al. 1997), and GFP (Molecular Probes; Eugene, OR). Sections were incubated with these primary antibodies for 12 hr at 4C, with the antibodies against α-SMA, osteopontin, and GFP diluted 1:500 and with those against Bmi-1, Smad4, Runx2, and Osterix diluted 1:100. Sections were reacted with Histofine Simple Stain rat MAX-PO (MULTI; NICHIREI Co., Tokyo, Japan) for 1 hr at room temperature. The immune complexes were visualized by using diaminobenzidine (EnVision kit; DAKO, Carpinteria, CA). The immunostained sections were then counterstained with hematoxylin. In the place of primary antibodies, non-immune mouse or rabbit sera were diluted to the same strength for use as negative controls. The staining controls did not show any specific immunoreactivity.
For detection of cell proliferation, several animals were intraperitoneally injected with bromodeoxyuridine (BrdU; Sigma-Aldrich) at a dosage of 2.5 mg/100 g body weight 2 hr before fixation. After sections had been prepared and pretreated with 2N HCl for 30 min at 37C, followed by 0.1% trypsin in PBS for 15 min at room temperature, they were incubated with 1:1000 diluted monoclonal antibody against BrdU (Becton Dickinson Immunocytometry System; Becton Dickinson, Franklin Lakes, NJ). Immunoreactivity was visualized with the reagents from a Histofine Simple Stain rat MAX-PO and EnVision kit.
Enzyme Histochemistry
Sections were stained for tartrate-resistant acid phosphatase (TRAP), a marker of osteoclasts and osteoclast-like cells, as described previously (Hosoya et al. 2008).
Results
Bone-like Tissue Formation in the Dental Pulp
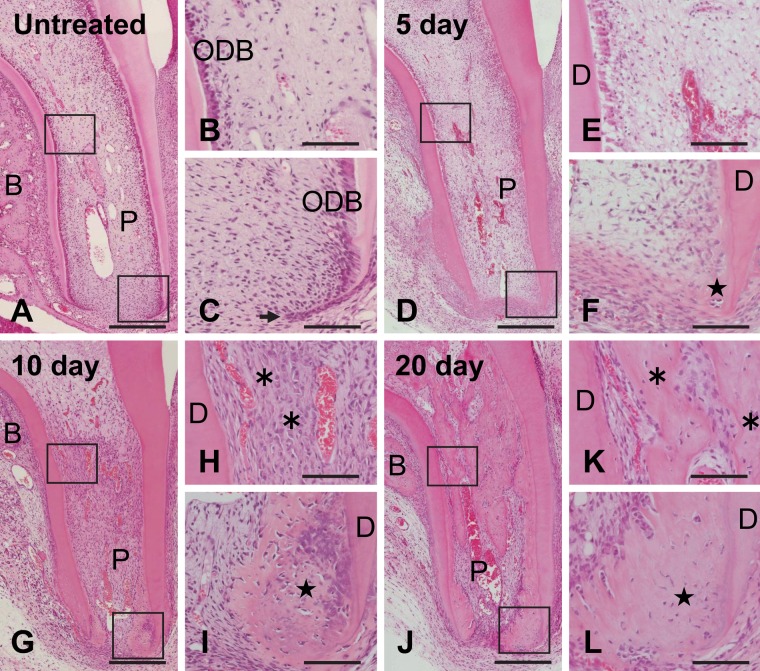
The upper first molar of 4-week-old rats was in the process of root formation, as evidenced by the presence of Hertwig’s epithelial root sheath (HERS) at the root apex (Fig. 1C). The tooth root was widely opened, and columnar odontoblasts were aligned on the predentin at the periphery of the pulp. Dental pulp cells and blood vessels were seen in the inner region of the pulp (Fig. 1A–C).
Figure 1.
Histological observations of untreated (A–C) and subcutaneously transplanted (D–L) teeth. Higher magnifications of the boxed regions in A, D, G, and J are shown in B–C, E–F, H–I, and K–L, respectively. (A–C) The tooth root of this molar from a 4-week-old rat is developing, and the root apex remains widely open. The arrow in C shows Hertwig’s epithelial root sheath. (D, E) At 5 days after transplantation, the upper region of the pulp (P) becomes necrotic, and angioinvasion has occurred up to the root-canal orifice. (F) Eosin-stained matrix is seen adjacent to the apical dentin (D). (G) At 10 days after transplantation, vascularization reaches the coronal pulp tissue. (H) Woven bone-like tissue (asterisks in H and K) is observed at the orifice of the root canal. (I) Matrix (star in F, I, and L) containing numerous cells is formed at the root apex. (J–L) By 20 days, the mineralized area has expanded in the pulp cavity. B, alveolar bone; ODB, odontoblast. Bar: A, D, G, J = 250 µm; B–C, E–F, H–I, K–L = 70 µm.
At 5 days after transplantation, the transplanted tooth was surrounded by fibrous granulation tissue. The predentin was scarcely observed, and no typical odontoblasts were seen on the surface of the dentin matrix. Vascularization from the root apex was evident in the radicular part of the pulp (Fig. 1D). The upper region of the pulp was necrotic, as evidenced by scattered pyknotic nuclei of odontoblasts and dental pulp cells. However, there were no signs of internal resorption (Fig. 1E). At the root apex, eosin-stained extracellular matrix was found on the surface of the dentin facing the pulp cavity (Fig. 1F).
At 10 days after transplantation, blood flow had reached as far as the upper region of the pulp, and this region showed recovered cellular activity. The cell density in the pulp was increased compared with that in the untreated physiological pulp (Fig. 1G). At the root canal orifice, woven bone-like tissue had formed apart from the dentin. Large round cells had aligned on the surface of this matrix (Fig. 1H). At the root apex, matrix formation had expanded inwardly from the dentin surface. This matrix contained numerous cells and did not have any tubular structures (Fig. 1I). In the bifurcation region, alveolar bone had regenerated apart from the outer surface of the tooth root (Fig. 1G; Hosoya et al. 2008).
At 20 days, the amount of newly formed matrix showed a significant increase in the pulp and the bifurcation (Fig. 1J). In the upper region of the pulp, matrix resembling bone containing numerous bone lacuna-like structures, each surrounding a cell, was observed and was connected to the dentin in some areas (Fig. 1K). We consider this matrix to have initiated from the woven bone-like tissue that formed at the root canal orifice, and we refer to this matrix as root bone-like tissue in this article. At the root apex, newly formed matrix containing numerous cells showed histological features similar to those observed at 10 days, with the exception that it was poorly stained with hematoxylin (Fig. 1L). We also term this matrix apical bone-like tissue. Except for these two bone-like tissues, thin newly formed matrix with few cells was seen adjacent to dentin in the lower pulp. After 30 days, the root bone-like tissue formed bone marrow–like tissue, whereas there was no evidence of vascularization in the apical bone-like tissue (Suppl. Fig. S1A–C).
Origin of Osteoblast-like Cells in the Pulp Cavity
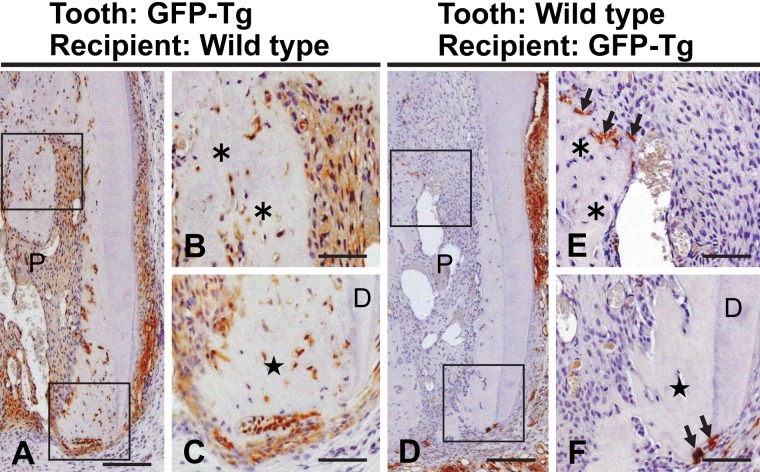
Transplantation of a GFP-positive tooth into the subcutaneous tissue of a GFP-negative rat showed that the cells lining the apical and the root bone-like tissues were immunopositive for GFP. GFP-positive cells were also localized in the dental pulp and the cementum side of the connective tissue (Fig. 2A–C). In the converse experiment using a GFP-negative rat tooth and GFP-transgenic rat hypodermis, some GFP-positive cells indicating a host-cell origin were detected on the surface of the newly formed bone-like tissue (Fig. 2D–F). Because these positive cells seemed to have multiple nuclei, we further analyzed the cell type of these invading cells. A section prepared from the transplantation model using the GFP-negative rat tooth was first stained with TRAP. Subsequently, the same section was immersed in 100% ethanol for 10 min to remove the red color of TRAP staining and then was examined immunohistochemically for GFP. In this section, the TRAP-positive cells corresponded to those cells with GFP immunoreactivity (Suppl. Fig. S2A,B), indicating that the invading cells had been derived from hematopoietic lineage cells and differentiated into osteoclast-like cells in the pulp cavity after transplantation.
Figure 2.
Distribution of green fluorescent protein (GFP)–positive cells in a transplanted tooth from a GFP-positive Tg rat into a wild-type recipient (A–C) and in one from a GFP-negative rat into a GFP-Tg recipient (D–F) at 20 days after transplantation. Higher magnifications of the boxed regions in A and D are shown in B–C and E–F, respectively. (A–C) Most cells in the dental pulp (P) and near the cementum are immunopositive for GFP. (D–F) GFP-positive cells (arrows in E–F) indicating host-cell origin are detected on the surface of the root bone-like tissue (asterisks) and on the resorptive surface of the apical bone-like tissue (stars). D, dentin. Bar: A, D = 170 µm; B–C, E–F = 60 µm.
Transplantation of a GFP-positive tooth into a GFP-negative hypodermis showed that these formative cells were derived from the transplant. Therefore, mesenchymal cells from the recipient did not participate in the bone-like tissue formation within the dental pulp. However, it was impossible to distinguish between the dental pulp and the PDL as the origin of these osteoblast-like cells by using this transplantation model. Then, we next chemically removed the PDL tissue from a tooth, prior to the transplantation, to assess its involvement in the formation of the bone-like tissue. Transplantation of a GFP-positive tooth lacking its PDL into a GFP-negative hypodermis was followed by the formation of both apical and root bone-like tissues at 20 days. In addition, appropriate removal of the PDL was confirmed by the lack of the GFP label near the outer surface of the tooth root (Fig. 3A–C), showing that all newly formed bone-like tissue was of dental pulp origin.
Figure 3.
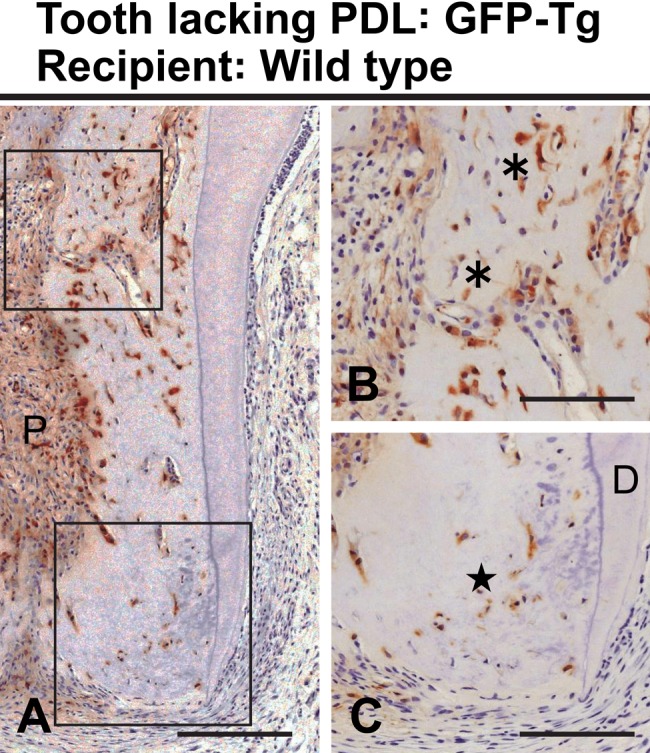
Bone-like tissue formation at 20 days after transplantation of a tooth having its periodontal ligament removed. Higher magnifications of the boxed regions in A are shown in B–C. (A–C) Bone-like tissues are evident at the upper (asterisks in B) and the apical (star in C) root canal. Immunoreactivity of green fluorescent protein (GFP) is localized in the dental pulp (P) of this GFP-transgenic rat tooth. D, dentin. Bar: A = 150 µm; B–C = 100 µm.
Cell Proliferation in the Transplanted Tooth Pulp
The numbers of BrdU-positive cells in the pulp of untreated and transplanted teeth are summarized in Supplementary Table S1.
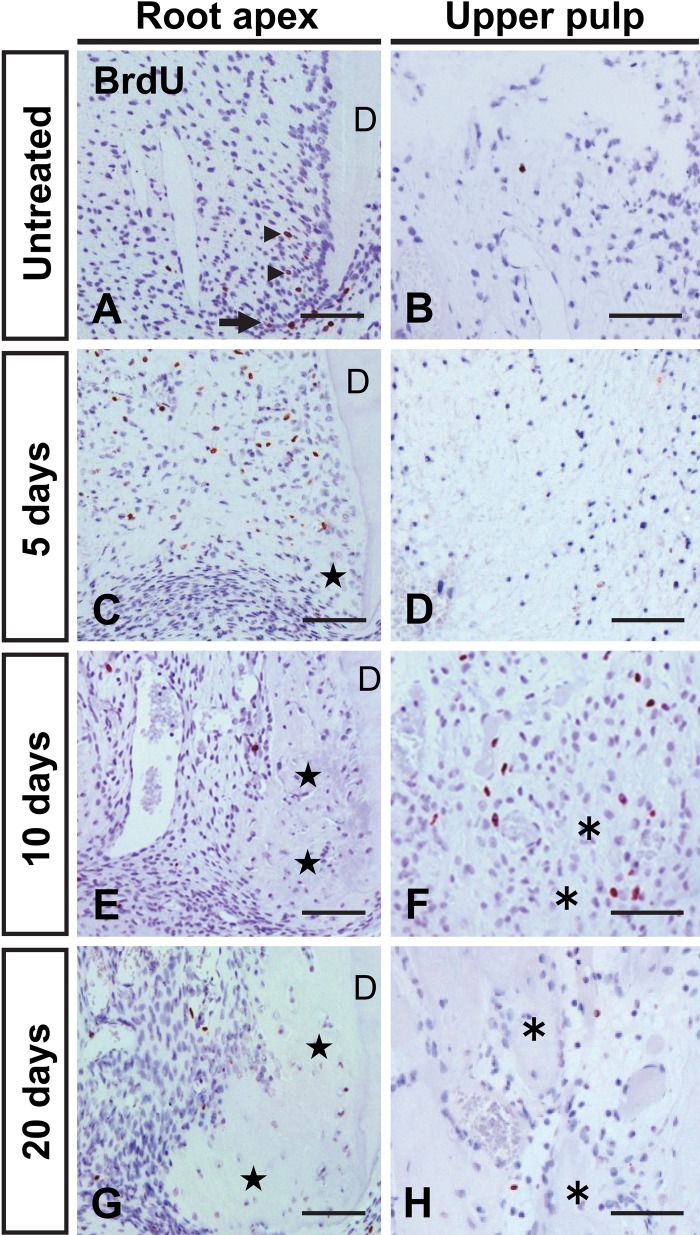
BrdU-positive cells, indicating DNA synthesis, were detected in the vicinity of the apical dentin in the untreated tooth. Some HERS cells as well as dental follicle cells near the HERS were also immunopositive for BrdU (Fig. 4A). On the other hand, these positive cells were scarce in the upper pulp cavity (Fig. 4B). At 5 days after transplantation, numerous BrdU-positive cells appeared at the root apex, but cells in the necrotic upper pulp showed no reactivity (Fig. 4C, D). The area of cell proliferation shifted from the apex to the upper pulp at 10 days (Fig. 4E, F). After 20 days, the number of BrdU-positive cells decreased as the areas of bone-like tissue expanded (Fig. 4G,H).
Figure 4.
Immunohistochemical localization of BrdU in the areas of the root apex (A, C, E, G) and upper root (B, D, F, H) in an untreated (A, B) and a subcutaneously transplanted tooth at 5 (C, D), 10 (E, F), and 20 (G, H) days after transplantation. (A, B) In the untreated tooth, some cells (arrowheads in A) near the apical dentin show BrdU immunoreactivity. The arrow in A indicates Hertwig’s epithelial root sheath. (C–F) Numerous BrdU-positive cells are apparent in the area of the root apex at 5 days after transplantation and are observed near the root bone-like tissue (asterisks) at 10 days. (G, H) Positive cells have decreased in number by 20 days after transplantation. Stars in C, E, and G indicate the apical bone-like tissue. Bar = 50 µm.
Differentiation of Osteoblast-like Cells
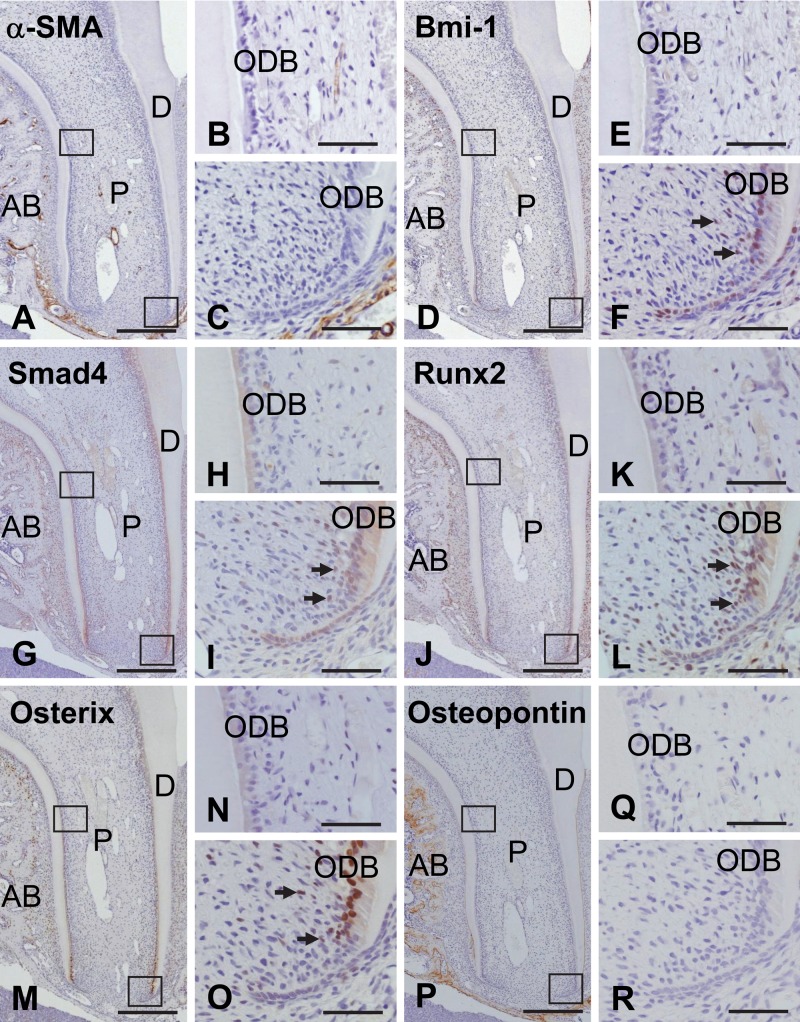
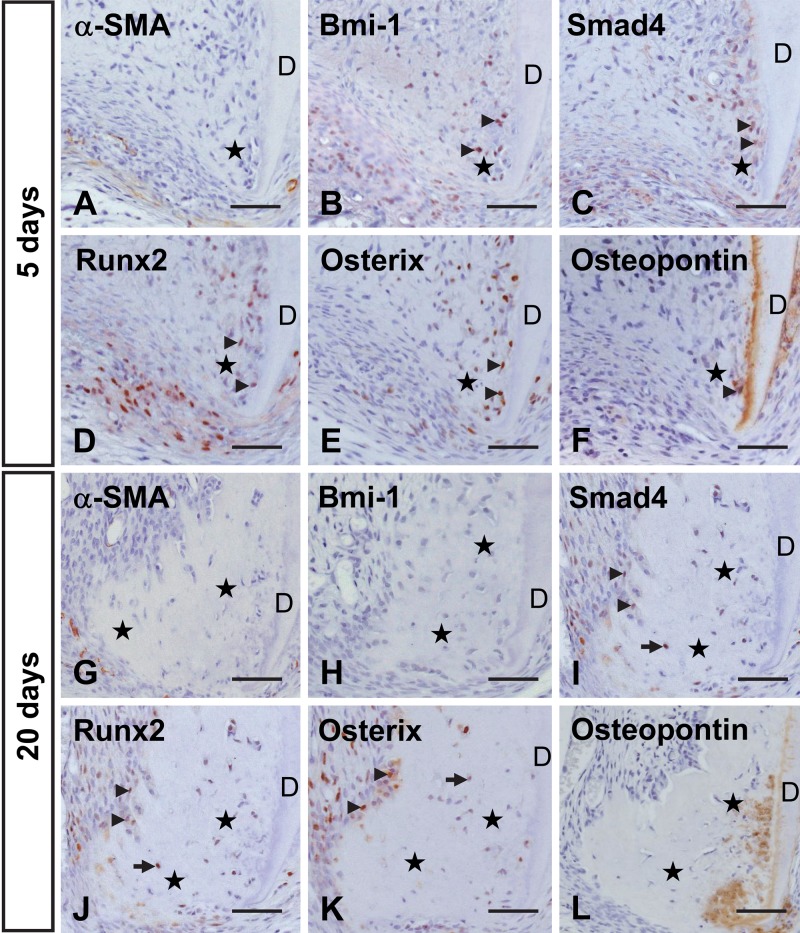
In the dental pulp of the 4-week-old untreated tooth, no immunoreactivity for α-SMA was revealed except for that of the vascular endothelial cells (Fig. 5A–C). Localization of Bmi-1, Smad4, Runx2, and Osterix was confined to the apical odontoblasts within the odontoblast layer. Their immunoreactivities were also detected in some cells in the apical subodontoblast cell-rich layer (Fig. 5D–O). Osteopontin did not show any immunoreactivity in the dental pulp and dentin, whereas intense staining indicating its presence was detected in the alveolar bone and cementum (Fig. 5P–R). In the root apex at 5 days after transplantation, cells positive for Bmi-1 were recognized within and near the apical bone-like tissue, but there was no evidence of α-SMA immunolocalization (Fig. 6A, B). In addition, Smad4, Runx2, and Osterix showed the same localization pattern as Bmi-1 (Fig. 6C–E), and cells and matrix positive for osteopontin were detected (Fig. 6F), suggesting that apical odontoblasts and/or subodontoblast-layer cells might have maintained their cellular activity and undergone mineralization. At 20 days, there was no evidence of α-SMA or Bmi-1 in cells within or lining the apical bone-like tissue (Fig. 6 G,H), whereas these cells were positive for Smad4, Runx2, and Osterix (Fig. 6I, K). Osteopontin was localized within the matrix of the apical bone-like tissue along the dentin (Fig. 6L).
Figure 5.
Immunohistochemical localization of α-smooth muscle actin (α-SMA) (A–C), Bmi-1 (D–F), Smad4 (G–I), Runx2 (J–L), Osterix (M–O), and osteopontin (P–R) in the pulp cavity of a molar from a 4-week-old rat. Higher magnifications of the boxed regions in A, D, G, J, M, and P are shown in B–C, E–F, H–I, K–L, N–O, and Q–R, respectively. (A–C) α-SMA immunoreactivity is not visible in the pulp (P) except near the blood vessels. (D–F) Bmi-1 is localized in odontoblasts (ODB) and in some cells in the subodontoblastic layer (arrows) at the root apex within the pulp. (G–O) Smad4, Runx2, and Osterix demonstrate a similar pattern of immunoreactivity as Bmi-1. (P–R) Osteopontin-positive cells are not seen in the pulp cavity. AB, alveolar bone; D, dentin. Bar: A, D, G, J, M, P = 250 µm; B–C, E–F, H–I, K–L, N–O, Q–R = 70 µm.
Figure 6.
Immunohistochemical localization of α-smooth muscle actin (α-SMA) (A, G), Bmi-1 (B, H), Smad4 (C, I), Runx2 (D, J), Osterix (E, K), and osteopontin (F, L) in the area of root apex at 5 (A–F) and 20 (G–L) days after transplantation. (A) α-SMA-positive cells are not detected at the root apex. (B–E) Some cells (arrowheads) within and around the newly formed apical bone-like tissue (stars) are immunopositive for Bmi-1, Smad4, Runx2, and Osterix. (F) Osteopontin is localized in cells and matrix near the dentin (D). (G, H) Cells around the apical bone-like tissue hardly show immunoreactivity for α-SMA or Bmi-1. (I–K) Cells positive for Smad4, Runx2, and Osterix include lining cells (arrowheads) and cells (arrows) embedded in the apical bone-like tissue (stars). (L) The matrix of the apical bone-like tissue near the dentin demonstrates immunoreactivity for osteopontin. Bar = 50 µm.
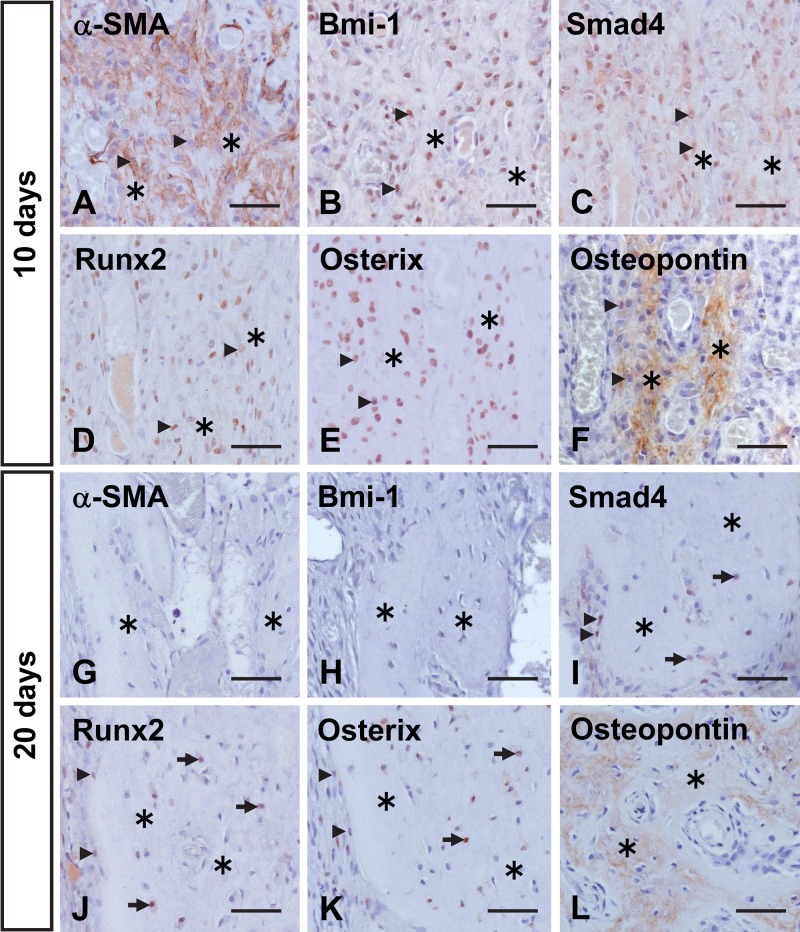
In the upper pulp, cells positive for these differentiation marker proteins were scarce before 5 days posttransplantation (not shown). However, at 10 days after transplantation, numerous α-SMA-positive cells were detected around the root bone-like tissue (Fig. 7A). Bmi-1, Smad4, Runx2, and Osterix were also strongly expressed in cells lining this matrix (Fig. 7B–E). Osteopontin was detected in osteoblast-like cells and their secreted matrix (Fig. 7F). At 20 days after transplantation, immunoreactivity indicating these marker proteins was generally weak. Cells lining the root bone-like tissue and those embedded within the matrix showed no immunoreactivity for α-SMA and Bmi-1 but did show weak expression of Smad4, Runx2, and Osterix (Fig. 7G–K). Cells and matrix of the root bone-like tissue exhibited osteopontin immunoreactivity (Fig. 7L).
Figure 7.
Immunohistochemical localization of α-smooth muscle actin (α-SMA) (A, G), Bmi-1 (B, H), Smad4 (C, I), Runx2 (D, J), Osterix (E, K), and osteopontin (F, L) in the upper-pulp area at 10 (A–F) and 20 (G–L) days after transplantation. (A) Numerous α-SMA-positive cells (arrowheads) are visible in the upper-pulp cavity. (B–E) Intense staining for Bmi-1, Smad4, Runx2, and Osterix is seen in lining cells (arrowheads) and cells (arrows) embedded in the root bone-like tissue (asterisks). (F) Osteopontin is localized to bone-like tissue matrix and its lining cells. (G, H) There is no expression of α-SMA or Bmi-1 in the upper pulp, except for α-SMA immunoreactivity in its vascular endothelial cells. (I–K) Smad4, Runx2, and Osterix demonstrate a similar pattern of immunoreactivity as that at 10 days after transplantation; however, weaker staining of these cells is observed. (L) The matrix of the root bone-like tissue is immunopositive for osteopontin. Bar = 50 µm.
Discussion
Odontoblasts in the dental pulp continuously form dentin under physiological conditions. However, at 5 days after tooth transplantation, apical bone-like tissue containing numerous cells started to form adjacent to the apical dentin. In addition, formation of the root bone-like tissue was noted apart from the dentin at the root canal orifice by 10 days after transplantation. When these two bone-like tissues were compared, the root bone-like tissue seemed to be quite similar to normal bone, because of its bone marrow-forming ability after 30 days. In addition, α-SMA-positive cells were detected around the root bone-like tissue but not around the apical one. MC3T3-E1, an osteoblast-like cell line, synthesizes α-SMA during the undifferentiated stage (Menard et al. 2000). In addition, α-SMA-positive cells are found in the dental follicle near the alveolar bone during tooth development (Hosoya et al. 2006), suggesting that α-SMA might be a useful marker for osteoblast progenitors in their early state. Therefore, in the present study, the formation process of the root bone-like tissue also resembles that of normal bone.
Next, we analyzed the origin of these two different types of osteoblast-like cells that appeared in the pulp cavity. The transplantation of a GFP-positive tooth into a GFP-negative hypodermis showed that the osteoblast-like cells in the pulp cavity had been derived from the transplant. Then, we transplanted into the hypodermis a tooth lacking its PDL to distinguish the cell source between the dental pulp and PDL. As a result, the tooth missing its PDL demonstrated a bone-like tissue formation similar to that of the untreated tooth, indicating that formation of both bone-like tissues had been accomplished by pulp cells, not PDL ones. We propose that most cells in the pulp cavity had suffered serious damage due to the tooth transplantation and that the surviving undifferentiated cells could reestablish pulp vitality. Undifferentiated pulp cells might differentiate into osteoblast-like cells, not into odontoblast-like cells, except the final epithelial signals in severely injured pulp (Nanci 2008).
Odontoblasts at the lowest part of the dental pulp and some cells in the apical subodontoblast layer are immunopositive for osteoblast marker proteins such as Smad4, Runx2, and Osterix (Komori 2011; Long 2012) before transplantation. Because these immunoreactivities were also discerned in cells within and near the apical bone-like tissue at 5 days, it seemed that cells in the apical odontoblast and/or subodontoblast layers maintained their cellular activity and differentiated there into mineralizing cells. We suggest that the dental pulp at the root apex contained cells having characteristics of early state osteoblasts and could immediately differentiate into hard tissue-forming cells after pulp injury. On the other hand, in the upper pulp, the pulp tissue became necrotic because of an interruption of blood flow at 5 days after transplantation. At this time, numerous proliferating cells were detected in the pulp cavity at the root apex, indicating that undifferentiated pulp cells at the root apex had proliferated and replaced the necrotic cells in the upper pulp. These undifferentiated cells might be immunopositive for α-SMA and are thought to have differentiated into osteoblast-like cells at 10 days after transplantation. The 5-day delay of mineralization in the upper pulp, compared with that in the root apex, might be related to the degree of differentiation into hard tissue-forming cells.
In the present study, the xenograft tooth transplantation model showed that most of the newly formed hard tissue in the pulp cavity had bone-like characteristics. However, autologous tooth transplantation into the hypodermis induces a dentin sialoprotein (DSP)-positive dentin-like matrix, in addition to the two different bone-like tissues, in the pulp cavity (Hosoya et al. 2003). This DSP-positive matrix is also formed by tooth cells in the pulp cavity after tooth replantation (Zhao et al. 2007). These dentin-like matrices may have been formed by original odontoblasts that had been left behind or by pulp cells that differentiated into odontoblast-like cells. In general, xenograft transplantation takes a longer time to engraft within the host tissue. Thus, the type of newly formed matrix in the injured pulp might be related to the cellular activity, which would be dependent on the speed of vascularization. We propose that proper pulp regeneration after pulpal injury requires the maintenance of odontoblast linage cells.
It is unknown why pulp cells and odontoblasts calcify if the tooth is removed from the tooth socket. The central region of the pulp hardly becomes calcified under normal physiological conditions; however, tooth transplantation and replantation induce calcification, resulting in an almost stenotic state (Shimizu et al. 2000; Kawasaki et al. 2004). The dental pulp has a rich nerve supply, and most of these nerves are located within the wall of blood vessels in the pulp (Heyeraas et al. 1993). In addition, many neuromodulatory molecules are expressed in this pulpal tissue (Nosrat et al. 1998; Fried et al. 2000). Therefore, neurotrophins can be proposed to affect pulp cell proliferation and differentiation. Among these bioactive molecules, nerve growth factor (Arany et al. 2009), brain-derived neurotrophic factor, and neurotrophins (Mizuno et al. 2008) were shown to participate in pulp mineralization. This neuromodulatory system is interrupted by tooth extraction, and inhibition of mineralization might be relieved after tooth transplantation. Certainly, one can speculate that several key molecules are closely associated with pulp mineralization and that neurological activity may be a candidate for consideration as a regulator of the differentiation of hard tissue-forming cells in the pulp.
In conclusion, the cell source of the two distinct bone-like tissues that formed in the pulp cavity after subcutaneous transplantation was revealed to be the dental pulp itself. These bone-like tissues are thought to have originated from different types of cells. At the root apex, the apical bone-like tissue was formed in the pulp cavity adjacent to the dentin, indicating that this was produced by progenitor cells having already embarked on the road to hard tissue-forming cells. At the root canal orifice, the root bone-like tissue was induced apart from the dentin. This tissue would be formed by undifferentiated cells that have migrated from the apical pulp and differentiated into osteogenic cells. Therefore, these results suggest that various types of dental pulp cells participate in pulp regeneration and that some cells can form hard tissues after tooth transplantation. Our results also support the importance of maintaining pulp vitality and function, as well as suggest that dental pulp cells might be useful in a variety of tissue engineering applications for use as hard tissue-forming cells.
Supplementary Material
Acknowledgments
We thank PhoenixBio Co. for providing the GFP-transgenic rats. We are also grateful to Dr. Michiko Nakatsuka and Dr. Yasutomo Iwai, Department of Oral Anatomy, Osaka Dental University, for their valuable advice, comments, and discussion.
Footnotes
Supplementary material for this article is available on the Journal of Histochemistry & Cytochemistry Web site at http://jhc.sagepub.com/supplemental.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- Arany S, Koyota S, Sugiyama T. 2009. Nerve growth factor promotes differentiation of odontoblast-like cells. J Cell Biochem. 106:539–545 [DOI] [PubMed] [Google Scholar]
- Bronckers AL, D’Souza RN, Butler WT, Lyaruu DM, van Dijk S, Gay S, Woltgens JH. 1993. Dentin sialoprotein: biosynthesis and developmental appearance in rat tooth germs in comparison with amelogenins, osteocalcin and collagen type-I. Cell Tissue Res. 272:237–247 [DOI] [PubMed] [Google Scholar]
- D’Souza RN, Bachman T, Baumgardner KR, Butler WT, Litz M. 1995. Characterization of cellular responses involved in reparative dentinogenesis in rat molars. J Dent Res. 74:702–709 [DOI] [PubMed] [Google Scholar]
- D’Souza RN, Bronckers AL, Happonen RP, Doga DA, Farach-Carson MC, Butler WT. 1992. Developmental expression of a 53 KD dentin sialoprotein in rat tooth organs. J Histochem Cytochem. 40:359–366 [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang Y, Feng XH. 1998. Smads: transcriptional activators of TGF-beta responses. Cell. 95:737–740 [DOI] [PubMed] [Google Scholar]
- Fried K, Nosrat C, Lillesaar C, Hildebrand C. 2000. Molecular signaling and pulpal nerve development. Crit Rev Oral Biol Med. 11:318–332 [DOI] [PubMed] [Google Scholar]
- Giannopoulou C, Cimasoni G. 1996. Functional characteristics of gingival and periodontal ligament fibroblasts. J Dent Res. 75:895–902 [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. 2000. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 97:13625–13630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakamata Y, Tahara K, Uchida H, Sakuma Y, Nakamura M, Kume A, Murakami T, Takahashi M, Takahashi R, Hirabayashi M, et al. 2001. Green fluorescent protein–transgenic rat: a tool for organ transplantation research. Biochem Biophys Res Commun. 286:779–785 [DOI] [PubMed] [Google Scholar]
- Heyeraas KJ, Kvinnsland I, Byers MR, Jacobsen EB. 1993. Nerve fibers immunoreactive to protein gene product 9.5, calcitonin gene-related peptide, substance P, and neuropeptide Y in the dental pulp, periodontal ligament, and gingiva in cats. Acta Odontol Scand. 51:207–221 [DOI] [PubMed] [Google Scholar]
- Hiraga T, Ninomiya T, Hosoya A, Takahashi M, Nakamura H. 2009. Formation of bone-like mineralized matrix by periodontal ligament cells in vivo: a morphological study in rats. J Bone Miner Metab. 27:149–157 [DOI] [PubMed] [Google Scholar]
- Hosoya A, Hiraga T, Ninomiya T, Yukita A, Yoshiba K, Yoshiba N, Takahashi M, Ito S, Nakamura H. 2012. Thy-1-positive cells in the subodontoblastic layer possess high potential to differentiate into hard tissue-forming cells. Histochem Cell Biol. 137:733–742 [DOI] [PubMed] [Google Scholar]
- Hosoya A, Nakamura H, Akahane S, Yoshiba K, Yoshiba N, Ninomiya T, Hoshi K, Sahara N, Kasahara E, Ozawa H. 2006. Immunohistochemical study of osteodentin in the unerupted rat incisor. J Oral Biosci. 48:132–137 [Google Scholar]
- Hosoya A, Nakamura H, Ninomiya T, Yoshiba K, Yoshiba N, Nakaya H, Wakitani S, Yamada H, Kasahara E, Ozawa H. 2006. Immunohistochemical localization of alpha–smooth muscle actin during rat molar tooth development. J Histochem Cytochem. 54:1371–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoya A, Ninomiya T, Hiraga T, Zhao C, Yoshiba K, Yoshiba N, Takahashi M, Okabe T, Wakitani S, Yamada H, et al. 2008. Alveolar bone regeneration of subcutaneously transplanted rat molar. Bone. 42:350–357 [DOI] [PubMed] [Google Scholar]
- Hosoya A, Yoshiba K, Yoshiba N, Hoshi K, Iwaku M, Ozawa H. 2003. An immunohistochemical study on hard tissue formation in a subcutaneously transplanted rat molar. Histochem Cell Biol. 119:27–35 [DOI] [PubMed] [Google Scholar]
- Inoue H, Ohsawa I, Murakami T, Kimura A, Hakamata Y, Sato Y, Kaneko T, Takahashi M, Okada T, Ozawa K, et al. 2005. Development of new inbred transgenic strains of rats with LacZ or GFP. Biochem Biophys Res Commun. 329:288–295 [DOI] [PubMed] [Google Scholar]
- Iohara K, Zheng L, Wake H, Ito M, Nabekura J, Wakita H, Nakamura H, Into T, Matsushita K, Nakashima M. 2008. A novel stem cell source for vasculogenesis in ischemia: subfraction of side population cells from dental pulp. Stem Cells. 26:2408–2418 [DOI] [PubMed] [Google Scholar]
- Karaoz E, Demircan PC, Saglam O, Aksoy A, Kaymaz F, Duruksu G. 2011. Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow–derived mesenchymal stem cells. Histochem Cell Biol. 136:455–473 [DOI] [PubMed] [Google Scholar]
- Katagiri T, Takahashi N. 2002. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 8:147–159 [DOI] [PubMed] [Google Scholar]
- Kawasaki N, Hamamoto Y, Nakajima T, Irie K, Ozawa H. 2004. Periodontal regeneration of transplanted rat molars after cryopreservation. Arch Oral Biol. 49:59–69 [DOI] [PubMed] [Google Scholar]
- Kinner B, Zaleskas JM, Spector M. 2002. Regulation of smooth muscle actin expression and contraction in adult human mesenchymal stem cells. Exp Cell Res. 278:72–83 [DOI] [PubMed] [Google Scholar]
- Komori T 2011. Signaling networks in RUNX2-dependent bone development. J Cell Biochem. 112:750–755 [DOI] [PubMed] [Google Scholar]
- Li L, Kwon HJ, Harada H, Ohshima H, Cho SW, Jung HS. 2011. Expression patterns of ABCG2, Bmi-1, Oct-3/4, and Yap in the developing mouse incisor. Gene Expr Patterns. 11:163–170 [DOI] [PubMed] [Google Scholar]
- Lian JB, Javed A, Zaidi SK, Lengner C, Montecino M, van Wijnen AJ, Stein JL, Stein GS. 2004. Regulatory controls for osteoblast growth and differentiation: role of Runx/Cbfa/AML factors. Crit Rev Eukar Gene. 14:1–41 [PubMed] [Google Scholar]
- Long F. 2012. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 13:27–38 [DOI] [PubMed] [Google Scholar]
- Menard C, Mitchell S, Spector M. 2000. Contractile behavior of smooth muscle actin-containing osteoblasts in collagen-GAG matrices in vitro: implant-related cell contraction. Biomaterials. 21:1867–1877 [DOI] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. 2003. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 100:5807–5812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno N, Shiba H, Inui T, Takeda K, Kajiya M, Hasegawa N, Kawaguchi H, Kurihara H. 2008. Effect of neurotrophin-4/5 on bone/cementum-related protein expressions and DNA synthesis in cultures of human periodontal ligament cells. J Periodontol. 79:2182–2189 [DOI] [PubMed] [Google Scholar]
- Mukai M, Yoshimine Y, Akamine A, Maeda K. 1993. Bone-like nodules formed in vitro by rat periodontal ligament cells. Cell Tissue Res. 271:453–460 [DOI] [PubMed] [Google Scholar]
- Mutoh N, Nakatomi M, Ida-Yonemochi H, Nakagawa E, Tani-Ishii N, Ohshima H. 2011. Responses of BrdU label-retaining dental pulp cells to allogenic tooth transplantation into mouse maxilla. Histochem Cell Biol. 136:649–661 [DOI] [PubMed] [Google Scholar]
- Nakamura H, Yamada M, Fukae M, Yanagisawa T, Ozawa H. 1997. The localization of CD44 and moesin in osteoclasts after calcitonin administration in mouse tibiae. J Bone Miner Metab. 15:184–192 [Google Scholar]
- Nanci A. 2008. Ten Cate’s oral histology: development, structure, and function. St. Louis, MO: Mosby: p. 379–395 [Google Scholar]
- Nohutcu RM, McCauley LK, Koh AJ, Somerman MJ. 1997. Expression of extracellular matrix proteins in human periodontal ligament cells during mineralization in vitro. J Periodontol. 68:320–327 [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Fried K, Ebendal T, Olson L. 1998. NGF, BDNF, NT3, NT4 and GDNF in tooth development. Eur J Oral Sci. 106(Suppl 1):94–99 [DOI] [PubMed] [Google Scholar]
- Robertson A, Lundgren T, Andreasen JO, Dietz W, Hoyer I, Noren JG. 1997. Pulp calcifications in traumatized primary incisors: a morphological and inductive analysis study. Eur J Oral Sci. 105:196–206 [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. 2003. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 113:685–700 [DOI] [PubMed] [Google Scholar]
- Shimizu A, Nakakura-Ohshima K, Noda T, Maeda T, Ohshima H. 2000. Responses of immunocompetent cells in the dental pulp to replantation during the regeneration process in rat molars. Cell Tissue Res. 302:221–233 [DOI] [PubMed] [Google Scholar]
- Tate Y, Yoshiba K, Yoshiba N, Iwaku M, Okiji T, Ohshima H. 2006. Odontoblast responses to GaAlAs laser irradiation in rat molars: an experimental study using heat-shock protein-25 immunohistochemistry. Eur J Oral Sci. 114:50–57 [DOI] [PubMed] [Google Scholar]
- van Beurden HE, Von den Hoff JW, Torensma R, Maltha JC, Kuijpers-Jagtman AM. 2005. Myofibroblasts in palatal wound healing: prospects for the reduction of wound contraction after cleft palate repair. J Dent Res. 84:871–880 [DOI] [PubMed] [Google Scholar]
- Yamada M, Kurihara H, Kinoshita K, Sakai T. 2005. Temporal expression of alpha–smooth muscle actin and drebrin in septal interstitial cells during alveolar maturation. J Histochem Cytochem. 53:735–744 [DOI] [PubMed] [Google Scholar]
- Zhang HW, Ding J, Jin JL, Guo J, Liu JN, Karaplis A, Goltzman D, Miao D. 2010. Defects in mesenchymal stem cell self-renewal and cell fate determination lead to an osteopenic phenotype in Bmi-1 null mice. J Bone Miner Res. 25:640–652 [DOI] [PubMed] [Google Scholar]
- Zhao C, Hosoya A, Kurita H, Hu T, Hiraga T, Ninomiya T, Yoshiba K, Yoshiba N, Takahashi M, Kurashina K, et al. 2007. Immunohistochemical study of hard tissue formation in the rat pulp cavity after tooth replantation. Arch Oral Biol. 52:945–953 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.