Abstract
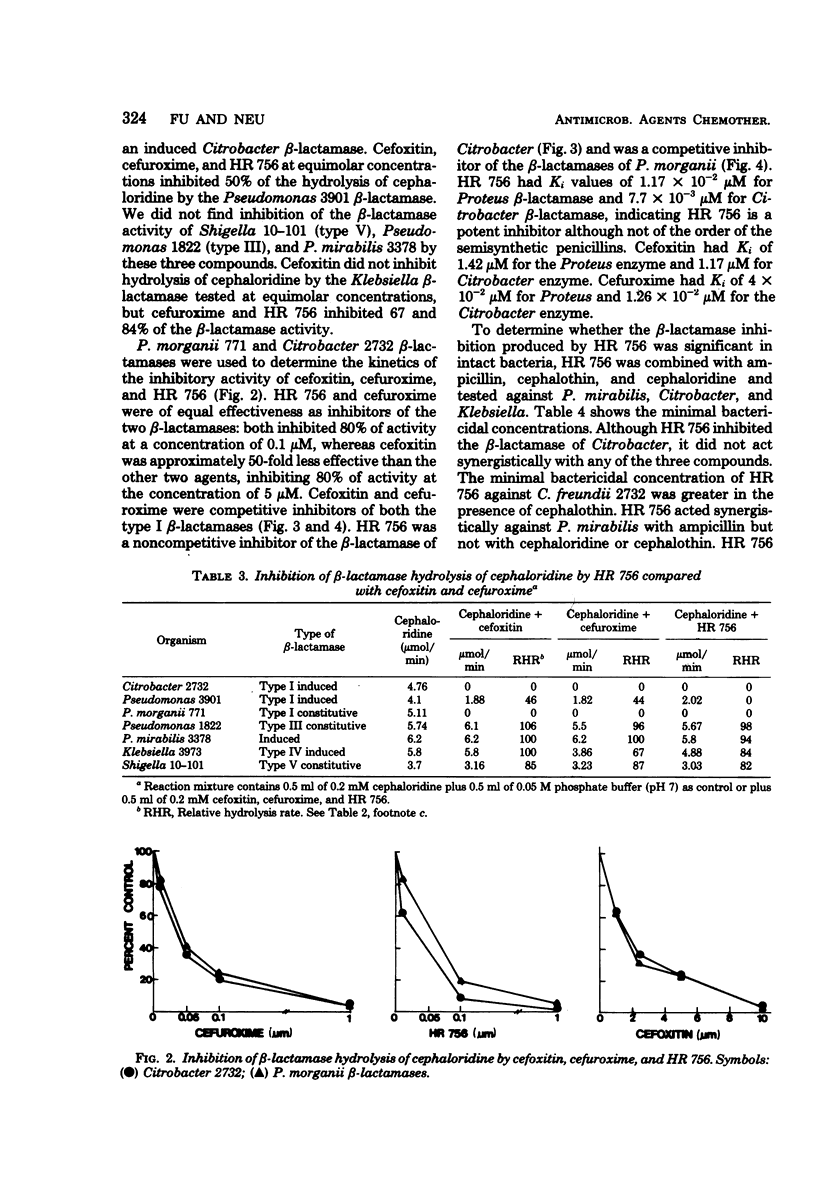
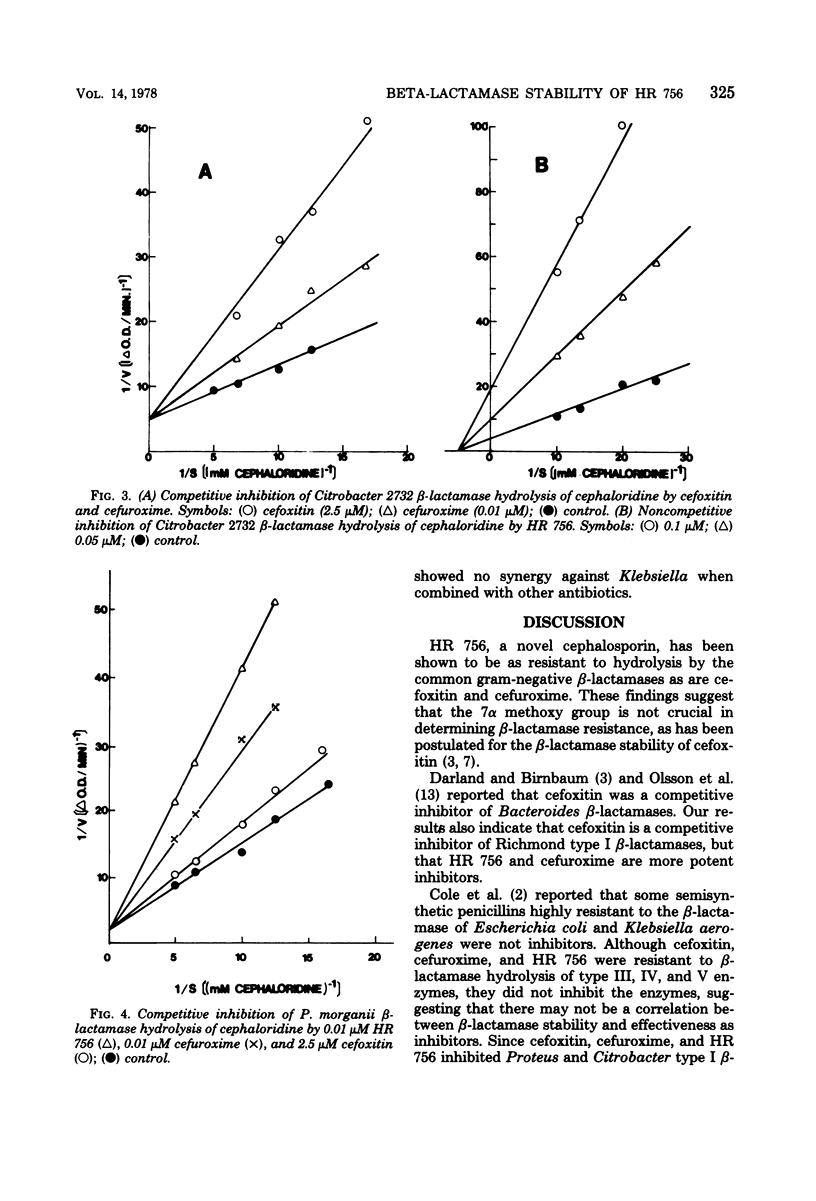
The stability to β-lactamase hydrolysis of HR 756, a new cephalosporin antibiotic, was compared to the β-lactamase stability of cefoxitin and cefuroxime. HR 756, cefoxitin, and cefuroxime were not hydrolyzed by Richmond type I, III, IV, and V β-lactamases. Antibacterial activity of HR 756 correlated well with resistance to β-lactamase hydrolysis except against Pseudomonas aeruginosa. HR 756, cefoxitin, and cefuroxime inhibited type I β-lactamases, but not type III, IV, or V enzymes. HR 756 was the most active inhibitor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. G., Butterworth D., Cole M., Hanscomb G., Hood J. D., Reading C., Rolinson G. N. Naturally-occurring beta-lactamase inhibitors with antibacterial activity. J Antibiot (Tokyo) 1976 Jun;29(6):668–669. doi: 10.7164/antibiotics.29.668. [DOI] [PubMed] [Google Scholar]
- Cole M., Elson S., Fullbrook P. D. Inhibition of the -lactamases of Escherichia coli and Klebsiella aerogenes by semi-synthetic penicillins. Biochem J. 1972 Mar;127(1):295–308. doi: 10.1042/bj1270295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darland G., Birnbaum J. Cefoxitin resistance to beta-lactamase: a major factor for susceptibility of bacteroides fragilis to the antibiotic. Antimicrob Agents Chemother. 1977 Apr;11(4):725–734. doi: 10.1128/aac.11.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu K. P., Neu H. C. In vitro synergistic effect of netilmicin, a new aminoglycoside antibiotic. Antimicrob Agents Chemother. 1976 Sep;10(3):511–518. doi: 10.1128/aac.10.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood D., O'Grady F. Potent combinations of beta-lactam antibiotics using the beta-lactamase inhibition principle. Chemotherapy. 1975;21(6):330–341. doi: 10.1159/000221878. [DOI] [PubMed] [Google Scholar]
- Heymès R., Lutz A., Schrinner E. Experimental evaluation of HR756, a new cephalosporin derivative: pre-clinical study. Infection. 1977;5(4):259–260. doi: 10.1007/BF01640793. [DOI] [PubMed] [Google Scholar]
- Mahoney D. F., Koppel G. A., Turner J. R. Substrate inhibition of beta-lactamases, a method for predicting enzymatic stability of cephalosporins. Antimicrob Agents Chemother. 1976 Sep;10(3):470–475. doi: 10.1128/aac.10.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. Cefoxitin, a semisynthetic cephamycin antibiotic: antibacterial spectrum and resistance to hydrolysis by gram-negative beta-lactamases. Antimicrob Agents Chemother. 1974 Aug;6(2):170–176. doi: 10.1128/aac.6.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Sykes R. B., Ryan D. M., Foord R. D., Muggleton P. W. Cefuroxime - a new cephalosporin antibiotic. J Antibiot (Tokyo) 1976 Jan;29(1):29–37. doi: 10.7164/antibiotics.29.29. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C., Morris A. Inhibition of beta-lactamases by beta-lactam antibiotics. Antimicrob Agents Chemother. 1972 Dec;2(6):442–448. doi: 10.1128/aac.2.6.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson B., Nord C. E., Wadström T. Formation of beta-lactamase in Bacteroides fragilis: cell-bound and extracellular activity. Antimicrob Agents Chemother. 1976 May;9(5):727–735. doi: 10.1128/aac.9.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading C., Cole M. Clavulanic acid: a beta-lactamase-inhiting beta-lactam from Streptomyces clavuligerus. Antimicrob Agents Chemother. 1977 May;11(5):852–857. doi: 10.1128/aac.11.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]



