Abstract
STUDY DESIGN
Descriptive cadaveric laboratory study.
OBJECTIVE
To identify the fiber type composition of the rotator cuff and teres major muscles in human subjects.
BACKGROUND
The rotator cuff is commonly injured in athletics and is a major focus of sports medicine. Although the anatomy and architecture of each muscle has been described in great detail, these muscles have never been fiber typed using immunohistochemistry or gel electrophoresis. Fiber typing is important in modeling function, exercise training, and rehabilitation.
METHODS AND MEASURES
We harvested tissue samples for all 4 rotator cuff muscles, as well as the teres major muscle from cadavers. Tissues were frozen in liquid nitrogen and sectioned. Cryosections were labeled with commercially available antibodies against fast and slow isoforms of myosin heavy chain (MHC). We also harvested fresh (unembalmed) tissue from deceased subjects and labeled tissue sections with antibodies against fast or slow MHC and wheat germ agglutinin. Gel electrophoresis followed by silver staining was also used to identify and quantify MHC isoforms in fresh tissue samples.
RESULTS
All of the muscles were of mixed fiber type composition. As a whole, 44% of rotator cuff fibers labeled positively for slow MHC, with slow MHC content of 54% in supraspinatus, 41% in infraspinatus, 49% in teres minor, 38% in subscapularis, and 40% in teres major. Mixed MHC isoform distribution was confirmed by SDS-PAGE, which also indicated that the IIa and IIx isoforms were roughly equally present across the muscles.
CONCLUSIONS
Human rotator cuff muscles, at least in older subjects, have a mixed fiber type. Because we only examined older subjects, we must limit our interpretation to this population.
Keywords: immunohistochemistry, myosin heavy chain, shoulder, supraspinatus
The human rotator cuff consists of 4 muscles (supraspinatus, infraspinatus, subscapularis, and teres minor) that fuse to form tendons enclosing the humeral head. In addition to contributing to humeral movement, the rotator cuff functions to provide dynamic stability to the glenohumeral joint. Rotator cuff pathology is a frequent contributor to acute and chronic shoulder pain.44 While there is no consensus on the optimal management of rotator cuff pathology, exercise aimed at restoring muscular function is a common intervention, with demonstrated benefits in patients with symptomatic shoulders (for recent review, see Ainsworth and Lewis, 2007). Many of the factors that contribute to the muscular function, including the size of the muscle (cross-sectional area), the attachment of each muscle relative to the axis of movement (moment arm), and the arrangement of the fibers within a muscle (muscle architecture), have been described in great detail for the human rotator cuff,47 but fiber type has not.
Using physiologic methods, skeletal muscles and even individual motor units can be classified as type I (slow-twitch) or type II (fast-twitch).3 Type I fibers have slower maximum shortening velocities and are more resistant to fatigue when compared to type II fibers. Maximum shortening velocity of a single fiber is proportional to the myosin adenosine-triphosphatase (ATPase) activity (the rate at which myosin ATPase can hydrolyze ATP).2 Therefore, fibers can be identified based on histological staining for myosin ATPase.8 Most human muscle tissue samples are limited to those commonly biopsied due to accessibility (eg, vastus lateralis, gastrocnemius), so cadaveric muscle offers the obvious advantage of studying any muscle. Using cadaveric samples and ATPase staining, only 1 study to date has systematically examined the fiber type composition of human rotator cuff muscles.39 Using such methods, type II fibers can be divided into subtypes (eg, type IIa, IIx) on the basis of differences in staining. ATPase staining is a useful technique in healthy skeletal muscle; but the classification of each muscle fiber is based on the sensitivity of ATPase to pH and, therefore, ATPase staining may not be an accurate reflection of ATPase activity rates.35 ATPase staining might also be less accurate in analysis of cadaveric muscles due to postmortem changes that affect the pH-sensitive nature of ATPase activity.18 Talmadge and Roy41 developed a method of separating the predominant isoforms myosin heavy chain (MHC) using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). While this method allows for the determination of relative percentages of MHC isoforms, it does not provide any morphological information, nor does it work with embalmed tissue. More recently, immunohistochemistry has been used to label muscles fibers with antibodies for specific myosin isoforms (eg, MHCI and MHCII) in cadaveric muscle.24
Muscle architecture (muscle fiber arrangement and length within a muscle, cross-sectional area, moment arm of the muscle, etc) is by far the most important predictor of force generation.22 However, the fiber type composition of a muscle can affect a muscle’s speed of contraction,11 power,42 fatigability,11 and metabolism,11,29 and is associated with muscle stiffness,28 rate of atrophy,12,43 and even susceptibility to injury.23,46 Furthermore, differences in fiber type distribution are associated with differences in performance of a number of functional tasks. For example, slow fiber type in the lower extremities is significantly associated with exercise economy and functional performance during walking.4,16 Muscle fiber type affects muscle fiber conduction velocity,9,33 a parameter that can have significant influence on surface electromyography-based (EMG) estimates of neural strategies during movement and exercise, including motor unit recruitment and derecruitment.10 Thus, clinicians and researchers working with the rotator cuff would benefit from knowing the fiber type of the muscles they are rehabilitating or studying. Our aim was to identify the fiber type composition of human rotator cuff muscles, and the closely positioned teres major muscle, using 2 methods not yet applied to these muscles: immunohistochemistry and SDS-PAGE.
METHODS
Tissue Preparation
Fixed tissue was harvested from the proximal and distal aspects of the supraspinatus, infraspinatus, teres minor, subscapularis and teres major muscles of 6 cadavers (3 male, 3 female; mean ± SD age, 65 ± 12 years) that were embalmed using standard procedures of the State Anatomy Board of Maryland. Briefly, the cadavers were perfused via the brachial artery (in) and femoral artery (out) under pressure (34 kPa), with a mixture of the following chemical proportions: methanol (33%), phenol (27%), glycerin (34%), and formaldehyde (6%). Unfixed (unembalmed) tissue was harvested from proximal and distal aspects of muscles of 3 cadavers (2 male, 1 female; ages 72, 87, and 60 years) within 24 hours of death. We selected subjects that did not have long-term diseases or known myopathies. All harvested tissue was frozen liquid nitrogen-cooled isopentane and stored at −80°C until needed.
Immunofluorescent Labeling
Immunofluorescent labeling was performed on all 9 cadavers as previously described25 to assess fiber type composition. Sections of frozen muscles (10 µm thick) were cut on a cryotome (2800 Frigocut; Reichert-Jung, Arnsberg, Germany) and collected onto glass slides (Superfrost Plus; VWR, West Chester, PA). Sections were washed for 10 minutes in 100 mmol glycine phosphate-buffered saline (glycine PBS), blocked for 1 hour in 1% bovine serum albumin PBS (BSA-PBS), then incubated for 2 hours with primary antibodies diluted to 2 mg/ml in BSA-PBS monoclonal antibody to fast (M8421) or slow (M4276) myosin (Sigma, St Louis, MO). The tissue sections were then washed 3 times with 1% BSA-PBS for 10 minutes before incubation, with species-specific secondary antibodies coupled to Alexa dye 568 (Invitrogen, Carlsbad, CA) (dilution of 1:100). Unfixed tissue samples were double-labeled with primary and secondary antibodies to myosin as described, as well as fluorescein succinylated wheat germ agglutinin (M0208; Vector Laboratories, Burlingame, CA). All samples were mounted in VECTASHIELD (Vector Laboratories) and covered with glass cover slips (No 1, VWR), before examination under epifluorescent optics (Zeiss Axioskop 50; Carl Zeiss, Poughkeepsie, NY). Sections from both proximal and distal portions of the muscles were viewed at 20× and random pictures were taken from different fields. Each optical field contained an average ± SD of 61 ± 4.9 fibers, and at least 20 fields were counted per muscle (mean ± SD, 16.5 ± 1.5 fields from proximal portions and 15.5 ± 1.5 fields from distal portions). The number of positively labeled fibers for slow myosin were counted per field and presented as the mean ± SD.
SDS-PAGE and Silver Staining
Because our immunofluorescent labeling could discriminate only fast and slow myosin, it was possible that all of the fast-labeled fibers for a given muscle could have been of a single type II isoform (ie, type IIa or type IIx). To investigate this possibility, we performed SDS-PAGE, which allows identification of distinct subtypes of type II isoforms in humans,35 on the subjects from whom unfixed tissue was available.
Portions of the unfixed muscles were processed as described by Talmadge and Roy,41 with modifications for human samples.1 Muscle tissue was homogenized in ice-cold buffer (pH 6.8) containing sucrose (250 mmol), KCl (100 mmol), EDTA (10 mmol), and Tris (20 mmol). The resulting homogenate was centrifuged at 10 000 × g for 10 minutes at 4°C. The supernatants were discarded and the pellets were rehomogenized in a refrigerated wash buffer containing KCl (175 mmol), EDTA (2.0 mmol), Tris (20 mmol), plus 0.5% (Triton X-100). Centrifugation was repeated and the final pellet was suspended in a buffer (pH 7) of KCl (150 mmol) and Tris (20 mmol). Total protein of the final suspension was determined using the Bradford Assay (Bio-Rad, Hercules, CA). Samples were diluted 1:1 with glycerol and stored at −20°C until used for SDS-PAGE.
Minigels were prepared according to Talmadge and Roy,41 with stacking and separating gels containing 4% and 7% acrylamide:Bis, respectively, and both stacking and separating gels containing 30% glycerol. Stored samples were thawed at 4°C and diluted in 2× sample buffer (Bio-Rad, Hercules, CA). Gel lanes were loaded with 0.5 µg of total protein, and samples were run at 125 V for 22 to 28 hours at 4°C. The gels were subsequently stained using commercially available kits (Silver Stain Plus, Bio-Rad, Hercules, CA) per the manufacturer’s instructions. Stained gels were fixed, scanned, and analyzed densitometrically using Scion Image (Scion Corporation, Frederick MD) to determine the relative content of the 3 human myosin heavy chain isoforms.1
Statistics
Statistical analyses were performed using SPSS Version 15.0, with the threshold for significance set at P≤.05. Differences in slow MHC fibers among the 5 muscles were evaluated using 1-way, repeated-measures analyses of variance (ANOVAs). In the event of a significant main effect of muscle, post hoc comparisons were made using the sequential Bonferroni test. Statistical evaluations of the SDS-PAGE data were precluded by the small number of unfixed samples available (n = 3).
RESULTS
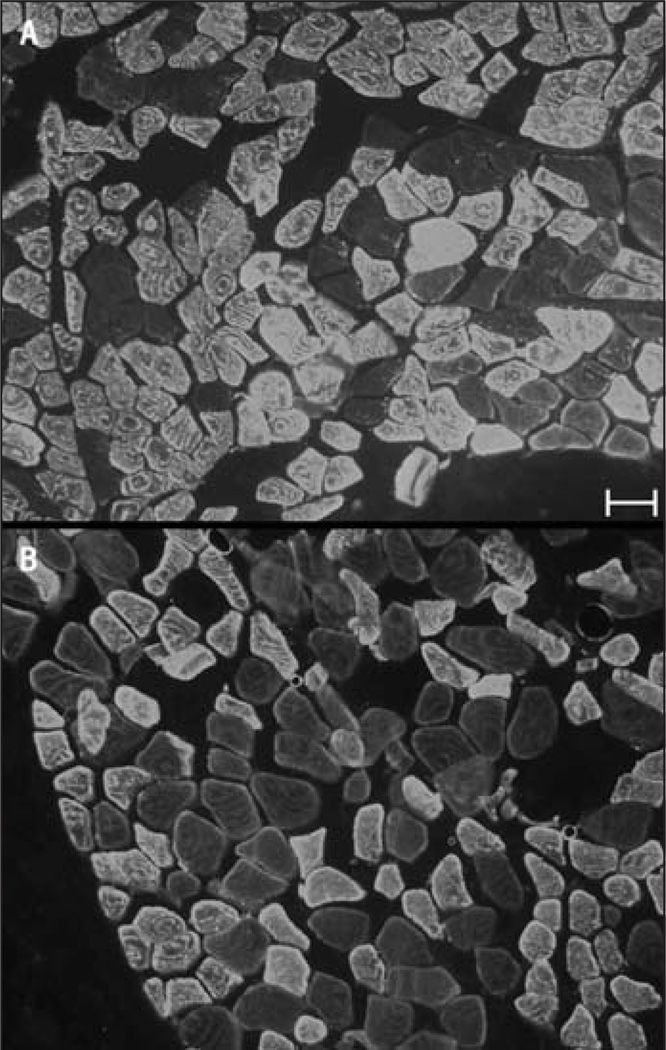
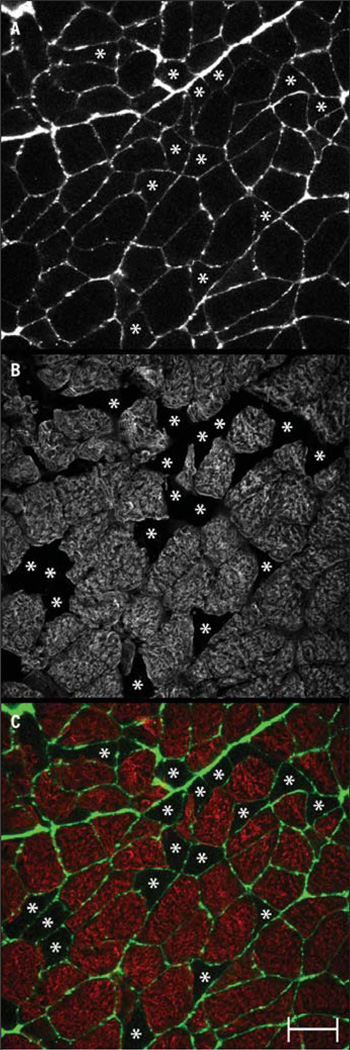
Labeling for myosin isoforms works well in embalmed tissue,24 likely because of the high natural abundance of myosin in each muscle fiber. Labeling with antibodies against fast and slow myosin worked well in our fixed (embalmed) tissue samples (FIGURE 1). Fibers in the fixed tissue that did not label were readily visible by increasing the gain on the microscope (FIGURE 1), but it is desirable to have some labeling around each cell to ensure that unlabeled fibers are not missed when counting the total number of fibers per field. Labeling the cell membranes or extracellular matrix proved unfeasible with fixed tissue, as none of the antibodies against these molecules work in embalmed tissue. Because it was necessary to obtain fresh tissue to perform gel electrophoresis, we tried, successfully, to label a second molecule, wheat germ agglutinin, a carbohydrate-binding protein that selectively recognizes sialic acid and sugar residues, which are predominantly found on the plasma membrane (FIGURE 2).
FIGURE 1.
Representative labeling of cross-sections from (A) a fixed supraspinatus muscle labeled with antibodies against fast myosin heavy chain and from (B) a fixed teres major muscle labeled with antibodies against slow myosin heavy chain. Scale bar is 50 µm.
FIGURE 2.
Representative cross-section of labeling from an unfixed subscapularis muscle labeled with antibodies to (A) fluorescein succinylated wheat germ agglutinin and (B) fast myosin. The overlay of wheat germ agglutinin (green) and fast myosin (red) labeling is shown in color (C). Asterisks are placed to show muscle fibers not labeled for fast myosin (ie, slow fibers). Scale bar is 50 µm.
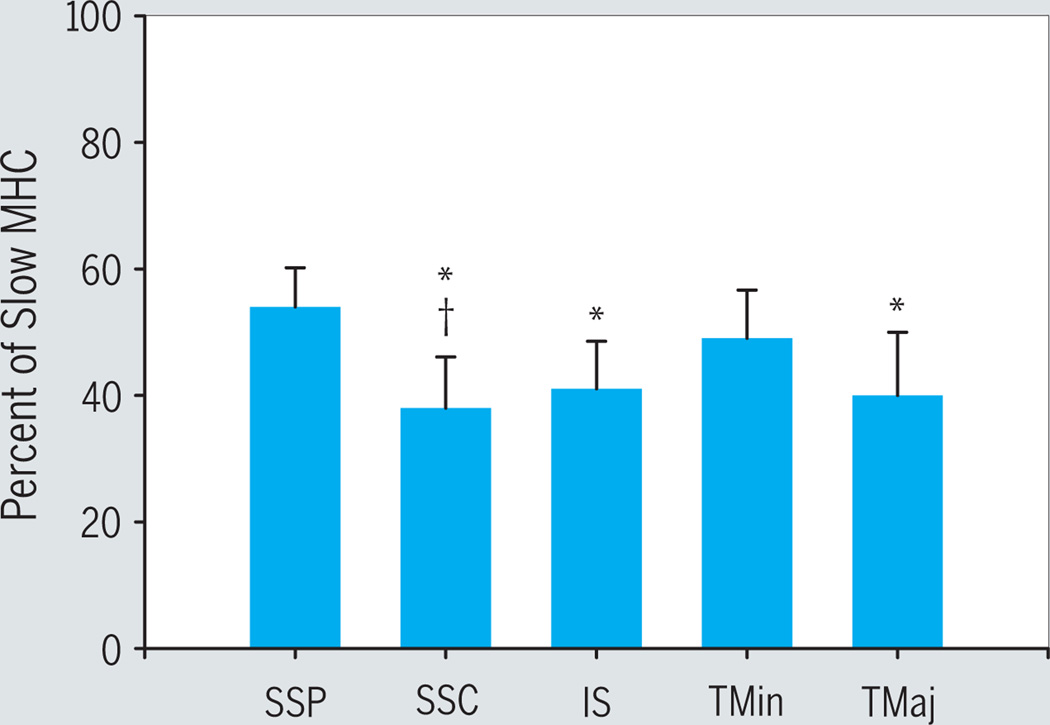
Based on immunofluorescent labeling from all 9 subjects, all muscles displayed a mixed fiber type (FIGURE 3). We analyzed multiple sections from each muscle (approximately 40 sections per muscle, with an average ± SD of 61 ± 5 fibers per field). On average (±SD) for all muscles, 44% ± 7% of fibers labeled positive for slow myosin. Specifically, average ± SD slow myosin content was 54% ± 6% in supraspinatus, 38% ± 8% in subscapularis, 41% ± 8% in infraspinatus, 49% ± 8% in teres minor, and 40% ± 10% in teres major. The percentage of slow MHC fibers in supraspinatus was significantly greater than those of the infraspinatus, subscapularis, and teres major. The number of slow MHC fibers in the subscapularis was also significantly less than that of the teres minor (FIGURE 3). No differences were found when comparing the proximal versus distal portions of the muscles (data not shown), so the data from the 2 portions were combined in the analysis.
FIGURE 3.
Histogram showing mean ± SD of slow myosin heavy chain (MHC) fiber type determined from immunofluorescent labeling of cross-sections from all 9 subjects. Abbreviations: IS, infraspinatus; SSC, subscapularis; SSP, supraspinatus; TMaj, teres major; TMin, teres minor. *Significantly less than SSP; §Significantly less than TMin.
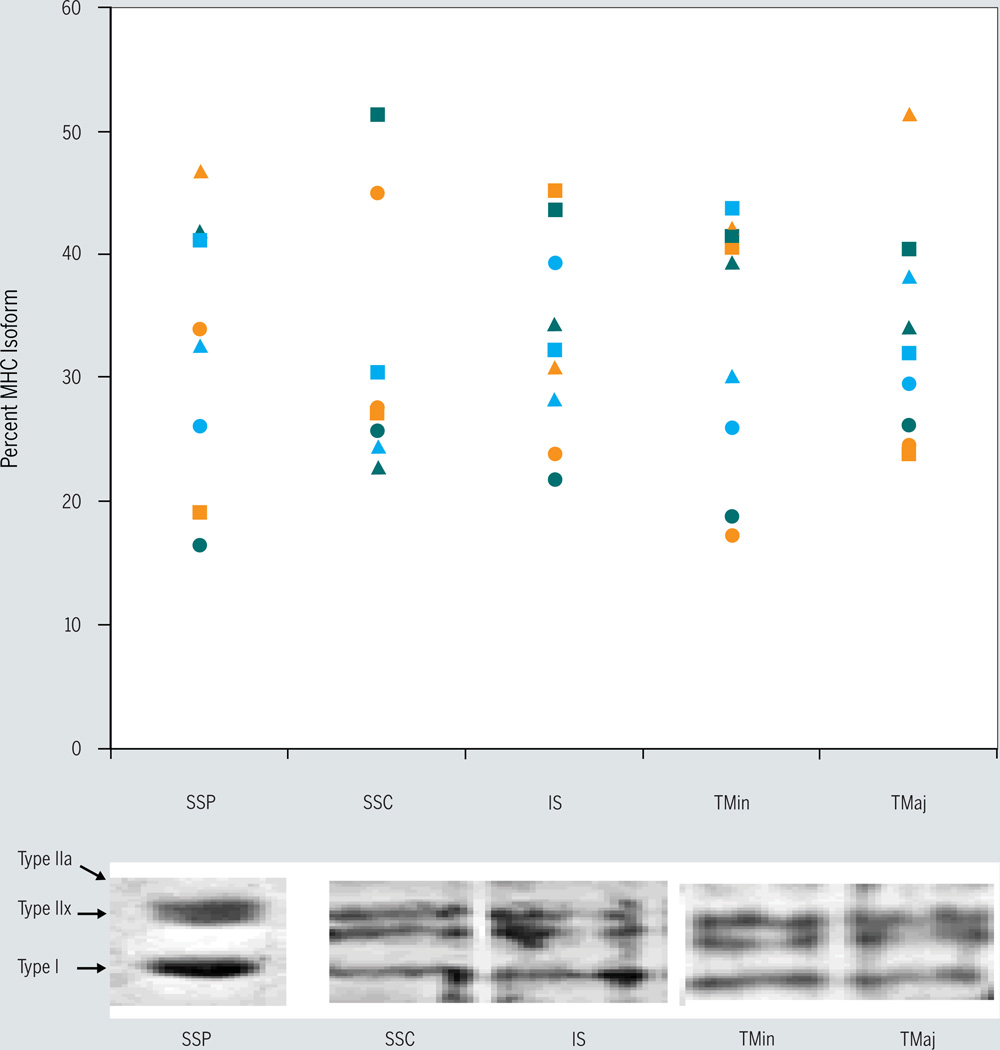
Gel electrophoresis and staining of the subsample of muscles from the 3 unembalmed subjects revealed that all of the subjects expressed all 3 human MHC isoforms in each of the muscles tested (FIGURE 4). Although no statistical analyses were performed on the SDS-PAGE data, these findings suggest that it is extremely unlikely that the fast-labeled fibers from the muscles of the embalmed subjects were of a single type II isoform.
FIGURE 4.
Myosin heavy chain (MHC) fiber type distribution from each individual from whom unfixed muscle tissue was obtained (n = 3) (top). Each color (orange, blue, and green) represents a different subject: ▲ = type I, ■ = type IIa, ● = type IIx. Separation of MHC isoforms into 3 distinct bands on representative gels (bottom). Abbreviations: IS, infraspinatus; SSC, subscapularis; SSP, supraspinatus; TMaj, teres major; TMin, teres minor.
DISCUSSION
Our main finding is that all of the rotator cuff muscles and the teres major have a mixed fiber type distribution, consistent with findings in other human muscles.13,14,40 We are aware of only 1 other study that has examined the fiber type of rotator cuff muscles.39 That study included only male cadavers (n = 4) of ages 17, 51, 57, and 75 years; but only their subscapularis and teres major data included samples from all 4 specimens.6 Thus, the data reported here represent a marked increase in the available information related to the fiber type composition of the human rotator cuff. Despite the differences in methodologies (ATPase staining versus immunofluorescence and SDS-PAGE), intersubject variability, and small sample sizes, the fiber type proportions reported in the present study are roughly equivalent to those found in the ATPase study. The proportion of slow fibers found in the samples here ranged from 40% to 54%, whereas it ranged from 37% to 50% in the earlier study. Because of the small subset used in the SDS-PAGE experiments, comparisons regarding type IIa and IIx composition between the 2 studies are more problematic, but remain within the range of variability observed in both studies (for a muscle-by-muscle comparison).6
Muscle architecture is a very important determinant of muscle function. The architecture of skeletal muscle refers to the “arrangement of muscle fibers within a muscle relative to the axis of force generation.”22 Architectural differences between muscles are notably variable. Architectural parameters (ie, fiber pennation and fiber length), together with muscle size, can be used to accurately predict muscle function (reviewed by Lieber and Friden22). The architecture of a particular muscle is consistent between individuals of the same species,22 suggesting that it is difficult to alter; however, it can change with age.27 Even so, architecture of the human rotator cuff is not the focus of this work and has been well described elsewhere.47
Rotator cuff muscles and the teres major are integral to proper shoulder function, as they contribute to both internal and external humeral rotation and function dynamically to stabilize the humeral head on the glenoid fossa. Failure of stabilization can lead to impingement due to superior migration of the humeral head.5 As the muscles of the rotator cuff are frequently damaged, particularly the tendon of the supraspinatus,47 therapists must often design rehabilitation protocols to restore their function. An optimal rehabilitation protocol should make use of the physiological, as well as biomechanical, characteristics of the involved musculature. This study is among the first to provide data regarding a physiological muscle parameter/muscle fiber type in the muscles of the rotator cuff.
Different fatigue properties are associated with the 3 primary human MHC isoforms, with fatigability increasing in the following order: type I, type IIa, and type IIx.14 Given that Ellenbecker and Roetert7 reported greater fatigue of external compared to internal rotators in elite tennis players, it was somewhat surprising that we found that the subscapularis contained type I myosin in amounts comparable to the infraspinatus and less than the teres minor, the main external rotators in the rotator cuff. However, fiber type composition is influenced by the physical activity of the individual. For example, muscle disuse is typically associated with a type I to type II shift.14,30,37 Conversely, increased muscle activity is generally associated with a fast-to-slow transition, characterized by an increase in type IIa fibers, with a decrease in type IIx fibers.14,30,37 Multiple studies in animal models (for review, see Pette & Vrbová)31 and recent data in humans,38 however, suggest that, if an endurance exercise protocol is followed for sufficient time, a further shift resulting in an increase in type I fibers can occur. In any case, the habitual use or exercise of specific muscles is associated with an overall shift from more fatigable to less fatigable isoforms. Thus, it may be that the demands of elite tennis training and competition stress the internal more than the external rotators, leading tennis players to adapt by increasing the type I fiber content of the subscapularis to a greater extent than the other rotator cuff muscles. Alternatively, the discrepancies between functional fatigue and fiber type may suggest that parameters such as metabolic enzyme activity are greater determinants of fatigue than fiber type, as suggested by Gregory et al.13 Likely, both fiber type and these other factors are major contributors.
In addition to losing muscle mass and strength, injured or postoperative rotator cuff muscles are likely to be more fatigable, due to fiber type changes that occur with disuse atrophy. Fatigue of these muscles markedly compromises normal glenohumeral and scapular kinematics.6 Protocols to restore muscular endurance, as well as strength, are, therefore, likely necessary to restore optimal function. Furthermore, fiber type composition can affect susceptibility to muscle injury during active lengthening (eccentric contractions), with type II fibers more likely to be damaged.23,46 Individuals, such as base-ball pitchers, who subject their rotator cuff muscles to frequent, high-intensity lengthening contractions may enhance their resistance to injury through endurance exercise to facilitate type II to type I fiber transitions.
The results of this study have implications for other tools that could be used to assess shoulder muscle function. Recently, interest has grown in using T2*-weighted imaging to assess muscle activation in rehabilitation.36 Such measurements, however, have been shown to be markedly influenced by intramuscular factors, including metabolic profiles and fiber type. Generally, fast, glycolytic muscles exhibit greater T2 and T2* changes for a given degree of muscle activity than do slow, more aerobic muscles,32,45 although no study has linked training-induced changes in fiber type to directional changes in T2. The more frequently used technique of electromyography (EMG) is also employed to assess the degree to which different rotator cuff muscles are active during selected tests or exercises.26 Each fiber type, however, exhibits a different muscle conduction velocity,9,33 which is a key factor in the EMG signal.10 Although not demonstrated here, the present data suggest that EMG signals from the different rotator cuff muscles are, on the whole, unlikely to be differentially influenced by fiber type composition, although specific intraindividual differences are always a possibility. However, due to the changes in fiber type associated with activity and training noted above, differences in EMG following various exercise interventions that might be attributed to neural mechanisms could be due to adaptations in the muscles themselves. Although our findings must be interpreted with caution due to the small sample size, the mixed nature of the rotator cuff muscles reported here and elsewhere39 indicates that there is room for such confounding fiber type changes to occur. By contrast, a muscle that had a very high type I content prior to a training program would be unlikely to become markedly more type I dominant.
Some limitations of the present study must be noted. First and foremost, only a small number of subjects were studied, particularly with regard to the SDS-PAGE data. Nevertheless, the 5 male and 4 female subjects examined here represent a 225% increase in the existing data (4 subjects total, to our knowledge) in the area. Second, our subjects were relatively advanced in age. An overall type II to type I shift in MHC appears to occur in some,17,20 but not all,15,21 muscles with aging; although this effect is typically stronger with regard to percent fiber type area rather than percent fiber type number. This phenomenon has not been studied in the rotator cuff, but if similar age-related changes in MHC occur in the rotator cuff muscles, one might expect that younger subjects would exhibit even greater type II MHC content than that reported here. In addition, the habitual physical activity and training status of the subjects is not known, and could have affected the results reported here. Of note, one of the few studies to evaluate the effects of age on fiber type in an upper extremity muscle, the biceps brachii, found no age-related differences.19 A third limitation is that we only had access to commercial antibodies that identified type I or type II MHC. These antibodies, unlike those developed by Schiaffino et al,34 do not distinguish between type IIa and IIx MHC. We attempted to address this limitation by performing SDS-PAGE and silver staining to assess the relative percentages of the 3 predominant human MHC isoforms in a small subset of muscles from unembalmed cadavers. Both type IIa and IIx MHC were detected in each muscle from each unembalmed cadaver.
CONCLUSION
Using 2 separate, complementary techniques to determine fiber type in skeletal muscle, we found that the rotator cuff muscles in humans have a heterogeneous fiber type composition. These findings have implications both for clinical rehabilitative exercise protocols designed for these muscles and for the interpretation of research studies using EMG and imaging to assess neuromuscular changes following various interventions.
KEY POINTS.
FINDINGS
Using the techniques of immunohistochemistry and myosin separation by electrophoresis to identify fiber types of the human rotator cuff muscles, we established the fiber type distribution in human rotator cuff muscles.
IMPLICATIONS
Knowledge of fiber type composition is important for physical therapists and other healthcare professionals to optimize muscle training and rehabilitation protocols.
CAUTION
The advanced age of the cadaver specimens may limit the extrapolation of our findings regarding fiber type composition to younger individuals.
ACKNOWLEDGEMENTS
We wish to thank Dr Robert Staron for his willingness to share both his laboratory and expertise in the processing of the muscle samples for SDS-PAGE, and Dr Robert J. Bloch for use of his laboratory. Further gratitude is due to Drs Patrick Reed and Scott K. Stackhouse for helpful comments on earlier drafts of the manuscript.
Funding Sources: NIH-NIAMS K01AR053235 and MDA 4278 to RML.
REFERENCES
- 1.Bamman MM, Clarke MS, Talmadge RJ, Feeback DL. Enhanced protein electrophoresis technique for separating human skeletal muscle myosin heavy chain isoforms. Electrophoresis. 1999;20:466–468. doi: 10.1002/(SICI)1522-2683(19990301)20:3<466::AID-ELPS466>3.0.CO;2-7. http://dx.doi.org/10.1002/(SICI)1522-2683(19990301)20:3<466::AID-ELPS466>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Barany M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967;50(Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burke RE, Levine DN, Zajac FE., 3rd Mammalian motor units: physiological-histochemical correlation in three types in cat gastrocnemius. Science. 1971;174:709–712. doi: 10.1126/science.174.4010.709. [DOI] [PubMed] [Google Scholar]
- 4.De Deyne PG, Hafer-Macko CE, Ivey FM, Ryan AS, Macko RF. Muscle molecular phenotype after stroke is associated with gait speed. Muscle Nerve. 2004;30:209–215. doi: 10.1002/mus.20085. http://dx.doi.org/10.1002/mus.20085. [DOI] [PubMed] [Google Scholar]
- 5.Deutsch A, Altchek DW, Schwartz E, Otis JC, Warren RF. Radiologic measurement of superior displacement of the humeral head in the impingement syndrome. J Shoulder Elbow Surg. 1996;5:186–193. doi: 10.1016/s1058-2746(05)80004-7. [DOI] [PubMed] [Google Scholar]
- 6.Ebaugh DD, McClure PW, Karduna AR. Scapulothoracic and glenohumeral kinematics following an external rotation fatigue protocol. J Orthop Sports Phys Ther. 2006;36:557–571. doi: 10.2519/jospt.2006.2189. http://dx.doi.org/10.2519/jospt.2006.2189. [DOI] [PubMed] [Google Scholar]
- 7.Ellenbecker TS, Roetert EP. Testing isokinetic muscular fatigue of shoulder internal and external rotation in elite junior tennis players. J Orthop Sports Phys Ther. 1999;29:275–281. doi: 10.2519/jospt.1999.29.5.275. [DOI] [PubMed] [Google Scholar]
- 8.Engel WK. The essentiality of histo- and cytochemical studies of skeletal muscle in the investigation of neuromuscular disease 1962. Neurology. 1998;51:655–617. [PubMed] [Google Scholar]
- 9.Farina D, Ferguson RA, Macaluso A, De Vito G. Correlation of average muscle fiber conduction velocity measured during cycling exercise with myosin heavy chain composition, lactate threshold, and VO2max. J Electromyogr Kinesiol. 2007;17:393–400. doi: 10.1016/j.jelekin.2006.03.003. http://dx.doi.org/10.1016/j.jelekin.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96:1486–1495. doi: 10.1152/japplphysiol.01070.2003. http://dx.doi.org/10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- 11.Fitts RH. The cross-bridge cycle and skeletal muscle fatigue. J Appl Physiol. 2008;104:551–558. doi: 10.1152/japplphysiol.01200.2007. http://dx.doi.org/10.1152/japplphysiol.01200.2007. [DOI] [PubMed] [Google Scholar]
- 12.Fitts RH, Metzger JM, Riley DA, Unsworth BR. Models of disuse: a comparison of hindlimb suspension and immobilization. J Appl Physiol. 1986;60:1946–1953. doi: 10.1152/jappl.1986.60.6.1946. [DOI] [PubMed] [Google Scholar]
- 13.Gregory CM, Vandenborne K, Dudley GA. Metabolic enzymes and phenotypic expression among human locomotor muscles. Muscle Nerve. 2001;24:387–393. doi: 10.1002/1097-4598(200103)24:3<387::aid-mus1010>3.0.co;2-m. http://dx.doi.org/10.1002/1097-4598(200103)24:3<387::AID-MUS1010>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 14.Grossman EJ, Roy RR, Talmadge RJ, Zhong H, Edgerton VR. Effects of inactivity on myosin heavy chain composition and size of rat soleus fibers. Muscle Nerve. 1998;21:375–389. doi: 10.1002/(sici)1097-4598(199803)21:3<375::aid-mus12>3.0.co;2-z. http://dx.doi.org/10.1002/(SICI)1097-4598(199803)21:3<375::AID-MUS12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 15.Houmard JA, Weidner ML, Gavigan KE, Tyndall GL, Hickey MS, Alshami A. Fiber type and citrate synthase activity in the human gastrocnemius and vastus lateralis with aging. J Appl Physiol. 1998;85:1337–1341. doi: 10.1152/jappl.1998.85.4.1337. [DOI] [PubMed] [Google Scholar]
- 16.Hunter GR, Bamman MM, Larson-Meyer DE, et al. Inverse relationship between exercise economy and oxidative capacity in muscle. Eur J Appl Physiol. 2005;94:558–568. doi: 10.1007/s00421-005-1370-z. http://dx.doi.org/www.homefrontla.com10.1007/s00421-005-1370-z. [DOI] [PubMed] [Google Scholar]
- 17.Jakobsson F, Borg K, Edstrom L. Fibre-type composition, structure and cytoskeletal protein location of fibres in anterior tibial muscle. Comparison between young adults and physically active aged humans. Acta Neuropathol. 1990;80:459–468. doi: 10.1007/BF00294604. [DOI] [PubMed] [Google Scholar]
- 18.Jump SS, Schuenke MD, Staron RS. Postmortem alterations in the pH range of myofibrillar ATPase activation/inactivation. Histochem Cell Biol. 2003;119:161–168. doi: 10.1007/s00418-002-0492-1. http://dx.doi.org/10.1007/s00418-002-0492-1. [DOI] [PubMed] [Google Scholar]
- 19.Klein CS, Marsh GD, Petrella RJ, Rice CL. Muscle fiber number in the biceps brachii muscle of young and old men. Muscle Nerve. 2003;28:62–68. doi: 10.1002/mus.10386. http://dx.doi.org/10.1002/mus.10386. [DOI] [PubMed] [Google Scholar]
- 20.Lee WS, Cheung WH, Qin L, Tang N, Leung KS. Age-associated decrease of type IIA/B human skeletal muscle fibers. Clin Orthop Relat Res. 2006;450:231–237. doi: 10.1097/01.blo.0000218757.97063.21. http://dx.doi.org/10.1097/01.blo.0000218757.97063.21. [DOI] [PubMed] [Google Scholar]
- 21.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 22.Lieber RL, Friden J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000;23:1647–1666. doi: 10.1002/1097-4598(200011)23:11<1647::aid-mus1>3.0.co;2-m. http://dx.doi.org/10.1002/1097-4598(200011)23:11<1647::AID-MUS1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 23.Lieber RL, Friden J. Selective damage of fast glycolytic muscle fibres with eccentric contraction of the rabbit tibialis anterior. Acta Physiol Scand. 1988;133:587–588. doi: 10.1111/j.1748-1716.1988.tb08446.x. [DOI] [PubMed] [Google Scholar]
- 24.Lovering RM, Anderson LD. Architecture and fiber type of the pyramidalis muscle. Anat Sci Int. 2008 Feb 28; doi: 10.1111/j.1447-073X.2007.00226.x. http://dx.doi.org/10.1111/j.1447-073X.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lovering RM, De Deyne PG. Contractile function, sarcolemma integrity, and the loss of dystrophin after skeletal muscle eccentric contraction-induced injury. Am J Physiol Cell Physiol. 2004;286:C230–C238. doi: 10.1152/ajpcell.00199.2003. http://dx.doi.org/10.1152/ajpcell.00199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malanga GA, Jenp YN, Growney ES, An KN. EMG analysis of shoulder positioning in testing and strengthening the supraspinatus. Med Sci Sports Exerc. 1996;28:661–664. doi: 10.1097/00005768-199606000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Narici MV, Maganaris CN, Reeves ND, Capodaglio P. Effect of aging on human muscle architecture. J Appl Physiol. 2003;95:2229–2234. doi: 10.1152/japplphysiol.00433.2003. http://dx.doi.org/10.1152/japplphysiol.00433.2003. [DOI] [PubMed] [Google Scholar]
- 28.Petit J, Filippi GM, Emonet-Denand F, Hunt CC, Laporte Y. Changes in muscle stiffness produced by motor units of different types in peroneus longus muscle of cat. J Neurophysiol. 1990;63:190–197. doi: 10.1152/jn.1990.63.1.190. [DOI] [PubMed] [Google Scholar]
- 29.Pette D, Spamer C. Metabolic properties of muscle fibers. Fed Proc. 1986;45:2910–2914. [PubMed] [Google Scholar]
- 30.Pette D, Staron RS. Mammalian skeletal muscle fiber type transitions. Int Rev Cytol. 1997;170:143–223. doi: 10.1016/s0074-7696(08)61622-8. [DOI] [PubMed] [Google Scholar]
- 31.Pette D, Vrbova G. What does chronic electrical stimulation teach us about muscle plasticity. Muscle Nerve. 1999;22:666–677. doi: 10.1002/(sici)1097-4598(199906)22:6<666::aid-mus3>3.0.co;2-z. http://dx.doi.org/10.1002/(SICI)1097-4598(199906)22:6<666::AID-MUS3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 32.Prior BM, Ploutz-Snyder LL, Cooper TG, Meyer RA. Fiber type and metabolic dependence of T2 increases in stimulated rat muscles. J Appl Physiol. 2001;90:615–623. doi: 10.1152/jappl.2001.90.2.615. [DOI] [PubMed] [Google Scholar]
- 33.Sadoyama T, Masuda T, Miyata H, Katsuta S. Fibre conduction velocity and fibre composition in human vastus lateralis. Eur J Appl Physiol Occup Physiol. 1988;57:767–771. doi: 10.1007/BF01076001. [DOI] [PubMed] [Google Scholar]
- 34.Schiaffino S, Gorza L, Sartore S, et al. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989;10:197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- 35.Scott W, Stevens J, Binder-Macleod SA. Human skeletal muscle fiber type classifications. Phys Ther. 2001;81:1810–1816. [PubMed] [Google Scholar]
- 36.Segal RL. Use of imaging to assess normal and adaptive muscle function. Phys Ther. 2007;87:704–718. doi: 10.2522/ptj.20060169. http://dx.doi.org/10.2522/ptj.20060169. [DOI] [PubMed] [Google Scholar]
- 37.Shepstone TN, Tang JE, Dallaire S, Schuenke MD, Staron RS, Phillips SM. Short-term high- vs. low-velocity isokinetic lengthening training results in greater hypertrophy of the elbow flexors in young men. J Appl Physiol. 2005;98:1768–1776. doi: 10.1152/japplphysiol.01027.2004. http://dx.doi.org/10.1152/japplphysiol.01027.2004. [DOI] [PubMed] [Google Scholar]
- 38.Short KR, Vittone JL, Bigelow ML, et al. Changes in myosin heavy chain mRNA and protein expression in human skeletal muscle with age and endurance exercise training. J Appl Physiol. 2005;99:95–102. doi: 10.1152/japplphysiol.00129.2005. http://dx.doi.org/10.1152/japplphysiol.00129.2005. [DOI] [PubMed] [Google Scholar]
- 39.Srinivasan RC, Lungren MP, Langenderfer JE, Hughes RE. Fiber type composition and maximum shortening velocity of muscles crossing the human shoulder. Clin Anat. 2007;20:144–149. doi: 10.1002/ca.20349. http://dx.doi.org/10.1002/ca.20349. [DOI] [PubMed] [Google Scholar]
- 40.Staron RS, Hagerman FC, Hikida RS, et al. Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem. 2000;48:623–629. doi: 10.1177/002215540004800506. [DOI] [PubMed] [Google Scholar]
- 41.Talmadge RJ, Roy RR. Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol. 1993;75:2337–2340. doi: 10.1152/jappl.1993.75.5.2337. [DOI] [PubMed] [Google Scholar]
- 42.Thorstensson A, Grimby G, Karlsson J. Force-velocity relations and fiber composition in human knee extensor muscles. J Appl Physiol. 1976;40:12–16. doi: 10.1152/jappl.1976.40.1.12. [DOI] [PubMed] [Google Scholar]
- 43.Tischler ME, Henriksen EJ, Munoz KA, Stump CS, Woodman CR, Kirby CR. Spaceflight on STS-48 and earth-based unweighting produce similar effects on skeletal muscle of young rats. J Appl Physiol. 1993;74:2161–2165. doi: 10.1152/jappl.1993.74.5.2161. [DOI] [PubMed] [Google Scholar]
- 44.Uhthoff HK, Sarkar K. An algorithm for shoulder pain caused by soft-tissue disorders. Clin Orthop Relat Res. 1990:121–127. [PubMed] [Google Scholar]
- 45.Vandenborne K, Walter G, Ploutz-Snyder L, Dudley G, Elliott MA, De Meirleir K. Relationship between muscle T2* relaxation properties and metabolic state: a combined localized 31Pspectroscopy and 1H-imaging study. Eur J Appl Physiol. 2000;82:76–82. doi: 10.1007/s004210050654. [DOI] [PubMed] [Google Scholar]
- 46.Vijayan K, Thompson JL, Norenberg KM, Fitts RH, Riley DA. Fiber-type susceptibility to eccentric contraction-induced damage of hindlimb-unloaded rat AL muscles. J Appl Physiol. 2001;90:770–776. doi: 10.1152/jappl.2001.90.3.770. [DOI] [PubMed] [Google Scholar]
- 47.Ward SR, Hentzen ER, Smallwood LH, et al. Rotator cuff muscle architecture: implications for glenohumeral stability. Clin Orthop Relat Res. 2006;448:157–163. doi: 10.1097/01.blo.0000194680.94882.d3. http://dx.doi.org/10.1097/01.blo.0000194680.94882.d3. [DOI] [PubMed] [Google Scholar]