Table 3.
Additions to β-Substituted Acyclic Dienones: Scopea
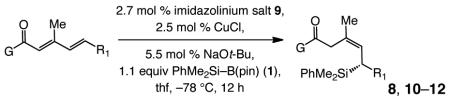
| entry | allylsilane product | yield (%);b | Z:E c | erd |
|---|---|---|---|---|
| 1e |
|
81 | 93:7 | >99:1 |
| 2 | 81 | 95:5 | 99:1 | |
| 3 | 75 | 91:9 | >99:1 | |
| 4 |
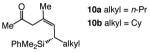
|
85 | 89:11 | >99:1 |
| 5 | 72 | 89:11 | >99:1 | |
| 6 |
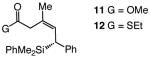
|
73 | >98:2 | >99:1 |
| 7f | 69 | >98:2 | 99:1 |
Reactions performed under N2 atm; >98% conv, <2% α,β-unsaturated carbonyl product, and >98% 1,6-addition in all cases.
Yields of purified products (±5%).
Determined by analysis of 400 MHz 1H NMR spectra of unpurified mixtures (±2%).
Determined by HPLC analysis; er values correspond to the Z alkene product.
Reaction time = 6.0 h.
Performed at −50°C for 48 h. See the SI for details.
