Abstract
Objectives: To determine the prevalence of premenstrual symptoms (PMS) due to primary dysmenorrhea among a sample of university female students, and to explore possible association with vitamin D and parathyroid (PTH) levels, as well as frequency of consumption of dairy products. Design: A cross-sectional study. Setting: One Jordanian university. Subjects: A total of 177 female students aged between 18 and 24 years who experienced primary dysmenorrhea participated in the study and completed a self administered questionnaire to collect information concerning demographics, menstruation- related information, associated specified premenstrual symptoms, and consumption of dairy products. Plasma 25-hydroxyvitamin vitamin D level and intact parathyroid hormone level were measured. Results: Of the 177 participants 91.5% had two or more symptoms among which fatigue, mood swings, anxiety, abdominal bloating, and depression were the most prevalent symptoms. There was no evident association between presence of symptoms and vitamin D status, PTH level or dairy products consumption. Headaches and social withdrawal were significantly lower in those women who consumed high amounts of dairy products. Conclusion: Premenstrual symptoms are very common in young women with primary dysmenorrhea. PMS has no relation to levels of vitamin D, parathyroid hormone or dairy products consumption. Headache and social withdrawal may be affected by dairy product consumption.
Keywords: premenstrual symptoms, primary dysmenorrheal, vitamin D, parathyroid hormone, dairy products
1. Introduction
Premenstrual symptoms, often collectively referred to as premenstrual syndrome (PMS), and dysmenorrhea, are common gynecological problems affecting the lifestyle and performance of young women. Different types of studies from both developed and developing countries have found a consistently high prevalence of both dysmenorrhea and premenstrual symptoms in women of different ages and nationalities [1,2,3,4,5]. The majority of cases of dysmenorrhea are primary, which is defined as painful menses in women with normal pelvic anatomy that usually begins during adolescence [6]. Premenstrual syndrome (PMS)/premenstrual dysphoric disorder (PMDD) is defined as a group of disorders characterized by emotional, behavioral and physical symptoms that occur in the luteal phase of the menstrual cycle and subside following menstruation [7], but remain without definitive physical or laboratory criteria for diagnosis and are associated with some degree of inter-cycle variability [8]. Physical symptoms of this disorder include headaches, breast tenderness, abdominal bloating, peripheral edema and general fatigue, while psychological or behavioral disorders include irritability, mood swings, food cravings, social withdrawal, anxiety, and depression. While definitive diagnosis of these disorders remains debatable, a prospective record of cycle related symptoms is the gold standard for diagnosis by establishing a relationship between the symptoms and the late luteal phase of the menstrual cycle. Retrospective, self-reporting of symptoms is found to be reasonably sensitive [9,10].
The prevalence of PMS is variable [11]. Prevalence as high as 75–85% is mentioned if one or several symptoms is considered, 10–15% if medical care is requested and 2–5% with social activities interruption [12,13,14]. The causes of premenstrual symptoms are uncertain [15]. Many theories have been tested regarding possible causes of premenstrual symptoms. While many of the hormonal and biochemical profiles of women with premenstrual symptoms and those who are symptom free were similar, fluctuation in gonadal hormone levels may trigger the symptoms [16]. PMDD is thought to be related to serotonergic synapse abnormality and women with PMDD are found to have lower serotogenic function in the luteal phase [17,18]. A correlation between premenstrual symptoms and dysmenorrhea was made by Isaa and Tomko [19,20]. The severity of dysmenorrheal pain and premenstrual symptoms varies among women but it can be severe enough to cause a substantial negative impact on their daily activity. Current understanding of the pathogenesis in primary dysmenorrhea implicates excessive imbalanced amounts of prostanoids and possibly eicosanoids released from the endometrium [21,22]. The majority of subjects benefit from administration of nonsteroidal anti-inflammatory drugs (NSAIDs) [13,23]. A strong negative correlation between dairy product intake and dysmenorrhea and its associated symptoms among university female students was demonstrated whereby the severity of primary dysmenorrhea decreased with increasing daily intake of dairy products [24]. These results suggested that dietary calcium, among other substances, may have a functional role in the etiology of dysmenorrhea and a possible relation between calcium and vitamin D and premenstrual symptoms has also been suggested by Bertone-Johson and colleagues [25]. The aim of this study was to explore the prevalence of premenstrual symptoms in young college students known to suffer from with dysmenorrhea and to investigate any relation with vitamin D and parathyroid hormone levels or consumption of dairy products by these women.
2. Experimental Section
2.1. Participants and Setting
One hundred and seventy seven, single and healthy college students who experienced primary dysmenorrhea responded to our advertisement and accepted to take part in the study during the period from March to May 2010. The Institutional Review Board Committee approved this study. Written informed consent was obtained from all participants.
Participants with primary dysmenorrhoea were instructed to complete a guided self-assessment questionnaire. Information regarding participants’ age, age at menarch, menstrual cycle regularity, amount of blood loss and pain severity was collected. Blood loss is described as light (below average), moderate and heavy if associated with clots. Pain severity was graded as mild, severe and very severe as follows: Mild: pain that is tolerated without need for medications; Severe: pain that resolved with analgesics; Very severe: pain that is not relieved with analgesics and may interfere with usual daily activity.
Participants were also requested to answer questions about their recurrent experience of 12 symptoms during the premenstrual phase that subside with onset of menstruation. Psychological symptoms of irritability, anxiety, depression, mood swings, social withdraw, change in appetite and cravings for sweet or salty foods are recorded, along with physical symptoms such as abdominal bloating, headaches, general fatigue, nausea, and breast tenderness [26]. The study group included participants having a minimum of two symptoms, one physical symptom and one psychological symptom, while those with only one symptom or no symptoms acted as control. Assessment of dairy products intake was measured by number of specified portions consumed per day, per week or per month of any type of dairy products. Consumption frequency was calculated as times per day. BMI was calculated at the same time.
2.2. Blood Samples
About 10 mL of venous blood was collected from each participant in heparinized tubes. Plasma was assayed for plasma 25-hydroxyvitamin vitamin D [25(OH) D] level and intact parathyroid hormone (PTH) level. An enzyme immunoassay (The Immunodiagnostic System (IDS), UK) was used. The tests were performed in the central laboratory of Specialty Hospital (ISO 15189:2007)/Amman, Jordan.
2.3. Definitions
Vitamin D status was divided into three diagnostic categories according to plasma 25(OH)D levels. Vitamin D deficiency (VDD) <25 mmol/L, Vitamin D insufficiency (VDI) 25–75 mmol/L and Vitamin D sufficiency (VDS) >75mmol/L. Normal reference value of PTH is 13.9–75 pg/mL. These reference ranges were provided by the manufacturer of the assay.
2.4. Statistical Analysis
Data were analyzed using the Statistical Package for Social Science (SPSS, version 16.0). Descriptive statistics that include frequency and, range and mean with standard deviation were carried out. Chi-square (X2) test was performed to investigate the association between premenstrual symptoms with characteristics of participants and other variables of interest (vitamin D status, parathyroid hormone level, daily dairy product intake). Findings with p value <0.05 were considered to be statistically significant.
3. Results
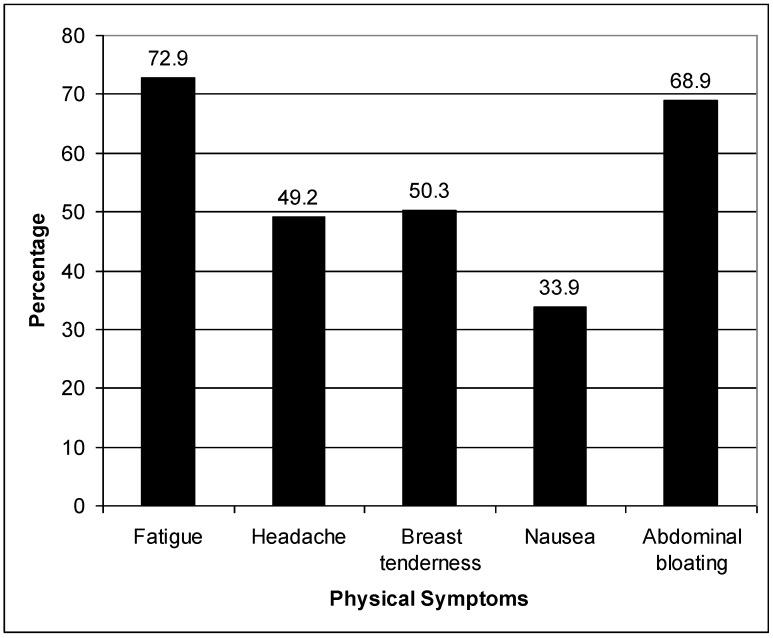
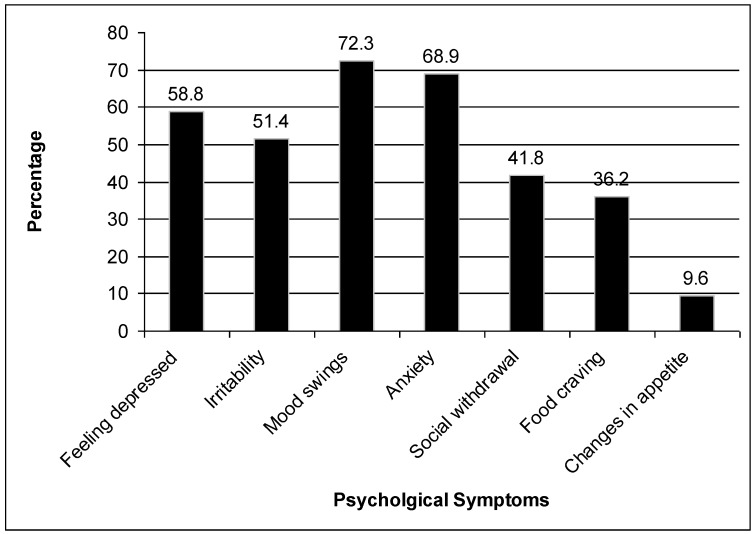
The age of the participants ranged between 18 and 24 years, with a mean of 21.8 ± 2.8 years. The mean body mass index value was 22.1 ± 1.1 Kg/m2. The mean age at menarche was 13.3 ± 1.4 years and dysmenorrhea started at an age of 15.3 ± 2.8. Approximately 17% of the participants had a menstrual cycle duration of less than five days, while 83% of them had menstrual cycle durations of five days or more. The majority of participants (77.8%) had regular menstrual cycles. Two or more premenstrual symptoms were detected in 91.5% of the participants. The most frequent symptoms are fatigue, mood swings, anxiety, abdominal bloating, and depression (Figure 1 and Figure 2). Approximately 22% of the participants graded their dysmenorrheal pain as mild, 68% as severe and only 9.6% of the participants reported very severe menstrual pain. Scoring of pain severity in relation to presence of premenstrual symptoms were highly significant (p value <0.001, Table 1). The relationship between premenstrual symptoms and vitamin D status, PTH level, daily dairy products intake was studied. No significant association between positive symptoms and vitamin D level, parathyroid hormone level or dairy products intake could be demonstrated (Table 2). Analysis of each symptom in relation to parathyroid hormone, Vitamin D and daily dairy products consumption did not reveal any significant association, apart from a negative association between dairy products intake and headache and social withdrawal (p value 0.036 and 0.044) respectively (Table 3, Table 4 and Table 5).
Table 1.
PMS in relation to menstrual pattern.
| Variable | Premenstrual symptomsn (%) a | Total | p value | |
|---|---|---|---|---|
| No | yes | |||
| Dysmenorrheal severity | <0.001 | |||
| Mild | 10 (25.6) | 29 (74.4) | 39 | |
| Severe | 5 (4.1) | 116 (95.9) | 121 | |
| Very Severe | 0 (0) | 17 (100) | 17 | |
| Regularity of menstruation | 0.39 | |||
| Irregular | 2 (5.1) | 37 (94.9) | 39 | |
| Regular | 13 (9.5) | 124 (90.5) | 137 | |
| Menstrual blood loss | 0.78 | |||
| Light | 1 (12.5) | 7 (87.5) | 8 | |
| Moderate | 13 (8.7) | 136 (91.3) | 149 | |
| Heavy | 1 (5) | 19 (95) | 20 | |
a Data presented as numbers and percent within parenthesis.
Table 2.
The association between parathyroid hormone, vitamin D, dairy products consumption and the risk of PMS.
| Variable | Premenstrual symptoms n (%) a | Total | p value | |
|---|---|---|---|---|
| No | yes | |||
| Parathyroid hormone level b | 0.93 | |||
| Normal | 11 (8.6) | 117 (91.4) | 128 | |
| Hyperparathyroid | 4 (8.2) | 45 (91.8) | 49 | |
| Vitamin D level c | 0.85 | |||
| Normal | 0 (0) | 3 (100) | 3 | |
| Insufficiency | 9 (9) | 91 (91) | 100 | |
| Deficiency | 6 (8.1) | 68 (91.9) | 74 | |
| Daily dairy products intake | 0.84 | |||
| ≤ 1 serving | 3 (6.7) | 42 (93.3) | 45 | |
| 1–2 servings | 9 (9.6) | 85 (90.4) | 94 | |
| >2 servings | 3 (7.9) | 35 (92.1) | 38 | |
a Data presented as numbers and percent within parenthesis; b Normal reference value of PTH is 13.9–75 pg/mL, Hyperparathyroid: >75 pg/mL; c Vitamin D deficiency: <25 mmol/L, Vitamin D insufficiency: 25–75 mmol/L and Vitamin D sufficiency: >75mmol/L.
Table 3.
Relation of symptoms to parathyroid hormone level.
| Symptom | Parathyroid hormone status n (%) a | p value | |
|---|---|---|---|
| Normal (n= 128) | Hyperparathyroidism (n= 49) | ||
| Fatigue | |||
| No | 33 (68.8%) | 15 (31.2%) | |
| Yes | 95 (73.6%%) | 34 (26.4%) | NS |
| Headache | |||
| No | 69 (76.7%) | 21 (23.3%) | |
| Yes | 59 (67.8%) | 28 (32.2%) | NS |
| Breast tenderness | |||
| No | 66 (75.0%) | 22 (25.0%) | |
| Yes | 62 (69.7%) | 27 (30.3%) | NS |
| Depression | |||
| No | 55 (75.3%) | 18 (24.7%) | |
| Yes | 73 (70.2%) | 31 (29.8%) | NS |
| Irritability | |||
| No | 63 (73.3%) | 23 (26.7%) | |
| Yes | 65 (71.4%) | 26 (28.6%) | NS |
| Mood swings | |||
| No | 38 (77.6%) | 11 (22.4%) | |
| Yes | 90 (70.3%) | 38 (29.7%) | NS |
| Anxiety | |||
| No | 42 (76.4%) | 13 (23.6%) | |
| Yes | 86 (70.5%) | 36 (29.5%) | NS |
| Social withdrawal | |||
| No | 78 (75.7%) | 25 (24.3%) | |
| Yes | 50 (67.6%) | 24 (32.4%) | NS |
| Nausea | |||
| No | 84 (71.8%) | 33 (28.2%) | |
| Yes | 44 (73.3%) | 16 (26.7%) | NS |
| Abdominal bloating | |||
| No | 40 (72.7%) | 15 (27.3%) | |
| Yes | 88 (72.1%) | 34 (27.9%) | NS |
| Food craving | |||
| No | 83 (73.5%) | 30 (26.5%) | |
| Yes | 45 (70.3%) | 19 (29.7%) | NS |
| Change in appetite | |||
| No | 13 (76.5%) | 4 (23.5%) | |
| Yes | 128 (72.3%) | 49 (27.7%) | NS |
a Data presented as numbers and percent within parenthesis.
Table 4.
Relation of symptoms to vitamin D levels.
| Symptom | Vitamin D status n (%) a | p value | ||
|---|---|---|---|---|
| Deficiency (n = 74) | Insufficiency (n = 100) | Normal (n = 3) | ||
| Fatigue | ||||
| No | 18 (37.5%) | 30 (62.5%) | 0 (0%) | |
| Yes | 56 (43.4%) | 70 (54.3%) | 3 (2.3%) | NS |
| Headache | ||||
| No | 37 (41.1%) | 53 (58.9%) | ||
| Yes | 37 (42.5%) | 47 (54.0%) | 3 (3.4%) | NS |
| Breast tenderness | ||||
| No | 40 (45.5%) | 48 (54.5%) | ||
| Yes | 34 (38.2%) | 52 (58.4%) | 3 (3.4%) | NS |
| Depression | ||||
| No | 36 (49.3%) | 36 (49.3%) | 1 (1.4%) | |
| Yes | 38 (36.5%) | 64 (61.5%) | 2 (1.9%) | NS |
| Irritability | ||||
| No | 35 (40.7%) | 50 (58.1%) | 1 (1.2%) | |
| Yes | 39 (42.9%) | 50 (54.9%) | 2 (2.2%) | NS |
| Mood swings | ||||
| No | 17 (34.7%) | 32 (65.3%) | ||
| Yes | 57 (44.5%) | 68 (53.1%) | 3 (2.3%) | NS |
| Anxiety | ||||
| No | 19(34.5%) | 36(65.5%) | 0 (0%) | |
| Yes | 55 (45.1%) | 64 (52.5%) | 3 (2.5%) | NS |
| Social withdrawal | ||||
| No | 43 (41.7%) | 60 (58.3%) | 0 (0%) | |
| Yes | 31 (41.9%) | 40 (54.1%) | 3 (4.1%) | NS |
| Nausea | ||||
| No | 52 (44.4%) | 64 (54.7%) | 1 (0.9%) | |
| Yes | 22 (36.7%) | 36 (60%) | 2 (3%) | NS |
| Abdominal bloating | ||||
| No | 23 (41.8%) | 31 (56.4%) | 1 (1.8%) | |
| Yes | 51 (41.8%) | 69 (56.6%) | 2 (1.6%) | NS |
| Food craving | ||||
| No | 52 (46%) | 60 (53.1%) | 1 (0.9%) | |
| Yes | 22 (34.4%) | 40 (62.5%) | 2 (3.1%) | NS |
| Change in appetite | ||||
| No | 68 (42.5%) | 89 (55.6%) | 3 (1.9%) | |
| Yes | 6(35.3%) | 11(64.7%) | 0 (0%) | NS |
a Data presented as numbers and percent within parenthesis.
Table 5.
Relation of symptoms to dairy product intake.
| Symptom | Daily dairy products consumption (serving) n (%) a | p value | ||
|---|---|---|---|---|
| ≤1 (n = 45) | ≤2 (n = 94) | >2 (n = 38) | ||
| Fatigue | ||||
| No | 11 (22.9%) | 24 (50.0%) | 13 (27.1%) | |
| Yes | 34 (26.4%) | 70 (54.3%) | 25 (19.4%) | NS |
| Headache | ||||
| No | 19 (21.1%) | 46 (51.1%) | 25 (27.8%) | |
| Yes | 26 (29.9%) | 48 (55.2%) | 13 (14.9%) | 0.036 |
| Breast tenderness | ||||
| No | 24(27.3%) | 46 (52.3%) | 18 (20.5) | |
| Yes | 21(23.6%) | 48(53.9%) | 20(22.5%) | NS |
| Depression | ||||
| No | 15 (20.5%) | 40 (54.8%) | 18 (24.7%) | |
| Yes | 30 (28.8%) | 54 (51.9%) | 20 (19.2%) | NS |
| Irritability | ||||
| No | 22 (25.6%) | 44 (51.2%) | 20 (23.3%) | |
| Yes | 23 (25.3%) | 50 (54.9%) | 18 (19.8%) | NS |
| Mood swings | ||||
| No | 13 (26.5%) | 25 (51.0%) | 11 (22.4%) | |
| Yes | 32 (25.0%) | 69 (53.9%) | 27 (21.1%) | NS |
| Anxiety | ||||
| No | 12 (21.8%) | 28 (50.9%) | 15 (27.3%) | |
| Yes | 33 (27.0%) | 66 (54.1%) | 23 (18.9%) | NS |
| Social withdrawal | ||||
| No | 19 (18.4%) | 60 (58.3%) | 24 (23.3%) | |
| Yes | 26 (35.1%) | 34 (45.9%) | 14 (18.9%) | 0.044 |
| Nausea | ||||
| No | 29 (24.8%) | 66 (56.4%) | 22 (18.8%) | |
| Yes | 16 (26.7%) | 28 (46.7%) | 16 (26.7%) | NS |
| Abdominal bloating | ||||
| No | 12 (21.8%) | 32 (58.2%) | 11 (20.0%) | |
| Yes | 33 (27.0%) | 62 (50.8%) | 27 (22.1%) | NS |
| Food craving | ||||
| No | 25 (22.1%) | 63 (55.8%) | 25 (22.1%) | |
| Yes | 20 (31.2%) | 31 (43.4%) | 13 (20.3%) | NS |
| Change in appetite | ||||
| No | 40 (25.0%) | 85 (53.1%) | 35 (21.9%) | |
| Yes | 5 (29.4%) | 9 (52.9%) | 3 (17.6%) | NS |
a Data presented as numbers and percent within parenthesis.
Figure 1.
Distribution of physical symptoms among females with premenstrual syndrome.
Figure 2.
Distribution of psychological symptoms among females with premenstrual syndrome.
4. Discussion
Premenstrual symptoms represent a wide group of physical and emotional symptoms of uncertain etiology and no definitive treatment that continues to adversely affect the quality of life and performance of a large segment of young women. These symptoms are highly underreported and years may pass before women seek medical help. The most notable finding of the current study is the very high incidence of premenstrual symptoms in young college students known with dysmenorrhoea (91.5%). The most common reported symptoms in our study of 177 young women were fatigue and mood swings at 72.9% and 72.3%, respectively. Thyse-Jacobs [27] in a review in 2000 hypothesized that disordered calciotropic hormone regulation is a major provocative factor in PMS. Calcium as a micronutrient is directly linked to the severity of the symptoms of PMS [28,29].Vitamin D and parathyroid hormone are key factors in calcium homeostasis. The issue of vitamin D and depression in general was discussed by Bertone-Johnson in 2009 [30] and the conclusion was that the evidence linking vitamin D and depression are largely circumstantial. However, low dietary vitamin D intake has been associated with the development of premenstrual symptoms [30]. In our study we found no difference in levels of vitamin D, whether normal, insufficient or deficient, between women with and those without premenstrual symptoms. Studies regarding vitamin D tested mostly the effect of supplements or dietary intake on premenstrual symptoms, where lower incidence is reported with high vitamin D intake [25,31]. This may not reflect contradictory results, however a variation in vitamin D utilization in women with and those without premenstrual symptoms reported by Tyse-Jacob in 2007 [32] suggested that variations in the levels of calcium regulating hormones all through the cycle are significant in patients with severe forms of PMS. As for parathyroid hormone, no difference in the incidence of hyperparathyroidism was detected in our subjects, while Thyse-Jacobs [33] linked transient secondary hyperparathyroidism to PMS. This difference in the results may reflect the difference in timing of blood sampling in our patients as compared to the Thyse-Jacobs study [33]. In our study the role of daily consumption of dairy products as a main source of calcium and other nutrients in relation to premenstrual symptoms failed to show any significant relation. Penland and Thyse-Jacobs [28,29] found that high calcium intake reduces the severity of premenstrual symptoms. As for each symptom, only headache and social withdrawal were significantly lower in those with high daily dairy product consumption. The strong relation between dysmenorrhoea and PMS may be related to disturbances in sex steroids production.
Limitations: The diagnosis depends on recall of symptoms rather than a prospective symptom calendar and the percentage of spontaneous anovulatory cycles in dysmenorrheic women of this study was not determined due to logistic difficulties. In addition, the menstrual cycle phase of women was not determined when the blood samples were drawn
5. Conclusions
Premenstrual symptoms rates are very high among female college students who experience dysmenorrhea. No relation between premenstrual symptoms and Vitamin D, PTH or daily dairy products consumption was revealed in this study. There is a statistically significant negative relationship between dairy products consumption and headache and social withdrawal.
Acknowledgments
The research is supported by grant (22/2010) from Deanship of Research/Jordan University of Science and Technology.
Conflict of Interest
The authors declare that they have no conflict of interest.
References and Notes
- 1.French L. Dysmenorrhea. Am. Fam. Physician. 2005;71:285–291. [PubMed] [Google Scholar]
- 2.Latthe P., Latthe M., Say L., Gülmezoglu M., Khan KS. WHO systematic review of prevalence of chronic pelvic pain: A neglected reproductive health morbidity. BMC Publ. Health. 2006;177 doi: 10.1186/1471-2458-6-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harlow SD., Campbell O.M.R. Epidemiology of menstrual disorders in developing countries: A systematic review. BJOG. 2004;111:6–16. doi: 10.1111/j.1471-0528.2004.00012.x. [DOI] [PubMed] [Google Scholar]
- 4.Tabassum S., Afridi B., Aman Z., Tabassum W., Durrani R. Premenstrual syndrome: Frequency and severity in young college girls. J. Pak. Med. Assoc. 2005;55:546–549. [PubMed] [Google Scholar]
- 5.Pinar G., Colak M., Oksuz E. Premenstrual syndrome in Turkish college students and its effects on life quality. Sex Reprod. Healthc. 2011;2:21–27. doi: 10.1016/j.srhc.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Harel Z. Dysmenorrhea in adolescents and young adults: Etiology and management. J. Pediatr. Adolesc. Gynecol. 2006;19:363–371. doi: 10.1016/j.jpag.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Freeman E.W. Premenstrual syndrome and premenstrual dysphoric disorder: Definitions and diagnosis. Psychoneuroendocrinology. 2003;28:S25–S37. doi: 10.1016/S0306-4530(03)00099-4. [DOI] [PubMed] [Google Scholar]
- 8.Hart W.G., Coleman G.J., Russell J.W. Assessment of premenstrual symptomatology: A re-evaluation of the predictive validity of self-report. J. Psychosom. Res. 1987;31:185–190. doi: 10.1016/0022-3999(87)90075-4. [DOI] [PubMed] [Google Scholar]
- 9.Futterman L.A., Rapkin A.J. Diagnosis of premenstrual disorders. J. Reprod. Med. 2006;51:S349–S358. [PubMed] [Google Scholar]
- 10.Bertone-Johnson E.R., Hankinson S.E., Johnson S.R., Manson J.E. A simple method of assessing premenstrual syndrome in large prospective studies. J. Reprod. Med. 2007;52:779–786. [PubMed] [Google Scholar]
- 11.Limosin F., Ades J. Psychiatric and psychological aspects of premenstrual syndrome. Encephale. 2001;27:501–508. [PubMed] [Google Scholar]
- 12.Reddish S. Dysmenorrhea. Aust. Fam. Physician. 2006;35:842–849. [PubMed] [Google Scholar]
- 13.Proctor M.L., Farquhar C.M. Dysmenorrhoea. Clin. Evid. 2006;6:2429–2448. [PubMed] [Google Scholar]
- 14.Banikarim C., Chacko M.R., Kelder S.H. Prevalence and impact of dysmenorrhea on hispanic female adolescents. Arch. Pediatr. Adolesc. Med. 2000;154:1226–1229. doi: 10.1001/archpedi.154.12.1226. [DOI] [PubMed] [Google Scholar]
- 15.Frackiewicz E.J., Shiovitz T.M. Evaluation and management of premenstrual syndrome and premenstrual dysphoric disorder. J. Am. Pharm. Assoc. 2001;41:437–447. doi: 10.1016/s1086-5802(16)31257-8. [DOI] [PubMed] [Google Scholar]
- 16.Cerin A., Collins A., Landgren B.M., Eneroth P. Hormonal and biochemical profiles of premenstrual syndrome. Treatment with essential fatty acids. Acta Obstet. Gynecol. Scand. 1993;72:337–343. doi: 10.3109/00016349309021108. [DOI] [PubMed] [Google Scholar]
- 17.Yonkers K.A., O’Brien P.M., Eriksson E. Premenstrual syndrome. Lancet. 2008;371:1200–1210. doi: 10.1016/S0140-6736(08)60527-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoue Y., Terao T., Iwata N., Okamoto K., Kojima H., Okamoto T., Yoshimura R., Nakamura J. Fluctuating serotonergic function in premenstrual dysphoric disorder and premenstrual syndrome: Findings from neuroendocrine challenge tests. Psychopharmacology (Berl.) 2007;190:213–219. doi: 10.1007/s00213-006-0607-9. [DOI] [PubMed] [Google Scholar]
- 19.Issa B.A., Yussuf A.D., Olatinwo A.W., Ighodalo M. Premenstrual dysphoric disorder among medical students of a Nigerian university. Ann. Afr. Med. 2010;9:118–122. doi: 10.4103/1596-3519.68354. [DOI] [PubMed] [Google Scholar]
- 20.Fujiwara T., Nakata R. Young Japanese college students with dysmenorrhea have high frequency of irregular menstruation and premenstrual symptoms. Open Med. Inform. J. 2007;1:8–11. doi: 10.2174/1874431100701010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnard N.D., Scialli A.R., Hurlock D., Bertron P. Diet and sex-hormone binding globulin, dysmenorrhea, and premenstrual symptoms. Obstet. Gynecol. 2000;95:245–250. doi: 10.1016/S0029-7844(99)00525-6. [DOI] [PubMed] [Google Scholar]
- 22.Marsden J.S., Strickland C.D., Clements M.T.L. Guanafensin as a treatment for primary dysmenorrheal. J. Am. Board Fam. Pract. 2004;17:240–246. doi: 10.3122/jabfm.17.4.240. [DOI] [PubMed] [Google Scholar]
- 23.Eccles R., Holbrook A., Jawad M. A double-blind, randomised, crossover study of two doses of a single-tablet combination of ibuprofen/paracetamol and placebo for primary dysmenorrhoea. Curr. Med. Res. Opin. 2010;26:2689–2699. doi: 10.1185/03007995.2010.525028. [DOI] [PubMed] [Google Scholar]
- 24.Abdul-Razzak K.K., Ayoub N.M., Abu-Taleb A.A., Obeidat B.A. The influence of dietary intake of dairy products on dysmenorrhea. J. Obstet. Gynaecol. Res. 2010;36:377–383. doi: 10.1111/j.1447-0756.2009.01159.x. [DOI] [PubMed] [Google Scholar]
- 25.Bertone-Johnson E.R., Chocano-Bedoya P.O., Zagarins S.E., Micka A.E., Ronnenberg A.G. Dietary vitamin D intake, 25-hydroxyvitamin D3 levels and premenstrual syndrome in a college-aged population. J. Steroid. Biochem. Mol. Biol. 2010;121:434–437. doi: 10.1016/j.jsbmb.2010.03.076. [DOI] [PubMed] [Google Scholar]
- 26.Mortola J.F., Girton L., Beck L., Yen S.S. Diagnosis of premenstrual syndrome by a simple, prospective, and reliable instrument: The calendar of premenstrual experiences. Obstet. Gynecol. 1990;76:302–307. [PubMed] [Google Scholar]
- 27.Thys-Jacobs S. Micronutrients and the premenstrual syndrome: The case for calcium. J. Am. Coll. Nutr. 2000;19:220–227. doi: 10.1080/07315724.2000.10718920. [DOI] [PubMed] [Google Scholar]
- 28.Penland J.G., Johnson P.E. Dietary calcium and manganese effects on menstrual cycle symptoms. Am. J. Obstet. Gynecol. 1993;168:1417–1423. doi: 10.1016/s0002-9378(11)90775-3. [DOI] [PubMed] [Google Scholar]
- 29.Thys-Jacobs S., Starkey P., Bernstein D., Tian J. Calcium carbonate and the premenstrual syndrome: Effects on premenstrual and menstrual symptoms. Premenstrual Syndrome Study Group. Am. J. Obstet. Gynecol. 1998;179:444–452. doi: 10.1016/S0002-9378(98)70377-1. [DOI] [PubMed] [Google Scholar]
- 30.Bertone-Johnson E.R. Vitamin D and the occurrence of depression: Causal association or circumstantial evidence? Nutr. Rev. 2009;67:481–492. doi: 10.1111/j.1753-4887.2009.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khajehei M., Abdali K., Parsanezhad M.E., Tabatabaee H.R. Effect of treatment with dydrogesterone or calcium plus vitamin D on the severity of premenstrual syndrome. J. Gynaecol. Obstet. 2009;105:158–161. doi: 10.1016/j.ijgo.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Thys-Jacobs S., McMahon D., Bilezikian J.P. Cyclical changes in calcium metabolism across the menstrual cycle in women with premenstrual dysphoric disorder. J. Clin. Endocrinol. Metab. 2007;92:2952–2959. doi: 10.1210/jc.2006-2726. [DOI] [PubMed] [Google Scholar]
- 33.Thys-Jacobs S., Alvir M.J. Calcium-regulating hormones across the menstrual cycle: Evidence of a secondary hyperparathyroidism in women with PMS. J. Clin. Endocrinol. Metab. 1995;80:2227–2232. doi: 10.1210/jc.80.7.2227. [DOI] [PubMed] [Google Scholar]