Abstract
EphAs and ephrin-As have been implicated in the morphogenesis of the developing brain. We found that EphA7 and ephrin-A5 are coexpressed in the dorsal midline (DM) of the diencephalon and anterior mesencephalon. Interestingly, programmed cell death (PCD) of the neural epithelial cells normally found in this region was reduced in ephrin-A5/ephrin-A2 dual-deficient embryos. In contrast, in vivo expression of ephrin-A5-Fc or full-length ephrin-A5 strongly induced apoptosis in neural epithelial cells and was accompanied by severe brain malformation during embryonic development. Expression of ephrinA5-Fc correlated with apoptosis of EphA7-expressing cells, whereas null mutation of ephrin-A5 resulted in the converse phenotype. Importantly, null mutation of caspase-3 or endogenous ephrin-A5 attenuated the PCD induced by ectopically overexpressed ephrin-A5. Together, our results suggest that brain region-specific PCD may occur in a region where EphAs cluster with neighboring ephrin-As through cell–cell contact.
Keywords: EphA, ephrin-A, apoptosis, early brain development
Evidence indicates that the magnitude of early elimination of neural progenitor cells is high during embryonic brain development.1, 2, 3, 4 This suggests that the total number of neurons in the brain is regulated in part by the selective elimination of stem cell progenitors. However, little is known about the signals that lead to apoptosis of proliferating neural progenitors. The number of cells in an organ is regulated by mitogenic or survival factors impinging on intrinsic determinants of cell proliferation and apoptosis.5, 6, 7 In addition, active and tightly regulated ‘suicidal' cell death is likely to serve as an efficient means to control the number of cells. Intuitively, the delicate balance between cell survival and death signals may determine the outcome for an individual neural progenitor, thereby adjusting the progenitor pool needed for proper brain development.3 On the other hand, cell–cell interactions are important for tissue organization and homeostasis, and they may be implicated in the morphogenesis of the developing brain.5, 8 For example, cell–cell contacts allow aggregation of various cell surface receptors and their ligands, thereby producing complex and sophisticated cellular responses. Therefore, it is quite interesting to see how apoptotic functions are regulated to affect the number of neural progenitors in the brain region in which cell–cell contacts aggregate cell surface receptors and their ligands during early brain development.
Erythropoietin-producing hepatocellular (Eph) receptor tyrosine kinases (RTKs) and their ephrin (Eph receptor interacting proteins) ligands are crucial for cell contact-dependent signaling, leading to various biological outcomes such as adhesion versus repulsion or increased versus decreased motility, which are dependent on cell context.9, 10, 11, 12 Accordingly, their critical roles have been found not only during developmental processes but also in the normal physiology and homeostasis of adult organs.13 However, Eph/ephrin interactions are not known to elicit a mitogenic response, and regulation of cell adhesion or motility has been considered their major function.13, 14 Nevertheless, evidence suggests that they are involved in either direct or indirect regulation of cell proliferation depending on certain biological contexts. For example, infusion of the ectodomain of EphB2 or ephrin-B2 into the lateral ventricle disrupts migration of neuroblasts but also increases cell proliferation in the subventricular zone.15 Infusion of EphA7-Fc or ephrin-A2-Fc results in an increase in cell proliferation in the lateral ventricle wall.16 Similarly, cells in the subventricular zone were shown to proliferate more and have a shorter cell cycle in ephrin-A2−/−. These observations indicate that Ephs/ephrins regulate neural progenitor proliferation in the neural stem cell niche, although it is unclear whether the Fc-fusion proteins acted as agonists or antagonists. Interestingly, ectopic expression of ephrin-A5 in cortical progenitors expressing EphA7 leads to apoptosis, resulting in a decrease in cortical size, whereas opposite phenotypes are observed in EphA7−/−.17 More recently, Eph receptor signaling was shown to promote germ-cell death in Caenorhabditis elegans.18 These suggest that Eph/ephrin signaling may be a physiological trigger for an apoptotic cascade that regulates the number of neural progenitors or germ cells during embryonic development. The difference that Ephs/ephrins exert an antiproliferative effect on adult neural progenitors but a pro-apoptotic effect on embryonic cortical progenitors is noteworthy. Interestingly, some reports have revealed that Ephs/ephrins interact with tropomyosin receptor kinase (Trk) receptors and p75 neurotrophin receptor (p75NTR).19, 20, 21 Therefore, the pleiotrophic functions of Ephs and ephrins, depending on their cellular context, may originate in their capacity to interact with diverse cell surface receptors such as death receptors or neurotrophin receptors.
Caspases play a central role in coordinating cell proliferation and apoptosis during early neurogenesis and have been implicated in the development and evolution of the mammalian brain.22 Most caspase-3 and caspase-9 null mice are embryonic lethal, display an excencephalic defect, and show decreased neural progenitor apoptosis.23, 24, 25, 26 In addition, studies with Fas-associated death domain (FADD) or caspase-8 null mice showed that FADD is essential for brain development in addition to a role in Fas-mediated apoptosis in the immune system.27, 28, 29, 30, 31 Recently, it was shown that these apoptotic components have a role in cleaving the necrosome and that a balance between death receptor-mediated apoptosis and necrosis is critical for embryonic development.32, 33 However, little is known about the extrinsic cues activating cell death receptors or caspase cascade to trigger neuronal progenitor apoptosis in a specific brain region. Importantly, Ephs and ephrins have been shown to be pro-apoptotic through caspases in certain cellular contexts. EphA2 is required for ultraviolet-induced apoptosis, and caspase-8 is essential in this apoptotic pathway.34 These suggest that the pro-apoptotic effect of EphA/ephrin-A may be linked to the activation of the caspase cascade. However, much less attention has been paid to earlier cell death events affecting neural epithelial cells (NECs) where Ephs/ephrins are abundantly expressed and their signaling may be linked to caspase activation in a brain region-specific manner.
The dorsal midline (DM) of the diencephalon and anterior mesencephalon contains NECs that coexpress EphA7 and ephrin-A5.35 In this region, some cells transmit robust and persistent Eph/ephrin signaling by clustering at sites of cell contact if coexpressed Ephs and ephrins segregate into distinct domains in the same cell and mainly engage in trans-interactions through cell–cell contact. A critical issue is whether this persistent Eph/ephrin signaling plays a role in inducing programmed cell death (PCD) of NECs during early brain development. Our results presented in this report support this hypothesis.
Results
Absence of ephrin-A2 and ephrin-A5 reduces PCD in the DM of the diencephalon and anterior mesencephalon
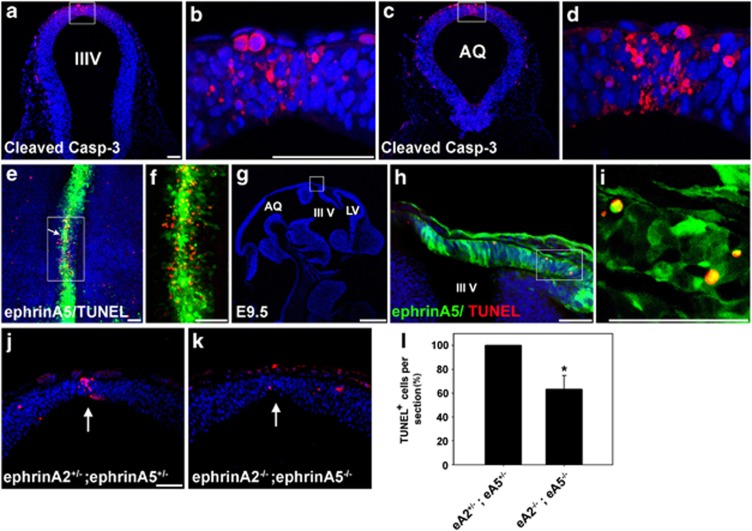
After the apposing neural folds have contacted and adhered to each other, many DM NECs undergo apoptosis.36 We found that a small number of DM cells in the diencephalon or anterior mesencephalon of E9.5 mouse embryos displayed activated caspase-3 (Figures 1a–d), suggesting that these cells undergo region-specific apoptosis. Interestingly, some of the ephrin-A5-expressing cells in the DM appeared to be colocalized with the apoptotic NECs, which were detected using TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) staining (Figures 1e–i). Importantly, a 0.4-fold decrease in TUNEL labeling was detected in the DM of ephrin-A2−/−;ephrinA5−/− embryos compared with that of ephrin-A2+/−;ephrin-A5+/− embryos (Figures 1j–l). Together, these data suggest that EphA/ephrin-A signaling is implicated in region-specific PCD, in particular in the DM during early brain development.
Figure 1.
PCD is observed in the DM of the diencephalon and anterior mesencephalon. (a and c) Transverse sections of an E9.5 embryo stained with anti-activated caspase-3 antibody. (b and d) Higher magnification photographs of the DM region in (a) and (c), respectively. IIIV, third ventricle; AQ, cerebral aqueduct. (e) Whole-mount TUNEL staining of DM explants of the diencephalon and anterior mesencephalon. An explant was dissected out of the E9.5 embryo of the ephrinA5-GFP BAC transgenic line. This embryo expresses GFP under the ephrin-A5 promoter as described previously. The arrow shows the region between the diencephalon and mesencephalon. (f) High-magnification photograph of the region marked by the box in (e). (g) Sagittal section of an E11.5 embryo from the ephrin-A5-GFP BAC transgenic line. Frozen sections were further subjected to TUNEL staining followed by DAPI staining. LV, lateral ventricle. Anterior is toward the right. (h) High-magnification photograph of the DM region corresponding to the box in (g). (i) High-magnification photograph of the region corresponding to the box in (h). (j and k) Coronal sections of E9.5 littermate embryos of the indicated genotype were stained with the TUNEL assay. The embryos were generated by crossing heterozygous ephrin-A2+/− and heterozygous ephrin-A5+/− mice. (l) The data in (j) and (k) were quantified by counting the number of TUNEL-positive cells in each coronal section. Data represent the mean±S.E. for six independent experiments in which a pair of littermate embryos with similar brain sizes was used for serial 5 μm sections (eA2−/−;eA5−/− (n=6), eA2+/−;eA5+/− (n=6)). Twenty sections surrounding the mesencephalic and diencephalic boundary were counted for each experiment. Only TUNEL-positive cells present in the DM were counted and normalized to the number of sections. The number of TUNEL-positive cells in a double heterozygous embryo for ephrin-A2 and ephrin-A5 was considered ‘100% apoptosis' in each experimental set. *P<0.001. Scale bars=50 μm (a, b, e, f, h, i, and j) and 500 μm (g)
EphA7 and ephrin-A5 are coexpressed in the DM of the diencephalon and anterior mesencephalon
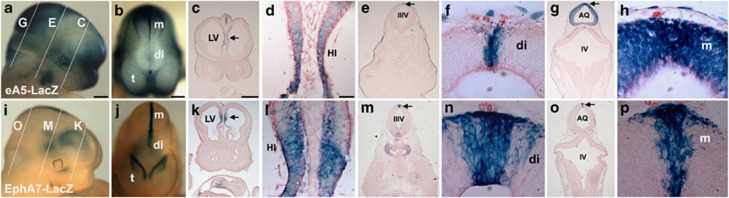
To investigate the pro-apoptotic roles of EphA/ephrin-A signaling along the DM of the developing embryo, we examined expression patterns of EphAs and ephrin-As using fusion proteins comprising the extracellular region of ephrin-A5 or EphA7 and the Fc portion of IgG (Supplementary Figure 1). Consistent with previous observations,37 detection of EphAs by staining for ephrin-A5-Fc was prominent in the dorsal anterior midbrain and the dorsal diencephalon (Supplementary Figure 1A–C). In contrast, the detection of ephrin-As by staining for EphA7-Fc was strong in the dorsal posterior midbrain (Supplementary Figure 1D–F). These suggest that various EphA and ephrin-A members are abundantly expressed in NECs during early brain development. However, this technique may not reveal a specific binding pattern at the DM of the diencephalon and anterior mesencephalon because Ephs/ephrins are largely bound to each other at sites of cell–cell contact and masked from the soluble fusion protein.37, 38 To examine the expression patterns of ephrin-A5 or EphA7 along the DM, we generated bacterial artificial chromosome (BAC) transgenic lines in which the ephrin-A5 or EphA7 promoter drives expression of the LacZ reporter, as described (Supplementary Figure 1G).39, 40 Consistent with previous studies, X-gal (X-gal, 5-bromo-4-chloro-3-indolyl-β-𝒟-galactopyranoside) staining of eA5-LacZ BAC embryos at E11.5 revealed that LacZ expression recapitulated the expression pattern of ephrin-A5 in brain regions such as the posterior midbrain, the DM of the diencephalon, and the medial region of the posterior telencephalon (Figures 2a and b). Similarly, reporter expression of the EphA7-LacZ BAC embryo is consistent with a previous report showing that EphA7 is expressed in the DM of the diencephalon and the lateral region of the posterior telencephalon (Figures 2i and j). Interestingly, our expression analysis using BAC transgenic embryos revealed partial overlap in expression between EphA7 and ephrin-A5, including in the hippocampal region (Figures 2c, d), the DM of the diencephalon (Figures 2e, f), and the DM of the anterior midbrain (Figures 2g, h). These suggest that at sites of cell–cell contact in the DM, Ephs and ephrins are activated, and thus their coexpression may be involved in the regulation of PCD of the NECs in this region.
Figure 2.
Coexpression of EphA7 and ephrin-A5 in the DM of the diencephalon and anterior mesencephalon. Whole-mount X-gal staining was performed using E11.5 mouse embryos. (a and b) Lateral and dorsal views, respectively, of the ephrin-A5 BAC transgenic embryo. (c, e, and g) Coronal sections corresponding to the lines in (a). (d, f, and h) High-magnification photographs of the regions indicated by the arrows in (c), (e), and (g), respectively. (i and j) Lateral and dorsal views, respectively, of the EphA7 BAC transgenic embryo. (k, m, and o) Coronal sections corresponding to the lines in (i). (l, n, and p) High-magnification photographs of the regions indicated by the arrows in (k), (m), and (o), respectively. di, diencephalon; m, mesencephalon; HI, hippocampus; LV, lateral ventricle; IIIV, third ventricle; AQ, cerebral aqueduct; IV, fourth ventricle. Scale bars=500 μm (a–c) and 50 μm (d)
In vivo expression of ephrin-A5-Fc results in increased apoptosis of NECs
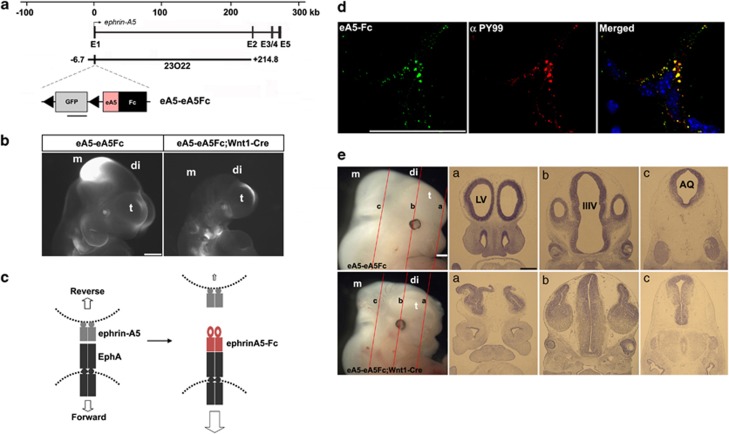
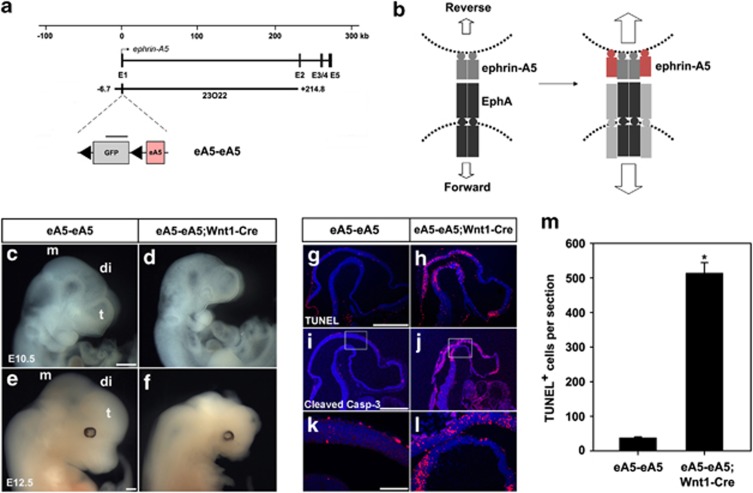
To investigate the pro-apoptotic role of the persistent activation of EphA signaling in the DM, we expressed ephrin-A5-Fc in the mouse embryo using the ephrin-A5 BAC that contains all the necessary genetic information to recapitulate the expression pattern of ephrin-A5.39 For this purpose, a targeting vector consisting of floxed green fluorescent protein (GFP) and ephrin-A5-Fc was inserted into the first exon of ephrin-A5 BAC in which only GFP expression occurs because of stop signals between GFP and ephrin-A5-Fc (Figure 3a). Transgenic mice carrying a recombinant BAC, eA5-eA5Fc, were crossed with Wnt1-Cre to induce specific expression of ephrin-A5-Fc in the DM of the diencephalon and mesencephalon (Figure 3b). As expected, GFP signals in double-transgenic embryos disappeared in the diencephalon and mesencephalon, whereas GFP continued to be expressed at lower levels in the medial region of the telencephalon (Figure 3b). However, overall GFP signals in the telencephalon of double-transgenic embryos were slightly reduced compared with those of control embryos. Region-specific apoptosis was also observed in the telencephalon at slightly earlier developmental stages such as E9.0 (Supplementary Figure 2A–D). Furthermore, the Wnt1-driven Cre activity was detected in the telencephalon of the Rosa26 reporter mouse (Supplementary Figure 2E–G). This suggests that Wnt1-Cre induces low-level expression of ephrin-A5-Fc in the telencephalon. In these transgenic embryos, ephrin-A5-Fc would be secreted from ephrin-A5-expressing cells and bind to their neighboring cells expressing EphAs (Figure 3c). One potential outcome was that the bound ephrin-A5-Fc would stimulate forward signaling through EphAs (Figure 3c). Consistently, dissociated neural progenitors prepared from the dorsal diencephalon displayed clustered labeling of the ephrin-A5-Fc-binding sites and they were colocalized with phosphotyrosine immunoreactivity, suggesting that ephrin-A5-Fc elicits the activation of EphA receptors in neural progenitors (Figure 3d). In addition, affinity probe detection using ephrin-A5-Fc indicated that ∼10% of EphA-expressing cells expressed ephrin-A5 (visualized by GFP signal; Supplementary Figure 3A) and that these cells expressing both EphAs and ephrin-A5 showed tyrosine phosphorylation of EphA receptors in response to ephrin-A5-Fc (Supplementary Figure 3B). However, immunofluorescence staining revealed a non-overlapping subcellular pattern of EphAs and ephrin-A5 in the majority of neural progenitors coexpressing these proteins, suggesting that coexpressed EphAs and ephrin-As are partially segregated and can be independently activated in trans (Supplementary Figure 3C). Strikingly, transgenic embryos expressing ephrin-A5-Fc under Wnt1-driven Cre displayed severe brain malformation during embyonic development and reduced brain size (Figure 3e and Supplementary Figure 4). Sections of eA5-eA5Fc;Wnt1-Cre embryonic brain revealed that the brain ventricles became stenosed and that the remaining neuroepithelial structures were thicker (Figure 3e).
Figure 3.
Severe brain malformation in mouse embryos expressing ephrin-A5-Fc. (a) Schematic map of the ephrin-A5 genomic locus with the ephrin-A5 BAC clone (RP23-23O22). The modified ephrin-A5 BAC clone, eA5-eA5Fc, is identical to the original ephrin-A5 BAC clone, except that it contains a floxed GFP followed by an ephrin-A5-Fc expression cassette inserted upstream into the translation start codon in the first exon of ephrin-A5. (b) GFP fluorescent image of the indicated transgenic embryo at E10.5. GFP images were compared after littermate embryos were obtained from crossing eA5-eA5Fc BAC transgenic mice with Wnt1-Cre mice. Three different transgenic lines were generated with the same result. (c) Schematic diagram showing that soluble ephrin-A5-Fc is secreted from ephrin-A5-expressing cells and that they bind to and activate EphA receptors in the neighboring NECs. (d) In vitro-dissociated NECs were cultured and treated with soluble ephrin-A5-Fc (unclustered) for 30 min at 37°C. Cells were fixed and immunostained using anti-phosphotyrosine antibody (αPY99) and anti-human IgG conjugated to Alexa488. (e) Comparison of E12.5 littermate embryos from a cross between eA5-eA5Fc BAC transgenic mice and Wnt1-Cre mice. Similar results were consistently observed in three different transgenic lines when each line was mated with Wnt1-Cre mice. (a–c) Coronal sections corresponding to each line shown in E12.5 embryos were stained using cresyl violet. t, telencephalon; di, diencephalon; m, mesencephalon; LV, lateral ventricle; IIIV, third ventricle; AQ, cerebral aqueduct. Scale bars=500 μm (b, e) and 50 μm (d)
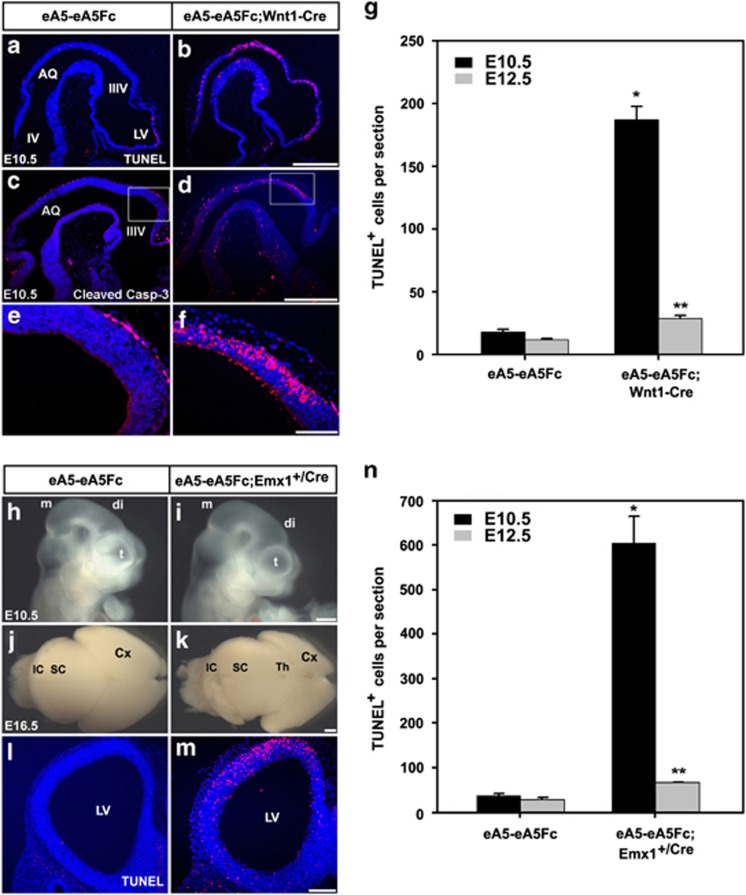
To explore the mechanism by which expression of ephrin-A5-Fc leads to abnormal brain development, we analyzed apoptosis of NECs. Interestingly, the TUNEL assay and immunohistochemical staining for activated caspase-3 revealed that apoptotic cells increased at least 10-fold in ephrin-A5-Fc embryos at E10.5 (Figures 4a–g). At later embryonic stages, such as E12.5, apoptosis in ephrin-A5-Fc;Cre embryos decreased compared with that at E10.5, but was significantly higher than that in wild-type littermates (Figure 4g). This decrease in apoptotic cells observed in E12.5 embryos may result from the engulfment process occurring at the earlier developmental stages. However, the mitotic rate at E10.5 was not different between wild-type littermates and ephrin-A5-Fc;Cre embryos (Supplementary Figure 5). Furthermore, measurement of the cell cycle exit index revealed that the differentiation rate of NECs in ephrin-A5-Fc embryos was not different from that of wild-type littermates (Supplementary Figure 6).
Figure 4.
Increased apoptosis in mouse embryos expressing ephrin-A5-Fc. To compare cell death in the cephalic region of E10.5 embryos according to genotype, sagittal sections were stained with the TUNEL assay (a and b) or anti-activated caspase-3 antibody (c and d). (e, f) High magnification of the dorsal diencephalic region corresponding to the boxes in (c) and (d), respectively. (g) The data in (a) and (b) were quantified by counting the number of TUNEL-positive cells in each sagittal section. Similar experiments were performed using E12.5 littermate embryos for quantitation (data not shown). Data represent the mean±S.E. of five independent experiments in which eight serial sections (5 μm) flanking the DM were counted for each condition. Only TUNEL-positive cells in the dorsal part of the mesencephalic and diencephalic regions were counted. *P<0.001; **P<0.01. (h–n) Littermate embryos from crossing eA5-eA5Fc BAC transgenic mice with Emx1+/Cre mice were analyzed with TUNEL staining. Lateral view of an E10.5 embryo (h and i) and dorsal view of an E16.5 embryonic brain (j and k). Note that the size of the cortical region was much smaller in the compound transgenic line expressing ephrin-A5-Fc in cortical progenitors. (l and m) Coronal sections of E10.5 embryos of the indicated genotype were stained with the TUNEL assay for comparison of cell death in the telencephalic region. (n) Experiments were performed essentially as described in (g) of this figure. Data represent the mean±S.E. for at least five independent experiments with eight sections counted for each condition. *P<0.001; **P<0.01. Scale bars=500 μm (b, d, i, and k) and 100 μm (f and m)
We examined whether expression of ephrin-A5-Fc induced apoptosis in other regions such as the telencephalon. For this purpose, eA5-eA5Fc mice were crossed with Emx1-Cre so that ephrin-A5-Fc was expressed in the dorsal medial region of the caudal telencephalon. As expected, the size of the telencephalon was reduced in ephrin-A5-Fc embryos, whereas the size of other brain regions including the diencephalon and mesencephalon was not (Figures 4h–k). Accordingly, apoptosis was increased ∼20-fold and ∼2.5-fold in telencephalic NECs of eA5-eA5Fc;Emx1-Cre embryos at E10.5 and E12.5, respectively (Figures 4l–n).
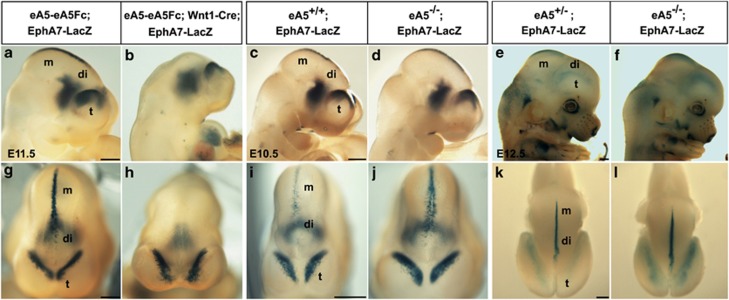
To analyze whether EphA-receptor-expressing cells undergo apoptosis in response to the expression of ephrin-A5-Fc, ephrin-A5-Fc mice were crossed with mice carrying EphA7-LacZ BAC, which recapitulates the endogenous expression pattern of EphA7, in particular at the DM of the diencephalon and mesencephalon as well as the lateral region of the caudal telencephalon (Figure 4a). Consistently, EphA7-LacZ BAC embryos showed complete loss of LacZ expression in the DM when expression of ephrin-A5-Fc was induced by Wnt1-Cre (Figures 5b and h). In contrast, LacZ expression in the telencephalon and ventral diencephalon was not altered. EphA7-LacZ;ephrin-A5+/− mice were crossed with ephrin-A5+/− mice, and apoptosis (indicated by a decrease in the number of LacZ-expressing cells) was analyzed in their progeny (Figures 5c–f and i–l). Interestingly, in ephrin-A5−/− embryos, the number of LacZ-expressing cells increased in the diencephalon and the caudal telencephalon, suggesting that EphA7-expressing cells undergo less apoptosis in the absence of ephrin-A5. Consistent with this, the majority of ephrin-A5−/− mutants showed an increase in the size of the tissue neighboring the DM (Supplementary Figure 7).
Figure 5.
The number of EphA7-expressing cells in the DM was inversely correlated with the expression level of ephrin-A5. Whole-mount X-gal staining was performed with littermate embryos with the indicated genotype obtained from each mating described below. (a and b) Lateral views of E11.5 embryos (n=6) from a cross between the eA5-eA5Fc;EphA7-LacZ transgenic line and the Wnt1-Cre transgenic line. (c–f) Lateral views of E10.5 (c and d) or E12.5 embryos (e and f) from a cross between eA5+/−;EphA7-LacZ mice and eA5+/− mice (eA5+/+;EphA7-LacZ at E10.5 (n=6), eA5−/−;EphA7-LacZ at E10.5 (n=8), eA5+/+;EphA7-LacZ at E12.5 (n=5), and eA5−/−;EphA7-LacZ at E12.5 (n=7)). (g–l) Dorsal views of the embryos shown in the top panels. t, telencephalon; di, diencephalon; m, mesencephalon. Scale bar=500 μm
Caspase-3 activation is required for ephrin-A5-induced PCD during early brain development
We examined whether overexpression of the membrane-tethered full-length ephrin-A5 triggered pro-apoptotic pathways in neural progenitors as we observed in ephrin-A5-Fc embryos. For this purpose, a targeting vector consisting of a floxed GFP and an ephrin-A5 cDNA was inserted into the ephrin-A5 BAC to generate an eA5-eA5 recombinant BAC (Figure 6a). In embryos overexpressing full-length ephrin-A5, ephrin-A5 tethered to the cell surface would transduce not only forward signaling through EphA receptors but also reverse signaling through ephrin-A5 itself (Figure 6b). Wnt1-Cre-mediated overexpression of ephrin-A5 also caused a drastic reduction in the size of the diencephalon and mesencephalon (Figures 6d and f). Similarly, the TUNEL assay and immunohistochemical staining revealed that severe apoptosis occurred in the diencephalic and mesencephalic tissues in eA5-eA5;Wnt1-Cre embryos (Figures 6h, j, l, and m).
Figure 6.
Severe brain malformation and enhanced apoptosis are observed in mouse embryos overexpressing full-length ephrin-A5. (a) Experiments were performed essentially as described for Figure 3a, except that full-length human ephrin-A5 cDNA was used instead of ephrin-A5-Fc. (b) Schematic diagram showing that human ephrin-A5 is expressed in mouse cells that endogenously express ephrin-A5 and that their membrane tethering subsequently elicits bidirectional signaling between two apposing NECs. (c–f) Littermate embryos at the indicated developmental stages were obtained from crossing eA5-eA5 BAC transgenic mice with Wnt1-Cre mice. Note that severe malformation of the cephalic region was evident in embryos overexpressing ephrin-A5 under control of Wnt1-Cre. The same results were observed in three different lines. (g–l) Apoptotic analysis using the embryos shown in (c) and (d), respectively. Sagittal sections were stained with the TUNEL assay (g and h) or anti-activated caspase-3 antibody (i and j) for comparison of cell death. (k and l) High magnification of the brain regions corresponding to the boxes in (i) and (j), respectively. (m) The data in (g) and (h) were quantified by counting the number of TUNEL-positive cells in each sagittal section as described in Figure 4g. Data represent the mean±S.E. for five independent experiments with eight sections (5 μm) counted for each condition. *P<0.001. t, telencephalon; di, diencephalon; m, mesencephalon; LV, lateral ventricle; IIIV, third ventricle; AQ, cerebral aqueduct. Scale bars=500 μm (c, e, g, and i) and 100 μm (k)
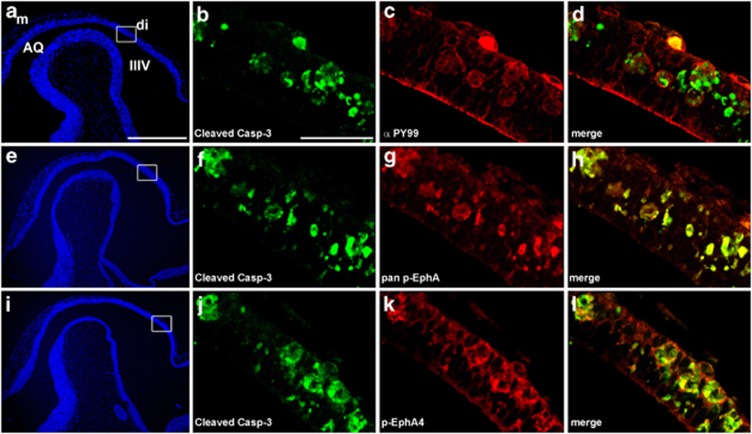
We next asked if EphA receptors in the apoptotic cells of eA5-eA5;Wnt1-Cre embryos are tyrosine phosphorylated. For this purpose, we used a phosphotyrosine antibody, phospho-EphA4 antibody, or pan-phospho-EphA antibody to examine colocalization with anti-activated caspase-3 antibody labeling. As shown in Figure 7, p-Eph receptors were detected in the apoptotic cells, suggesting that the membrane-tethered ephrin-A5 overstimulates forward signaling through EphAs and that this signaling process is linked to pro-apoptotic signaling.
Figure 7.
EphA receptors are strongly tyrosine phosphorylated in mouse embryos overexpressing full-length ephrin-A5. Sagittal sections were prepared from E10.5 embryos overexpressing ephrin-A5 under the control of Wnt1-Cre, and then stained with anti-activated caspase-3 antibody (green) and anti-pTyr antibody (a–d), anti-pan p-EphA antibody (e–h), or anti-p-EphA4 antibody (i–l). Note that the apoptotic cells stained with anti-activated caspase-3 show irregular cell morphology and a high level of tyrosine-phosphorylated EphA receptors. Scale bars=500 μm (a) and 50 μm (b)
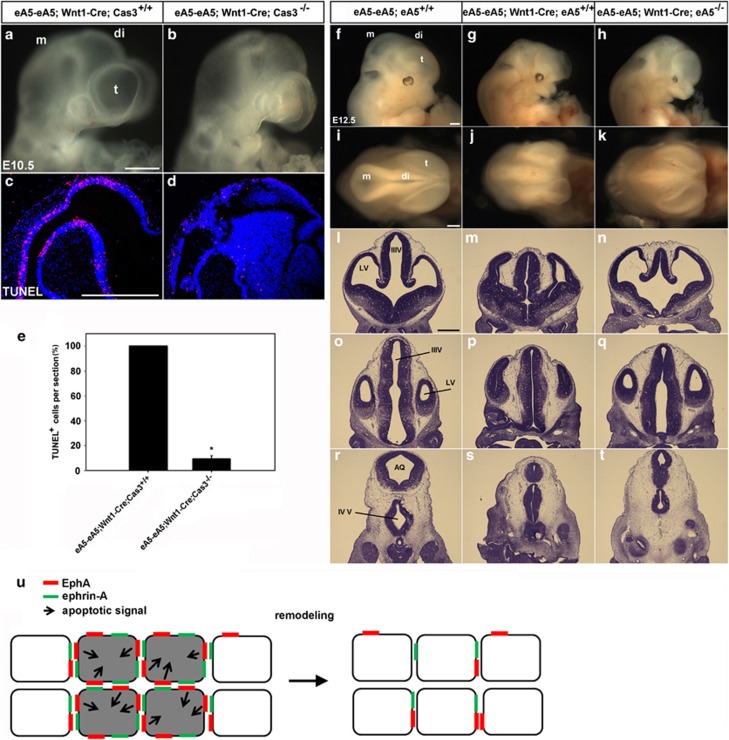
Because our data suggest that caspase-3 is activated in the EphA/ephrin-A-induced pro-apoptotic pathway, we generated a transgenic line in which Wnt1-Cre mediated overexpression of ephrin-A5 in caspase-3−/− embryos (Figures 8a–e). Such an apoptotic cell death phenotype was drastically diminished in these transgenic lines, although normal brain development was still impaired, which was likely because of the null mutation of the caspase-3 gene in other brain regions (Figures 8b and d). Importantly, fewer morphological defects in the telencephalon were observed in eA5-eA5;Wnt1-Cre;ephrin-A5−/− embryos compared with eA5-eA5;Wnt1-Cre embryos (Figures 8h, k). This result suggests that developmental defects in the telencephalon were rescued by null mutation of endogenous ephrin-A5, which was likely because of a low level of ectopic ephrin-A5 expression induced by Wnt1-Cre in the telencephalon. However, severe brain defects were observed in the diencephalon and mesencephalon of eA5-eA5;Wnt1-Cre;ephrin-A5−/− embryos (Figures 8q and t), suggesting that a high level of ectopic ephrin-A5 expression in this region is not easily compromised by the loss of endogenous ephrin-A5. Nevertheless, our data suggest that the level of Eph/ephrin signaling in a certain brain region regulates apoptotic death of NECs for proper development of the brain.
Figure 8.
Partial rescue of ephrin-A5-induced apoptosis and the brain defect by null mutation of caspase-3 or ephrin-A5. (a and b) E10.5 littermate embryos were obtained from crossing eA5-eA5;Cas3+/− mice with Wnt1-Cre;Cas3+/− mice. (c and d) TUNEL staining of sagittal sections from the embryos shown in (a) and (b), respectively. (e) Experiments were performed essentially as described in Figure 4g. Data represent the mean±S.E. for five independent experiments with 15 sections counted for each condition (eA5-eA5;Wnt1-Cre;Cas3+/+ at E10.5 (n=5), eA5-eA5;Wnt1-Cre;Cas3−/− at E10.5 (n=5)). *P<0.001. (f–h) Littermate embryos with the indicated genotype were obtained from crossing eA5-eA5Fc;eA5+/− mice with Wnt1-Cre;eA5+/− mice (eA5-eA5;Wnt1-Cre;ephrin-A5−/− (n=5)). (i–k) Dorsal views of the embryos shown in (f), (g), and (h), respectively. (l–t) Coronal sections corresponding to each line in (i–k) were stained with cresyl violet. (u) Model in which ephrin-A transduces apoptotic signaling into EphA-expressing cells at the site of cell–cell contact. Apoptotic signaling may be intense in a brain region where the coexpressed EphA and ephrin-A proteins segregate into distinct domains in the same cell, but they are also mainly engaged in trans-interactions through cell–cell contact. In this model, we hypothesize that the activated EphA receptors may interact with the apoptotic signaling cascade and that this cross-talk may occur in a specific brain region such as DM neural epithelial tissue coexpressing EphAs and ephrin-As. As a result, some NECs (represented by the gray color in this diagram) would be eliminated from this region, which may be a basis for the neuroepithelial tissue remodeling that occurs during early brain development. Scale bar=500 μm
Discussion
An important feature of Eph RTKs and ephrins is that their binding specificity is promiscuous: ephrin-As bind to all EphAs, whereas ephrin-Bs bind to all EphBs.37 Additional studies have indicated that the known repertoire of Eph/ephrin interactions can be expanded and that the actual degree of overlap between cognate Ephs and ephrins is larger than previously thought.12, 41 Consistently, it is not surprising to see that mutant mice that are null for single Eph or ephrin gene have no obvious morphological abnormalities, although these molecules are abundantly expressed in the developing brain.42 In contrast to the well-known complementarity of Eph and ephrin expression domains, some cells in the developing brain coexpress both Eph receptors and their cognate ligands. EphA7 is coexpressed with ephrin-A5 in the DM of the diencephalon and anterior mesencephalon.35 Ephrin-A5 in this DM was previously postulated to participate in cell adhesion because activation of full-length EphA7 is impaired by a truncated form of EphA7. This plausible mechanism explains why neural tube closure defects (NTCDs) are seen in ephrin-A5−/− embryos. However, we have not observed any NTCDs in ephrin-A5−/−, which have been crossed onto the C57BL/6 background. In fact, we had postulated that in vivo expression of ephrin-A5-Fc is a powerful tool to disrupt the adhesive interaction between EphA7 and ephrin-A5, resulting in more severe NTCDs. On the contrary, we found that no embryos expressing ephrin-A5-Fc in the DM showed NTCDs. The same results were observed in embryos expressing full-length ephrin-A5. Rather than NTCD, a gain of function of EphA signaling consistently induced a trigger for apoptosis, suggesting that Eph/ephrin signaling plays a role in determining the size of the NEC population. Importantly, the number of EphA7-expressing cells in the DM was increased in ephrin-A5−/− embryos. Based on our findings, a possible explanation for the previously reported NTCDs in ephrin-A5−/− is that the expanded number of NECs in the DM may influence the precise control of neural tube closure (NTC) and that their effects on NTC may depend on the genetic background of the mice.
An important issue is how the ephrin-A5-Fc ectopically expressed in transgenic embryos functions in the brain regions where EphAs and ephrin-As are coexpressed. Evidence suggests that EphA receptors are silenced through a cis-interaction with ephrin-As.43, 44 This cis-interaction appears to impair tyrosine phosphorylation of EphA receptors, thereby decreasing their sensitivity for ephrin-As in trans, an effect that was shown to be critical for retinocollicular mapping.43 If this hypothesis is correct, cis-interactions between EphAs and ephrin-As would provide a mechanism for NECs to survive through blocking their interaction with neighboring ephrin-As in trans. In our transgenic model, this cis-interaction between EphAs and ephrin-As would be disrupted by ectopically expressed ephrin-A5-Fc, leading to opposite phenotype such as activation of the pro-apoptotic pathway. Although we have not ruled out this possibility, some of our evidence is not consistent with this hypothesis. For example, some neural progenitors from the diencephalon coexpressed both EphA and ephrin-A5, and EphA receptors did not colocalize with ephrin-A5 in the same cell. Furthermore, expression of membrane-tethered ephrin-A5 resulted in increased numbers of apoptotic cells with a high level of tyrosine-phosphorylated EphA receptors, a phenomenon that can be better explained by trans-interactions. Therefore, our findings are more consistent with the hypothesis that coexpressed Eph and ephrin proteins segregate into distinct domains from which they signal distinct cellular effects, thereby allowing the utilization of both Ephs and ephrins as distinct receptors in the same cell.45 In this regard, we propose that the coexpressed EphA and ephrin-A proteins in brain regions such as the DM are engaged in trans-interactions through cell–cell contact to trigger pro-apoptotic signaling pathways (Figure 8u).
Clustering of ephrins through membrane attachment is important for the activity of Eph receptors.46 One question is whether ephrin-A5-Fc, a dimerized form of the ectodomain, can trigger signaling in Eph-expressing cells in the absence of clustering. Our immunohistochemical staining indicated that treatment of unclustered ephrin-A5-Fc could aggregate and activate EphA receptors in dissociated neural progenitors. This suggests that ephrin-A5-Fc expressed in vivo is likely to act as a conventional soluble factor similar to epidermal growth factor (EGF) or nerve growth factor (NGF). Interestingly, we observed that overexpression of ephrin-A5 in vivo consistently caused more severe brain malformation and apoptosis in mice, which can be explained by either a more potent signaling function because of membrane tethering or simultaneous bidirectional apoptotic signaling activation. Whether EphA7-Fc is able to aggregate ephrin-As and induce the caspase-3-dependent apoptotic pathway in vivo remains to be determined.
In general, signaling through Eph receptors is known to regulate contact-mediated repulsion in the nervous system.9, 11, 12 In the hindbrain, ectopic expression of Eph in the ephrin-expressing segment can cause Eph-expressing cells to sort out toward boundaries away from the ephrin-expressing cells.47, 48 This suggests that Eph/ephrin-mediated apoptosis is not induced during certain developmental processes such as cell sorting and establishing tissue boundaries in the hindbrain. Therefore, it is tempting to speculate that Eph/ephrin-triggered cell death may be a phenomenon unique to some NECs and that it provides a key mechanism of regulating their population size during formation of a specific brain tissue. A key issue is how Eph receptors or ephrins trigger apoptosis. Two plausible mechanisms may be considered. First, EphA receptors may cross-talk with death receptors such as tumor necrosis factor receptor 1 (TNFR-1) or Fas. Ephs/ephrins cross-talk with other cell surface receptors such as ErbB2,49 TrkB,20 and p75NTR.19 This possibility is supported by our preliminary findings that TNFR-1 or death receptor 5 (DR5) associate with EphA7 in 293 cells and that their interaction is critical for activating caspase-3. However, whether these death receptors are coexpressed with EphA7 in vivo or if their interaction is required for PCD of NECs during brain development is not yet clear. Second, Eph receptors may interact with caspase-8. Previous studies suggest that caspase-8-dependent signaling may play a role in the EphA-mediated cell death pathway, although its role in the developing brain has not been determined. Determining whether caspase-8 is required for EphA7-mediated PCD in the DM will be interesting. Whatever pro-apoptotic mechanism Eph/ephrin signaling mediates, it is interesting to note that region-specific expression of ephrin-A5-Fc or full-length ephrin-A5 somehow induced massive apoptosis and brain malformation. Two plausible explanations could be considered for these effects. First, the promiscuous binding capacity of ephrin-A5 may play a role in inducing massive apoptosis. For example, ephrin-A5 is likely to interact with various Eph receptors in the neighboring cells, subsequently inducing simultaneous activation of multiple Eph/ephrin signaling in the surrounding brain tissue. This effect is likely to be a cause for massive apoptosis, which would not be expected if ephrin-A5 was bound to only one member of Eph receptors. Second, massive apoptosis may play a mechanical role in inducing severe brain malformation. It has been known that endogenous apoptosis plays a role in shaping the normal embryonic brain. However, when massive apoptosis occurs in the embryonic brain, the entire tissue integrity is disrupted because the neighboring cells fill the space originally occupied by the dying cells, and this force may in turn change overall tissue tension and morphology. This excessive sculpting of the embryonic brain may be a reason for the severe malformation of brain morphology.
PCD may be a key process required for proper development of the nervous system. One question is why NECs in the DM of the developing brain undergo PCD. One hypothesis is that PCD occurs in the DM for remodeling of the epithelia and that this process is essential for eliminating the continuity between NECs and the surface ectoderm on each side after the apposing neural folds have contacted each other for fusion.36 Consistent with this hypothesis, inhibition of this DM remodeling in the chicken embryo was shown to prevent NTC.50 However, a recent study showed that apoptosis is not required for NTC in the mouse embryo.51 Alternatively, the DM of the developing brain may be an important signaling center for dorsoventral patterning, and an inappropriate number of NECs in this region may lead to malformation during brain development.52 In this respect, Eph/ephrin signaling may have evolved to link with the caspase signaling cascade, and this mechanism may be essential for maintaining an appropriately sized NEC population in the DM. Therefore, NEC apoptotic death deserves dedicated studies to examine how Eph/ephrin signaling cross-talks with the caspase signaling cascade in a region where Eph receptors and ephrins are bound to each other by clustering at sites of cell–cell contact. The evolutionary expansion of this Eph family in mammals may have been driven by selection for novel distribution and different expression levels. Their cross-interactions with various other RTKs, cell death receptors, or caspases may have provided a great advantage for more sophisticated regulation of apoptotic cell death versus cell proliferation during brain development.
Materials and Methods
Targeting vector for BAC modification, BAC transgenic mice, and knockout mice
To generate the targeting vector for ephrin-A5-LacZ BAC, homology arms A (426 bp) and B (686 bp) flanking the mouse ephrin-A5 translation start site (ATG) were synthesized with polymerase chain reaction (PCR) using the following primer sets: 5′-GGCCAAGTCGGCCGGTGTATGTCTCCCCGCAGCCGC-3′ (forward primer for A arm), 5′-GAGCTCGGAGATCGGGGATCCAG-3′ (reverse primer for A arm), 5′-GCGGCCGCTGCTCTTTCTGGTGCTCTGG-3′ (forward primer for B arm), and 5′-ATGCATCTTTCCACTCACCCATCCCT-3′ (reverse primer for B arm). Then, homology arms A and B were inserted into a plasmid vector (pGEM11z) containing a LacZ reporter with the SV40 polyadenylation site and a frt-Kana-frt cassette. The resulting plasmid was digested with SfiI/NsiI and then inserted specifically into the ephrin-A5 BAC genomic DNA (RP23-23O22) using a bacterial homologous recombination method as described previously.39 The targeting vector for EphA7-LacZ BAC was constructed essentially as described above, except that homology arms A (1010 bp) and B (1068 bp) were synthesized with PCR using the following primer sets: 5′-GTTTTAGTTCCCTGCGGACC-3′ (forward primer for A arm), 5′-CAAAAGCAACTTGGGGAGGAA-3′ (reverse primer for A arm), 5′-ACTTTTGATGGGAGGATGCC-3′ (forward primer for B arm), and 5′-GAACAAGAGGGCCTGCTCAT-3′ (reverse primer for B arm). The targeting vector was then inserted specifically into EphA7 BAC genomic DNA (RP23-457D20) as described previously.53 The targeting vectors for eA5-eA5Fc and eA5-eA5 BAC were constructed essentially the same as the targeting vector for ephrin-A5-LacZ BAC, except that a floxed GFP expression cassette followed by ephrin-A5-Fc or a full-length human ephrin-A5 expression cassette was used instead of a LacZ reporter. The targeting vectors were digested with NdeI and inserted specifically into ephrin-A5 BAC genomic DNA (RP23-23O22) as described previously.39
The modified recombinant BAC was injected into fertilized C57BL/6 mouse eggs as described previously.53 Each BAC transgenic line was identified with PCR analysis of DNA extracted from tail biopsy specimens. For ephrin-A5-LacZ, a 400-bp sequence was amplified with primers 5′-GTTACAATAAAGCAATAGCATCACA-3′ and 5′-GGTTGCTGCTGTTCCAGTAGAC-3′. For EphA7-LacZ, a 400-bp sequence was amplified with primers 5′-GTTACAATAAAGCAATAGCATCACA-3′ and 5′-AGGCTTCACAAATTACATTT-3′. For eA5-eA5Fc and eA5-eA5, a 360-bp sequence was amplified with primers 5′-TCATGTCTGGATCTCGACAAGGTAC-3′ and 5′-GGTTGCTGCTGTTCCAGTAGAC-3′.
Wnt1-Cre (003829), Emx1tm1(cre)Krj/J (005628), ephrin-A2tm1Jgfephrin-A5tm1Ddmo/J (005992), Gt(ROSA)26Sortm1(CAG-taulacZ)Bene (010633), and Casp3tm1Flv/J (006233) mouse lines were purchased from The Jackson Laboratory (Bar Harbor, ME, USA).
All mice were generated and maintained in accordance with the institutional guidelines approved by the Sookmyung Women's University Animal Care and Use Committee.
Neural progenitor cell culture
For neural progenitor culture, dissociated cells were obtained from the dorsal region of the diencephalon and mesencephalon of wild-type or eA5-EGFP BAC transgenic embryos at E10.5,39 seeded onto a coverglass coated with poly-ℒ-lysine (33 mg/ml, Sigma-Aldrich, St. Louis, MO, USA) and laminin (3 mg/ml, BD Bioscience, San Diego, CA, USA), and cultured in Neurobasal medium (Invitrogen, Carlsbad, CA, USA) containing N-2 supplement and B-27 for 24 h. Immunofluorescence staining was performed as described previously.54 Briefly, the cells were treated with soluble ephrin-A5-Fc or EphA7-Fc (unclustered, R&D Systems, Minneapolis, MN, USA) for 30 min at 37°C, fixed with 4% paraformaldehyde/2% sucrose in phosphate-buffered saline (PBS) for 20 min at room temperature, and rinsed three times for 5 min with washing buffer (0.2% Triton X-100 in PBS). Cells were blocked with washing buffer containing 2% bovine serum albumin for 30 min at room temperature and then incubated with primary antibodies for 1 h at room temperature. After washing, cells were incubated with fluorescence-conjugated secondary antibodies for 45 min at room temperature. Photos were taken with a confocal microscope (model FV300; Olympus, Tokyo, Japan).
Histology, immunohistochemistry, X-gal staining, and TUNEL assay
For histology, mouse embryos at the indicated developmental stage were collected and fixed overnight in 4% paraformaldehyde, dehydrated in ethanol, cleared in Histoclear, and embedded in paraffin. Sections were cut at 5 or 10 μm and collected on slides. The slides were rinsed in a Histoclear series in ethanol and an ethanol series in PBS to rehydrate the embryonic sections. Hematoxylin and eosin staining and cresyl violet staining were performed using 10-μm sections as previously described.55, 56 For immunohistochemistry using antibodies, sections were immersed in diluted antigen unmasking solution (VECTOR Laboratories, Burlingame, CA, USA) and boiled in a pressure cooker. After several minutes, sections were removed from the pressure cooker, soaked in cold water, and washed in PBS. Then, the sections were immersed in blocking buffer (2% horse serum, 0.3% Triton X-100 in PBS) for 1 h at room temperature, incubated with the primary antibody overnight at 4°C, and washed three times in PBS for 5 min. Subsequently, the slides were incubated with a suitable secondary antibody for 2 h at room temperature and washed in PBS. For X-gal staining, embryos to be stained were dissected in PBS, fixed in 0.2% glutaraldehyde, and washed and stained as described previously.53 TUNEL assays were performed according to the manufacturer's instructions (In Situ Cell Death Detection Kit, TMR red; Roche Diagnostics Corp., Indianapolis, IN, USA).
BrdU labeling for cell proliferation and cell cycle exit
Cell proliferation and cell cycle exit assays were performed as described previously.17, 57 For 5-bromo-2'-deoxyuridine (BrdU) labeling, timed pregnant female mice (E10.0 or E11.5) were injected intraperitoneally with a single pulse (300 or 50 mg/kg body weight) of BrdU (Sigma-Aldrich) and killed after 1 or 20 h. Embryos were fixed overnight in 4% paraformaldehyde, dehydrated in ethanol, cleared in Histoclear, embedded in paraffin, and sections were cut at 5 μm. Immunohistochemistry was performed using Ki67 and BrdU antibodies. Quantification of the labeling index and quitting fraction (exit from the cell cycle) were performed according to Chenn and Walsh.57 For analysis of cell proliferation and cell cycle exit, the data were quantified using the percentage of progenitor cells (Ki67+) labeled with BrdU, or the percentage of BrdU+ cells labeled only with BrdU (BrdU+ and Ki67−, no longer dividing).
Antibodies
Immunodetection was performed using monoclonal antibodies against neuronal class III-tubulin (Tuj1; 1 : 200; Covance, Princeton, NJ, USA) and BrdU (1 : 200; Sigma-Aldrich), and polyclonal antibodies against cleaved caspase-3 (Asp175; 1 : 250; Cell Signaling, Danvers, MA, USA), Ki67 (1 : 200; Novocastra, Rungis, France), ephrin-A5 (1 : 500; Abcam, Cambridge, UK), p-Tyr (PY99; 1 : 500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), p-EphA4 (Tyr-602; 1 : 100; ECM Biosciences, Versailles, KY, USA), and pan p-Eph (Tyr-596/602; 1 : 100; Millipore, Billerica, MA, USA).
Acknowledgments
We are indebted to Jonas Frisen and Pierre Vanderhaeghen for ephrin-A5 knock-out mice and human ephrin-A5 cDNA, respectively. This work was supported by a grant (no. 2009-0083149) from National Research Foundation of Korea (NRF), a grant (no. 2009K001246) from Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Education, Science and Technology, and a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare (A111706).
Glossary
- BAC
bacterial artificial chromosome
- BrdU
5-bromo-2′-deoxyuridine
- DM
dorsal midline
- DR5
death receptor 5
- EGF
epidermal growth factor
- Eph
erythropoietin-producing hepatocellular kinase
- ephrin
Eph receptor interacting proteins
- FADD
Fas-associated death domain
- GFP
green fluorescent protein
- NEC
neural epithelial cell
- NGF
nerve growth factor
- NTC
neural tube closure
- NTCD
neural tube closure defect
- PCD
programmed cell death
- PCR
polymerase chain reaction
- p75NTR
p75 neurotrophin receptor
- RTK
receptor tyrosine kinase
- TNFR-1
tumor necrosis factor receptor 1
- Trk
tropomyosin receptor kinase
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling
- X-gal
5-bromo-4-chloro-3-indolyl-β-𝒟-galactopyranoside
The authors declare no conflict of interest.
Footnotes
Supplementary information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by JM Hardwick
Supplementary Material
References
- Homma S, Yaginuma H, Oppenheim RW. Programmed cell death during the earliest stages of spinal cord development in the chick embryo: a possible means of early phenotypic selection. J Comp Neurol. 1994;345:377–395. doi: 10.1002/cne.903450305. [DOI] [PubMed] [Google Scholar]
- de la Rosa EJ, de Pablo F. Cell death in early neural development: beyond the neurotrophic theory. Trends Neurosci. 2000;23:454–458. doi: 10.1016/s0166-2236(00)01628-3. [DOI] [PubMed] [Google Scholar]
- Kuan CY, Roth KA, Flavell RA, Rakic P. Mechanisms of programmed cell death in the developing brain. Trends Neurosci. 2000;23:291–297. doi: 10.1016/s0166-2236(00)01581-2. [DOI] [PubMed] [Google Scholar]
- Yeo W, Gautier J. Early neural cell death: dying to become neurons. Dev Biol. 2004;274:233–244. doi: 10.1016/j.ydbio.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Buss RR, Sun W, Oppenheim RW. Adaptive roles of programmed cell death during nervous system development. Annu Rev Neurosci. 2006;29:1–35. doi: 10.1146/annurev.neuro.29.051605.112800. [DOI] [PubMed] [Google Scholar]
- Vermeulen K, Berneman ZN, Van Bockstaele DR. Cell cycle and apoptosis. Cell Prolif. 2003;36:165–175. doi: 10.1046/j.1365-2184.2003.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Raff MC. Social controls on cell survival and cell death. Nature. 1992;356:397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu Rev Neurosci. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- O'Leary DD, Wilkinson DG. Eph receptors and ephrins in neural development. Curr Opin Neurobiol. 1999;9:65–73. doi: 10.1016/s0959-4388(99)80008-7. [DOI] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10:165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitulescu ME, Adams RH. Eph/ephrin molecules – a hub for signaling and endocytosis. Genes Dev. 2010;24:2480–2492. doi: 10.1101/gad.1973910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover JC, Doetsch F, Garcia-Verdugo JM, Gale NW, Yancopoulos GD, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- Holmberg J, Armulik A, Senti KA, Edoff K, Spalding K, Momma S, et al. Ephrin-A2 reverse signaling negatively regulates neural progenitor proliferation and neurogenesis. Genes Dev. 2005;19:462–471. doi: 10.1101/gad.326905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depaepe V, Suarez-Gonzalez N, Dufour A, Passante L, Gorski JA, Jones KR, et al. Ephrin signalling controls brain size by regulating apoptosis of neural progenitors. Nature. 2005;435:1244–1250. doi: 10.1038/nature03651. [DOI] [PubMed] [Google Scholar]
- Li X, Johnson RW, Park D, Chin-Sang I, Chamberlin HM. Somatic gonad sheath cells and Eph receptor signaling promote germ-cell death in C. elegans. Cell Death Differ. 2012;19:1080–1089. doi: 10.1038/cdd.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YS, McLaughlin T, Sung TC, Santiago A, Lee KF, O'Leary DD. p75(NTR) mediates ephrin-A reverse signaling required for axon repulsion and mapping. Neuron. 2008;59:746–758. doi: 10.1016/j.neuron.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler KJ, Becker-Barroso E, Martinez A, Llovera M, Wentzel C, Poopalasundaram S, et al. A TrkB/EphrinA interaction controls retinal axon branching and synaptogenesis. J Neurosci. 2008;28:12700–12712. doi: 10.1523/JNEUROSCI.1915-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poopalasundaram S, Marler KJ, Drescher U. EphrinA6 on chick retinal axons is a key component for p75(NTR)-dependent axon repulsion and TrkB-dependent axon branching. Mol Cell Neurosci. 2011;47:131–136. doi: 10.1016/j.mcn.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S. Caspase function in programmed cell death. Cell Death Differ. 2007;14:32–43. doi: 10.1038/sj.cdd.4402060. [DOI] [PubMed] [Google Scholar]
- Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, et al. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, et al. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- Hakem R, Hakem A, Duncan GS, Henderson JT, Woo M, Soengas MS, et al. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Blaschke AJ, Flavell RA, Srinivasan A, Chun J. Decreased apoptosis in proliferative and postmitotic regions of the Caspase 3-deficient embryonic central nervous system. J Comp Neurol. 2000;423:1–12. [PubMed] [Google Scholar]
- Yeh WC, Pompa JL, McCurrach ME, Shu HB, Elia AJ, Shahinian A, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–1958. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- Juo P, Kuo CJ, Yuan J, Blenis J. Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr Biol. 1998;8:1001–1008. doi: 10.1016/s0960-9822(07)00420-4. [DOI] [PubMed] [Google Scholar]
- Los M, Wesselborg S, Schulze-Osthoff K. The role of caspases in development, immunity, and apoptotic signal transduction: lessons from knockout mice. Immunity. 1999;10:629–639. doi: 10.1016/s1074-7613(00)80062-x. [DOI] [PubMed] [Google Scholar]
- Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter ME. Programmed cell death: apoptosis meets necrosis. Nature. 2011;471:310–312. doi: 10.1038/471310a. [DOI] [PubMed] [Google Scholar]
- Zhang G, Njauw CN, Park JM, Naruse C, Asano M, Tsao H. EphA2 is an essential mediator of UV radiation-induced apoptosis. Cancer Res. 2008;68:1691–1696. doi: 10.1158/0008-5472.CAN-07-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg J, Clarke DL, Frisen J. Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature. 2000;408:203–206. doi: 10.1038/35041577. [DOI] [PubMed] [Google Scholar]
- Copp AJ, Greene ND, Murdoch JN. The genetic basis of mammalian neurulation. Nat Rev Genet. 2003;4:784–793. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- Flenniken AM, Gale NW, Yancopoulos GD, Wilkinson DG. Distinct and overlapping expression patterns of ligands for Eph-related receptor tyrosine kinases during mouse embryogenesis. Dev Biol. 1996;179:382–401. doi: 10.1006/dbio.1996.0269. [DOI] [PubMed] [Google Scholar]
- Yoo S, Kim Y, Noh H, Lee H, Park E, Park S. Endocytosis of EphA receptors is essential for the proper development of the retinocollicular topographic map. EMBO J. 2011;30:1593–1607. doi: 10.1038/emboj.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Park S. Identification of EphA7 BAC clone containing a long-range dorsal midline-specific enhancer. BMB Rep. 2011;44:113–117. doi: 10.5483/BMBRep.2011.44.2.113. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Saha N, Nikolov DB. Cell-cell signaling via Eph receptors and ephrins. Curr Opin Cell Biol. 2007;19:534–542. doi: 10.1016/j.ceb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulthard MG, Duffy S, Down M, Evans B, Power M, Smith F, et al. The role of the Eph-ephrin signalling system in the regulation of developmental patterning. Int J Dev Biol. 2002;46:375–384. [PubMed] [Google Scholar]
- Carvalho RF, Beutler M, Marler KJ, Knoll B, Becker-Barroso E, Heintzmann R, et al. Silencing of EphA3 through a cis interaction with ephrinA5. Nat Neurosci. 2006;9:322–330. doi: 10.1038/nn1655. [DOI] [PubMed] [Google Scholar]
- Kao TJ, Kania A. Ephrin-mediated cis-attenuation of Eph receptor signaling is essential for spinal motor axon guidance. Neuron. 2011;71:76–91. doi: 10.1016/j.neuron.2011.05.031. [DOI] [PubMed] [Google Scholar]
- Marquardt T, Shirasaki R, Ghosh S, Andrews SE, Carter N, Hunter T, et al. Coexpressed EphA receptors and ephrin-A ligands mediate opposing actions on growth cone navigation from distinct membrane domains. Cell. 2005;121:127–139. doi: 10.1016/j.cell.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Davis S, Gale NW, Aldrich TH, Maisonpierre PC, Lhotak V, Pawson T, et al. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994;266:816–819. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- Mellitzer G, Xu Q, Wilkinson DG. Eph receptors and ephrins restrict cell intermingling and communication. Nature. 1999;400:77–81. doi: 10.1038/21907. [DOI] [PubMed] [Google Scholar]
- Xu Q, Mellitzer G, Robinson V, Wilkinson DG. In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature. 1999;399:267–271. doi: 10.1038/20452. [DOI] [PubMed] [Google Scholar]
- Brantley-Sieders DM, Zhuang G, Hicks D, Fang WB, Hwang Y, Cates JM, et al. The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J Clin Invest. 2008;118:64–78. doi: 10.1172/JCI33154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil M, Jacobson MD, Raff MC. Is programmed cell death required for neural tube closure. Curr Biol. 1997;7:281–284. doi: 10.1016/s0960-9822(06)00125-4. [DOI] [PubMed] [Google Scholar]
- Massa V, Savery D, Ybot-Gonzalez P, Ferraro E, Rongvaux A, Cecconi F, et al. Apoptosis is not required for mammalian neural tube closure. Proc Natl Acad Sci USA. 2009;106:8233–8238. doi: 10.1073/pnas.0900333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhikov VV, Millen KJ. Roof plate-dependent patterning of the vertebrate dorsal central nervous system. Dev Biol. 2005;277:287–295. doi: 10.1016/j.ydbio.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Kim Y, Song E, Choi S, Park S. Engineering lacZ Reporter gene into an ephA8 bacterial artificial chromosome using a highly efficient bacterial recombination system. J Biochem Mol Biol. 2007;40:656–661. doi: 10.5483/bmbrep.2007.40.5.656. [DOI] [PubMed] [Google Scholar]
- Zimmer G, Kastner B, Weth F, Bolz J. Multiple effects of ephrin-A5 on cortical neurons are mediated by SRC family kinases. J Neurosci. 2007;27:5643–5653. doi: 10.1523/JNEUROSCI.0954-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim S, Kim Y, Shin J, Kim J, Park S. Regulation of EphA8 gene expression by TALE homeobox transcription factors during development of the mesencephalon. Mol Cell Biol. 2007;27:1614–1630. doi: 10.1128/MCB.01429-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh H, Park S. Ectopic expression of Ephrin-A5 under the EphA8 promoter at the anterior region of the superior colliculus. Exp Neurobiol. 2010;19:49–53. doi: 10.5607/en.2010.19.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.