Abstract
In cyanotic patients undergoing repair of heart defects, high level of oxygen during cardiopulmonary bypass (CPB) leads to greater susceptibility to myocardial ischemia and reoxygenation injury. This study investigates the effects of controlled reoxygenation CPB on gene expression changes in cyanotic hearts of patients undergoing surgical correction of tetralogy of Fallot (TOF). We randomized 49 cyanotic TOF patients undergoing corrective cardiac surgery to receive either controlled reoxygenation or hyperoxic/standard CPB. Ventricular myocardium biopsies were obtained immediately after starting and before discontinuing CPB. Microarray analyses were performed on samples, and array results validated with real-time PCR. Gene expression profiles before and after hyperoxic/standard CPB revealed 35 differentially expressed genes with three upregulated and 32 downregulated. Upregulated genes included two E3 Ubiquitin ligases. The products of downregulated genes included intracellular signaling kinases, metabolic process proteins, and transport factors. In contrast, gene expression profiles before and after controlled reoxygenation CPB revealed only 11 differentially expressed genes with 10 upregulated including extracellular matrix proteins, transport factors, and one downregulated. The comparison of gene expression following hyperoxic/standard vs. controlled reoxygenation CPB revealed 59 differentially expressed genes, with six upregulated and 53 downregulated. Upregulated genes included PDE1A, MOSC1, and CRIP3. Downregulated genes functionally clustered into four major classes: extracellular matrix/cell adhesion, transcription, transport, and cellular metabolic process. This study provides direct evidence that hyperoxic CPB decreases the adaptation and remodeling capacity in cyanotic patients undergoing TOF repair. This simple CPB strategy of controlled reoxygenation reduced the number of genes whose expression was altered following hyperoxic/standard CPB.
Keywords: gene expression, congenital heart disease, surgery, hypoxia, reoxygenation injury
reintroduction of high oxygen levels to cyanotic patients on cardiopulmonary bypass (CPB) leads to myocardial damage prior to ischemic cardioplegic arrest (31), suggesting that the injury seen following cardioplegic arrest may be in part due to CPB-induced reoxygenation injury, and this has also been demonstrated in other clinical studies (8, 9). One of the strategies proposed to avoid this injury is the use of “controlled reoxygenation” using normoxic CPB with a pump prime the Po2 (oxygen tension in the blood) of which is matched to the patient's preoperative saturation. This has been shown to ameliorate reoxygenation injury and leads to almost complete functional recovery (17, 20). In a recent prospective randomized trial we have demonstrated that controlled reoxygenation on starting CPB in cyanotic patients undergoing cardiac surgery significantly reduces oxidative stress and myocardial cell injury, compared with hyperoxic CPB (5). Hypoxia changes the cardiac protein pattern, mainly through altered gene expression (6, 15). We have recently shown that chronic hypoxia in cyanotic children with tetralogy of Fallot (TOF) induced the expression of genes associated with apoptosis and reduced the expression of genes associated with normal myocyte contractility and function (15) and may be responsible for the susceptibility of cyanotic children to reoxygenation injury during and after surgery.
To our knowledge, no previous attempts have been made to determine the gene expression profiles associated with reoxygenation injury in cyanotic heart disease. Transcriptional profiling is a powerful tool for delineating complex patterns of gene expression in response to severe systemic stimuli and injury (37), and several recent studies have used microarray technology to examine the global myocardial stress response during cardiac surgery (27, 36, 39). The aim of this study was to investigate the effects of reoxygenation injury on the myocardial gene expression profiles of cyanotic patients with a diagnosis of TOF undergoing corrective heart surgery. Furthermore, we evaluated whether controlling reoxygenation during CPB could prevent the effect of hyperoxic CPB on the myocardial gene expression profile in these patients.
MATERIALS AND METHODS
Patients, clinical, and biochemical methods.
We randomized 49 cyanotic patients undergoing TOF or pulmonary atresia repair between January 2004 and November 2009 at the Bristol Royal Hospital for Children to receive either controlled reoxygenation (50–80 mmHg, n = 24) or hyperoxic/standard (150–180 mmHg, n = 25). All patients were in a stable condition without preoperative respiratory or inotropic support. “Controlled reoxygenation CPB” referred to a pump prime, the Po2 of which is matched to the Po2 of the patient. “Hyperoxic/standard CPB” referred to a pump prime prepared to the current “best practice” protocols, which has a Po2 relatively hyperoxic for a cyanotic patient. Preoperative characteristics in the two groups are summarized in Table 1. The study was approved by the Hospital Research Ethics Committee, and parental informed consent was gained for all patients. Intraoperative anesthetic and operative techniques were standardized as previously reported (21). Cold blood (4–6°C) St Thomas' I-based cardioplegic solution (4:1 dilution blood/St Thomas' I crystalloid cardioplegia) was used for myocardial preservation, with the following composition (mM): 16 MgCl2, 2 CaCl2, 20 KCl, 147 NaCl, 1.0 procaine HCl. Additional cardioplegia was administered after each 20 min of aortic cross-clamping. Blood oxygen tension levels for assessing the efficacy of the controlled reoxygenation were measured in both groups immediately after intubation, at 10 and 30 min after starting CPB, at the end of the ischemic time, and 6 and 24 h postoperatively.
Table 1.
Baseline patients' characteristics and clinical outcomes
| Controlled Reoxygenation (n = 24) | Hyperoxic/Standard (n = 25) | P Value | |
|---|---|---|---|
| Age, mo | 10.5 ± 9.9 | 11.5 ± 6.5 | 0.1 |
| Weight, kg | 8.4 ± 2.3 | 8.2 ± 2.3 | 0.7 |
| Preoperative saturations, % | 80.3 ± 9.1 | 81.7 ± 5.9 | 0.5 |
| Echo RV wall thickness, mm | 6.5 ± 0.5 | 6.9 ± 0.7 | 0.2 |
| Echo RV wall indexed, mm/m2 | 15.8 ± 3.6 | 17.3 ± 3.7 | 0.4 |
| Echo VSD size, mm | 11.5 ± 3.0 | 10.3 ± 1.3 | 0.2 |
| CPB time, min | 107.4 ± 30.3 | 109.6 ± 29.9 | 0.4 |
| Cross-clamp time, min | 61.7 ± 19.6 | 68.7 ± 15.3 | 0.1 |
| Ventilation time, h | 21.4 (21.7–50.2) | 32.0 (30.1–73.2) | 0.03 |
| Dopamine support duration, h | 24.0 (20.9–38.6) | 37.0 (30.8–73.8) | 0.05 |
| Hospital stay, days | 7.5 (6.7–15.8) | 8.0 (6.9–17.8) | 0.2 |
| Lactate, mcg/l | >0.5 | ||
| T1 = preoperatively | 1.13 ± 0.3 | 1.03 ± 0.4 | |
| T2 = X-clamp off | 1.13 ± 0.3 | 1.04 ± 0.3 | |
| T3 = 30 min X-clamp off | 1.17 ± 0.4 | 1.05 ± 0.3 | |
| T4 = 2 h X-clamp off | 1.19 ± 0.4 | 1.57 ± 0.8* | |
| T5 = 6 h X-clamp off | 1.28 ± 0.9 | 1.09 ± 0.4 | |
| T6 = 24 h X-clamp off | 1.24 ± 0.4 | 1.28 ± 0.6 |
Data are means ± SD or median and interquartile range. RV, right ventricle; X-clamp, aortic cross clamp (cardioplegic ischemic time). *P < 0.05 vs. T1, T2, and T3 in the Hyperoxic/Standard group.
After the surgery, all patients were admitted to the pediatric intensive care unit (ICU) and were managed according to unit protocols (21, 32).
Serum lactate and 8-isoprostane levels (enzyme immunoassay; Cayman Chemicals, Ann Arbor, MI) were measured preoperatively, on removal of the aortic cross-clamp, and 30 min, 2, 6, and 24 h thereafter, as measurements of myocardial oxidative stress.
Continuous outcomes are summarized as an arithmetic mean and standard deviation if normally distributed or as median and interquartile range if skewed. Categorical data are presented as actual counts and percentages. Biochemical markers measured at multiple time points were analyzed by repeated-measures analysis of variance. A P value <0.05 was considered statistically significant.
Cardiac muscle biopsies.
Ventricular biopsy specimens (10 mg net weight) were collected from the apex of the right ventricle using a Trucut needle (21). The Pre-op biopsy was taken immediately after institution of CPB. The hyperoxic or controlled reoxygenation biopsies were collected at the end of ischemic time. Each specimen was immediately put in RNA Later solution (Qiagen, UK) and kept overnight at 4°C. The next day RNA Later solution was removed, and biopsies were kept at −80°C until RNA extraction.
RNA extraction.
Tissue was mechanically homogenized in lysis reagent (Qiagen, Crawley, UK) and Total RNA was purified with RNeasy Micro Kit (Qiagen) and eluted into 12 μl of RNase-free water. The concentration and purity of the total RNA samples were assessed by spectrophotometry (Nanodrop, Wilmington, DE). Only samples with a sufficiently high yield (≈1 μg of total RNA at a minimum concentration of 125 ng/μl) and purity (an A260/A280 ratio of close to 2) were further analyzed for integrity with a Bioanalyzer 2100 with RNA 6000 Nano Assay (Agilent Technologies, Stockport, UK). Samples that met the quality control criteria were used as templates for cRNA synthesis.
Gene microarrays.
For each experimental group (Pre-op, n = 7; Hyperoxic/standard, n = 5; and Controlled reoxygenation, n = 5), samples from individual patients were processed. Ventricular total RNAs (1 μg) from individual patients were used to generate biotinylated cRNAs. The quantity and size distribution of purified cRNA was assessed on a Bioanalyzer 2100 using RNA 6000 Nano Assay (Agilent Technologies) to ensure that the cRNA amplification was successful. Target fragmentation was achieved by incubation at 94°C for 35 min in fragmentation buffer (40 mM Tris-acetate, pH 8.1/100 mM KOAc/30 mM MgOAc). The size distribution of the fragmented labeled transcripts was assessed on the Agilent Technologies Bioanalyzer 2100 using the RNA 6000 Nano Assay. These cRNAs samples were used for hybridization to separate Affymetrix GeneChip arrays. For each experimental group, five samples from individual patients were processed. Hybridization of the labeled cRNA to the Affymetrix GeneChip Human Genome U133 Plus 2.0 Array was carried out for 16 h in the Affymetrix GeneChip Hybridization Oven 640. Then GeneChip arrays were stained and washed on the GeneChip Fluidics Station 450 (Affymetrix). The fluorescent signals were detected with an Affymetrix GeneChip Scanner 3000 and stored as high-resolution fluorescence intensity data file. These data were initially analyzed with Affymetrix GeneChip operating software GCOS 1.2, which generates an expression report file that lists the quality control parameters. All of these parameters were scrutinized to ensure that array data had reached the necessary quality standards (scaling factor of <3-fold; average background values at 20–100 and the ratio of 3′:5′ signal no more than 3 for housekeeping genes GAPDH and β-actin). All raw data have been submitted to the National Center for Biotechnology Information Gene Expression Omnibus database under the following accession numbers: GSE14956 and GSE38177.
Microarray data analysis.
Seven pre-op patients samples (4 pre-op hyperoxic and 3 pre-op controlled), five post-op hyperoxic samples and five post-op controlled samples were used. Separate microarrays were probed with independently generated target from each tissue from heart biopsies of either group at the beginning of surgery and at the end of ischemic time. The microarray data were normalized in accord with our standard protocol. Raw data (CEL files) were uploaded into ArrayStar software version 2.1 (DNASTAR) for normalization and statistical analysis. The robust multichip analysis algorithm (7) was used for background correction, quantile normalization, and median polish summarization. The statistical analysis was carried out using ArrayStar software followed by multiple testing corrections for false discovery rate. Transcripts were filtered on the basis of ≥1.6-fold difference.
Functional annotation analysis.
Differentially expressed genes were analyzed according to predefined pathways and functional categories annotated by KEGG (24) and GO (2) using the DAVID (Database for Annotation, Visualization, and Integrated Discovery) bioinformatics resource (11). All gene annotations were checked using online tools and databases (http://www.ncbi.nlm.nih.gov). Note that the databases are in a constant state of flux and that annotations are subject to updates, redefinition, and correction.
cDNA synthesis and real-time PCR.
For each experimental group (Hyperoxic/standard, n = 5 and controlled reoxygenation, n = 5), total RNA from individual patients was extracted. Complementary DNA was reverse transcribed from 1 μg of total RNA using Superscript III cDNA first-strand synthesis kit (Invitrogen, UK), diluted two fold and 1 μl used in real-time PCR reactions. Optimized primers of human SLC6A6 (QT00095655), NRAS1 (QT00076874), JUN1 (QT00242956), PIK3R1 (QT00023100), MAPK8 (QT01149512), NLK (QT00043148), COL1A2 (QT00072058), VCAN (QT00064064), PDE1A (QT00065877), MOSC1 (QT00084665), and CRIP3 (QT00089852) were purchased from Qiagen (UK). Amplification and detection of specific products were carried out with Roche Lightcycler 1.5 detection System. Each sample was performed in duplicate. 18S mRNA was used as endogenous control transcript in each sample. Relative expression ratios were calculated by the relative quantification real-time PCR method (34). Statistical analysis (unpaired t-test) was carried out using Instat 3 software, and a P value was calculated for comparison.
Western blotting.
For each experimental group (Hyperoxic/standard, n = 5 and controlled reoxygenation, n = 5), total proteins from individual patients were extracted. Western blotting was performed as previously described (15). Briefly, total protein extracts were prepared from ventricular biopsies, separated on SDS polyacrylamide gel, and transferred to Hybond nitrocellulose membrane (Amersham). Blocked membrane were incubated in primary polyclonal antibodies (rabbit anti-MOSC1, Lifespan Biosciences; rabbit anti-COL1A2, abcam; goat anti-TAUT, Santa Cruz Biotechnology), washed, and incubated in horseradish peroxidase-coupled anti-rabbit or anti-mouse secondary antibodies (Amersham). Membranes were exposed to Hyperfilm (Amersham), and protein bands were quantified with NIH Image J software. Statistical analysis (unpaired t-test) was carried out using Instat 3 software, and a P value was calculated for comparison.
RESULTS
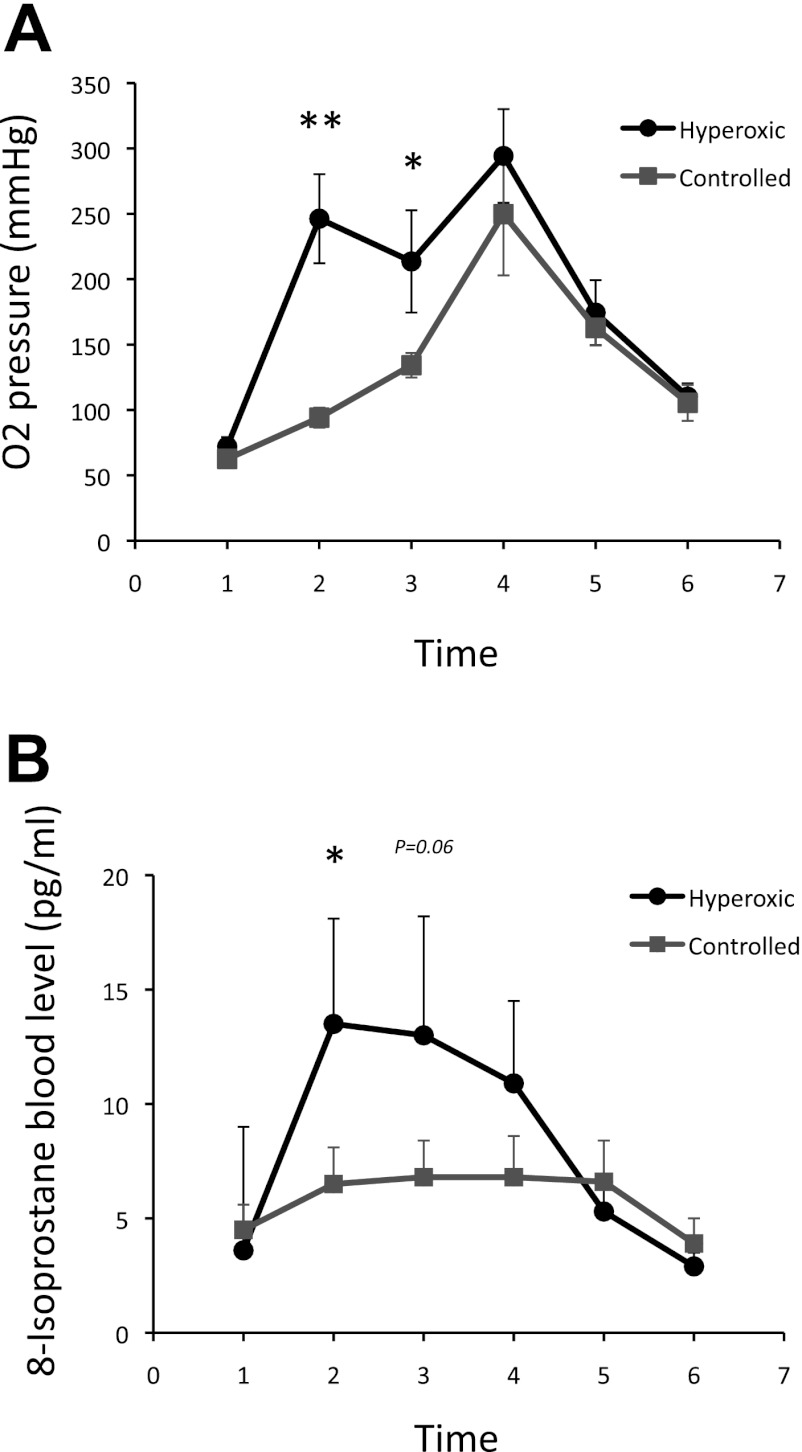
Operative and postoperative data for both groups are summarized in Table 1. The two groups were comparable in terms of age, weight, CPB, and cross-clamp time. The pro-operative echocardiographic data show no differences between the two groups regarding right ventricular wall thickness. As expected, the blood oxygen levels were significantly increased in the hyperoxic/standard compared with the controlled reoxygenation CPB group (P = 0.01) demonstrating the efficacy of the CPB treatment strategy (Fig. 1A).
Fig. 1.
Oxygen pressure (A) and 8-isoprostane level (B) changes up to 24 h postoperatively in the hyperoxic/standard compared with the controlled reoxygenation cardiopulmonary bypass (CPB) groups. Time 1, preoperatively; time 2, X-clamp off; time 3, 30 min X-clamp off; time 4, 2 h X-clamp off; time 5, 6 h X-clamp off; time 6, 24 h X-clamp off. **P < 0.01 and *P < 0.05 vs. time 1 in the Hyperoxic/Standard group.
There were no deaths and no major morbidities in both groups. Patients receiving hyperoxic/standard CPB had a longer ventilation time (P = 0.03) and duration of dopamine support (P = 0.05) compared with patients in the controlled reoxygenation CPB group. Postoperative lactate levels were significantly raised at 6 h from the end of the cross clamp time compared with preoperative levels in the hyperoxic/standard CPB group (P < 0.05).
The 8-isoprostane levels were also significantly increased at the end of the cross clamp time compared with preoperative levels in the hyperoxic/standard group, remaining elevated up to 2 h from the end of the ischemic time (P < 0.05, Fig. 1B).
Gene expression.
We have used the Affymetrix GeneChip Human Genome U133 Plus 2.0 Array in this study as it represents the most comprehensive whole human genome expression array. It provides comprehensive coverage of the transcribed human genome on a single array with variants from >47,000 well-substantiated human genes (http://www.affymetrix.com).
Genes expressed in cyanotic heart tissue before and after hyperoxic/standard or controlled reoxygenation CPB.
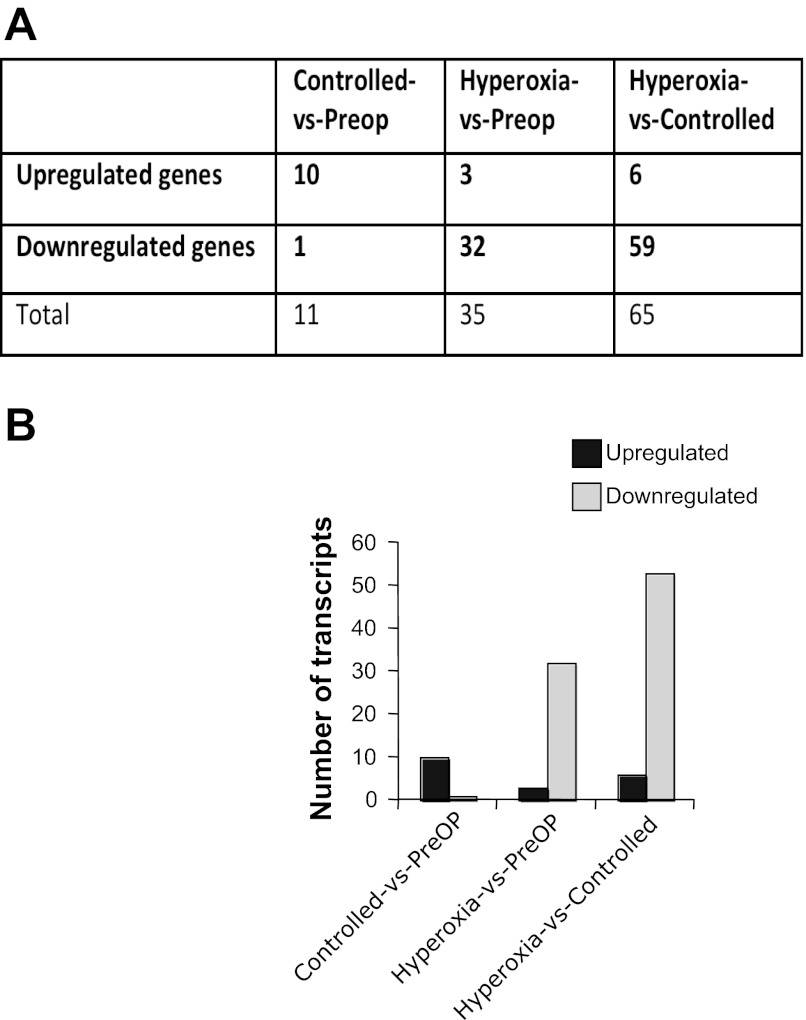
The comparison of gene expression profiles in the cyanotic hearts before and after hyperoxic/standard CPB revealed 35 differentially expressed genes with three upregulated and 32 downregulated (Fig. 2). The products of upregulated genes included two E3 Ubiquitin ligases HECTD1 and DTX3 (Table 2). The products of downregulated genes included intracellular signaling kinases MAPK8 and NLK (Wnt signaling pathway), metabolic process proteins (KLF9, biliverdin reductase A, PPAPDC1B), and transport factors: SLC39A8, SLC25A30 (mitochondrion protein) and GULP (Table 2). In contrast, the comparison of gene expression profiles before and after controlled reoxygenation CPB revealed only 11 differentially expressed genes with 10 upregulated including extracellular matrix proteins (COL1A2 and IDE), transport factors, and one downregulated (Cortactin; Fig. 2, Table 3). Controlled reoxygenation reduced the number of genes whose expression was altered following hyperoxic/standard CPB.
Fig. 2.
A: summary of the regulated genes (≥1.6-fold) in post-op vs. pre-op controlled reoxygenation, post-op vs. pre-op hyperoxic procedure, and post-op hyperoxic vs. post-op controlled reoxygenation. B: regulated transcripts in the 3 comparisons. The histogram shows the proportion of upregulated vs. downregulated genes.
Table 2.
Genes exhibiting 1.6-fold or greater expression change in ventricular biopsy of post-op vs. pre-op hyperoxic patients
| Gene | Symbol | NCBI Gene ID | Fold in Post-op | P Value |
|---|---|---|---|---|
| HECT domain containing 1 | HECTD1 | 25831 | 1.791 up | 0.0232 |
| Zinc finger protein 106 homolog | ZFP106 | 64397 | 1.703 up | 0.0172 |
| Deltex 3 homolog (Drosophila) | DTX3 | 196403 | 1.674 up | 0.00172 |
| Solute carrier family 25, member 30 | SLC25A30 | 253512 | 2.139 down | 0.00218 |
| Brain expressed X-linked 2 | BEX2 | 84707 | 2.032 down | 0.0198 |
| Prion protein (p27–30) | PRNP | 5621 | 1.993 down | 0.0117 |
| EF-hand calcium binding domain 2 | EFCAB2 | 84288 | 1.976 down | 0.0159 |
| PAP associated domain containing 1 | PAPD1 | 55149 | 1.890 down | 0.0181 |
| GULP, engulfment adaptor PTB domain containing 1 | GULP1 | 51454 | 1.889 down | 0.0178 |
| Ubiquitin-like modifier activating enzyme 6 | UBA6 | 55236 | 1.839 down | 0.0172 |
| TRK-fused gene | TFG | 10342 | 1.770 down | 0.0147 |
| Biliverdin reductase A | BLVRA | 644 | 1.759 down | 0.00761 |
| KIN, antigenic determinant of recA protein homolog | KIN | 22944 | 1.732 down | 0.00693 |
| CDNA clone IMAGE:5286843 | 1.724 down | 0.0203 | ||
| Mesoderm specific transcript homolog | MEST | 4232 | 1.695 down | 0.00819 |
| Solute carrier family 39 (zinc transporter), member 8 | SLC39A8 | 64116 | 1.678 down | 0.00967 |
| Branched chain aminotransferase 1, cytosolic | BCAT1 | 586 | 1.672 down | 0.0105 |
| Heparan sulfate (glucosamine) 3-O-sulfotransferase 1 | HS3ST1 | 9957 | 1.672 down | 0.0176 |
| Nemo-like kinase | NLK | 51701 | 1.672 down | 0.0249 |
| Mitogen-activated protein kinase 8 | MAPK8 | 5599 | 1.669 down | 0.00734 |
| RNA binding motif protein 7 | RBM7 | 10179 | 1.664 down | 0.0179 |
| 1.662 down | 0.00417 | |||
| Ras homolog gene family, member U | RHOU | 58480 | 1.660 down | 0.0165 |
| MIT, microtubule interacting and transport, domain containing 1 | MITD1 | 129531 | 1.660 down | 0.0201 |
| CDNA FLJ40901 fis, clone UTERU2003704 | 1.637 down | 0.00731 | ||
| TBP-like 1 | TBPL1 | 9519 | 1.635 down | 0.0249 |
| Calcyclin binding protein | CACYBP | 27101 | 1.631 down | 0.0234 |
| Carbohydrate (chondroitin) synthase 1 | CHSY1 | 22856 | 1.630 down | 0.0229 |
| PHD finger protein 14 | PHF14 | 9678 | 1.626 down | 0.0229 |
| Kruppel-like factor 9 | KLF9 | 687 | 1.618 down | 0.0201 |
| Ribonuclease H2, subunit B | RNASEH2B | 79621 | 1.617 down | 0.0222 |
| ATG10 autophagy related 10 homolog | ATG10 | 83734 | 1.606 down | 0.00197 |
| Chromosome 21 open reading frame 51 | C21orf51 | 54065 | 1.606 down | 0.0154 |
| Phosphatidic acid phosphatase type 2 domain containing 1B | PPAPDC1B | 84513 | 1.603 down | 0.0212 |
| COP9 constitutive photomorphogenic homolog subunit 8 | COPS8 | 10920 | 1.600 down | 0.0109 |
Table 3.
Genes exhibiting 1.6-fold or greater expression change in ventricular biopsy of post-op vs. pre-op controlled reoxygenation patients
| Gene | Symbol | NCBI Gene ID | Fold in Post-op | P Value |
|---|---|---|---|---|
| CDNA FLJ38472 fis, clone FEBRA2022148 | 2.246 up | 0.00654 | ||
| Ankyrin 3, node of Ranvier (ankyrin G) | ANK3 | 288 | 1.935 up | 0.0205 |
| Ubiquitously transcribed tetratricopeptide repeat, X chromosome | UTX | 7403 | 1.856 up | 0.0123 |
| HECT domain containing 1 | HECTD1 | 25831 | 1.815 up | 0.0232 |
| Golgi autoantigen, golgin subfamily a, 8B | GOLGA8B | 440270 | 1.807 up | 0.0228 |
| CDNA clone IMAGE:5263531 | 1.783 up | 0.021 | ||
| Collagen, type I, alpha 2 | COL1A2 | 1278 | 1.775 up | 0.00884 |
| RAN binding protein 2 | RANBP2 | 5903 | 1.752 up | 0.0206 |
| Phosphoinositide-3-kinase, class 2, alpha polypeptide | PIK3C2A | 5286 | 1.621 up | 0.0126 |
| Insulin-degrading enzyme | IDE | 3416 | 1.617 up | 0.0129 |
| Cortactin | CTTN | 2017 | 1.609 down | 0.0157 |
Comparing gene expression in cyanotic heart tissue following hyperoxic/standard versus controlled reoxygenation CPB.
Our data revealed 59 differentially expressed genes in hyperoxic-standard vs. controlled reoxygenation CPB, with six upregulated and 53 downregulated (Fig. 2). The upregulated genes included PDE1A, MOSC1, and CRIP3 (Table 4). The downregulated genes were functionally clustered into four major classes: extracellular matrix/cell adhesion, transcription, transport, and cellular metabolic process. They included Versican, Collagen I alpha 2, JUN, KLF9, SLC6A6, and BCAT1 (Table 4).
Table 4.
Genes exhibiting 1.6-fold or greater expression change in ventricular biopsy of post-op hyperoxic vs. postop controlled reoxygenation patients
| Gene | Symbol | NCBI Gene ID | Fold in Hyperoxic | P Value |
|---|---|---|---|---|
| Deiodinase, iodothyronine, type II | DIO2 | 1734 | 1.900 up | 0.0235 |
| Phosphodiesterase 1A, calmodulin-dependent | PDE1A | 5136 | 1.813 up | 0.00774 |
| Actin, alpha 1, skeletal muscle | ACTA1 | 58 | 1.755 up | 0.0168 |
| MOCO sulphurase C-terminal domain containing 1 | MOSC1 | 64757 | 1.740 up | 0.0168 |
| Cysteine-rich protein 3 | CRIP3 | 401262 | 1.700 up | 0.00171 |
| GABA(A) receptor-associated protein like 1 | GABARAPL1 | 23710 | 1.612 up | 0.0123 |
| CDNA FLJ38472 fis, clone FEBRA2022148 | 3.167 down | 0.00935 | ||
| CDNA FLJ40901 fis, clone UTERU2003704 | 2.658 down | 0.00731 | ||
| Branched chain aminotransferase 1, cytosolic | BCAT1 | 586 | 2.644 down | 0.0105 |
| CDNA FLJ38472 fis, clone FEBRA2022148 | 2.512 down | 0.00654 | ||
| Versican | VCAN | 1462 | 2.365 down | 0.0106 |
| Mesoderm specific transcript homolog | MEST | 4232 | 2.135 down | 0.00819 |
| GULP, engulfment adaptor PTB domain containing 1 | GULP1 | 51454 | 2.031 down | 0.0121 |
| Inturned planar cell polarity effector homolog | INTU | 27152 | 2.017 down | 0.0157 |
| Solute carrier family 25, member 30 | SLC25A30 | 253512 | 1.982 down | 0.00218 |
| Neuroligin 4, X-linked | NLGN4X | 57502 | 1.954 down | 0.00423 |
| Hypothetical protein LOC728555 /// hypothetical protein LOC730391 | LOC728555 /// LOC730391 | 728555 /// 730391 | 1.927 down | 0.0194 |
| Ras homolog gene family, member U | RHOU | 58480 | 1.895 down | 0.0165 |
| Neurotrimin | HNT | 50863 | 1.876 down | 0.0123 |
| Vacuolar protein sorting 35 homolog | VPS35 | 55737 | 1.853 down | 0.0217 |
| TIA1 cytotoxic granule-associated RNA binding protein | TIA1 | 7072 | 1.791 down | 0.0127 |
| Glycoprotein M6B | GPM6B | 2824 | 1.789 down | 0.0137 |
| Collagen, type I, alpha 2 | COL1A2 | 1278 | 1.782 down | 0.00884 |
| Insulin-degrading enzyme | IDE | 3416 | 1.755 down | 0.00773 |
| Calcitonin receptor-like | CALCRL | 10203 | 1.747 down | 0.00625 |
| Asparagine-linked glycosylation 13 homolog | ALG13 | 55849 | 1.745 down | 0.00513 |
| Ankyrin 3, node of Ranvier (ankyrin G) | ANK3 | 288 | 1.735 down | 0.0205 |
| ATG10 autophagy related 10 homolog | ATG10 | 83734 | 1.722 down | 0.00197 |
| Fer-1-like 3, myoferlin | FER1L3 | 26509 | 1.719 down | 0.00849 |
| Ubiquitin-like modifier activating enzyme 6 | UBA6 | 55236 | 1.708 down | 0.0172 |
| Solute carrier family 30 (zinc transporter), member 7 | SLC30A7 | 148867 | 1.707 down | 0.00933 |
| Quinolinate phosphoribosyltransferase (nicotinate-nucleotide pyrophosphorylase (carboxylating)) | QPRT | 23475 | 1.706 down | 0.0212 |
| CDNA clone IMAGE:5270438 | 1.698 down | 0.00574 | ||
| Solute carrier family 35 (UDP-glucuronic acid/UDP-N-acetylgalactosamine dual transporter), member D1 | SLC35D1 | 23169 | 1.697 down | 0.021 |
| Tetratricopeptide repeat domain 3 | TTC3 | 7267 | 1.697 down | 0.0118 |
| Zinc finger, SWIM-type containing 7 | ZSWIM7 | 125150 | 1.692 down | 0.0242 |
| Ectonucleoside triphosphate diphosphohydrolase 1 | ENTPD1 | 953 | 1.691 down | 0.0123 |
| Zinc finger protein 302 | ZNF302 | 55900 | 1.684 down | 0.0233 |
| Tau tubulin kinase 2 | TTBK2 | 146057 | 1.682 down | 0.0199 |
| Kruppel-like factor 9 | KLF9 | 687 | 1.679 down | 0.0201 |
| Family with sequence similarity 114, member A1 | FAM114A1 | 92689 | 1.678 down | 0.000927 |
| KIAA1199 | KIAA1199 | 57214 | 1.670 down | 0.0113 |
| Cdc2-related kinase, arginine/serine-rich | CRKRS | 51755 | 1.667 down | 0.00753 |
| Zinc finger protein 397 | ZNF397 | 84307 | 1.659 down | 0.0207 |
| Solute carrier family 6 (neurotransmitter transporter, taurine), member 6 | SLC6A6 | 6533 | 1.658 down | 0.015 |
| Transcribed locus | 1.655 down | 0.0101 | ||
| Pleckstrin homology domain interacting protein | PHIP | 55023 | 1.654 down | 0.0156 |
| Interleukin 1 receptor-like 1 | IL1RL1 | 9173 | 1.652 down | 0.0112 |
| 1.648 down | 0.00417 | |||
| Cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMP-N-acetylneuraminate monooxygenase) pseudogene | CMAH | 8418 | 1.646 down | 0.00472 |
| CDNA FLJ36491 fis, clone THYMU2018197 | 1.645 down | 0.0147 | ||
| NIMA (never in mitosis gene a)-related kinase 3 | NEK3 | 4752 | 1.638 down | 0.00289 |
| CDNA FLJ39389 fis, clone PLACE6003621 | 1.631 down | 0.00536 | ||
| Jun oncogene | JUN | 3725 | 1.630 down | 0.016 |
| Neuroblastoma RAS viral (v-ras) oncogene homolog | NRAS | 4893 | 1.621 down | 0.0189 |
| Aldehyde dehydrogenase 1 family, member A2 | ALDH1A2 | 8854 | 1.619 down | 0.00912 |
| Zinc finger protein 347 | ZNF347 | 84671 | 1.618 down | 0.00557 |
| Ankyrin repeat and SOCS box-containing 15 | ASB15 | 142685 | 1.617 down | 0.00804 |
| Brain expressed X-linked 2 | BEX2 | 84707 | 1.600 down | 0.0198 |
Gene ontology biologic process annotations.
Functional annotation clustering of the genes, identified as downregulated following hyperoxic/standard compared with controlled reoxygenation CPB, unmasked four overrepresented major classes involved in myocardium normal function (Table 5). Indeed, cell adhesion/extracellular matrix, transport, transcription, and cellular metabolic process were downregulated in cyanotic myocardium. These ontological changes indicate a decrease in the adaptation and remodeling capacity of cyanotic hearts subjected to hyperoxic/standard compared with controlled reoxygenation CPB.
Table 5.
Gene Ontology enrichment
| Pathway or GO ID | Term | Probes, n | P Value |
|---|---|---|---|
| KEGG | Erb signaling pathway | 3 | 3.0E-2 |
| GO:0031012 | extracellular matrix | 3 | 2.1E-1 |
| GO:0007155 | cell adhesion | 4 | 4.3E-1 |
| GO:0032268 | regulation of cellular protein metabolic process | 5 | 7.7E-2 |
| GO:0006351 | transcription | 7 | 5.1E1 |
| GO:0015031 | protein transport | 3 | 7.4E-1 |
Validation of microarray results with real-time PCR.
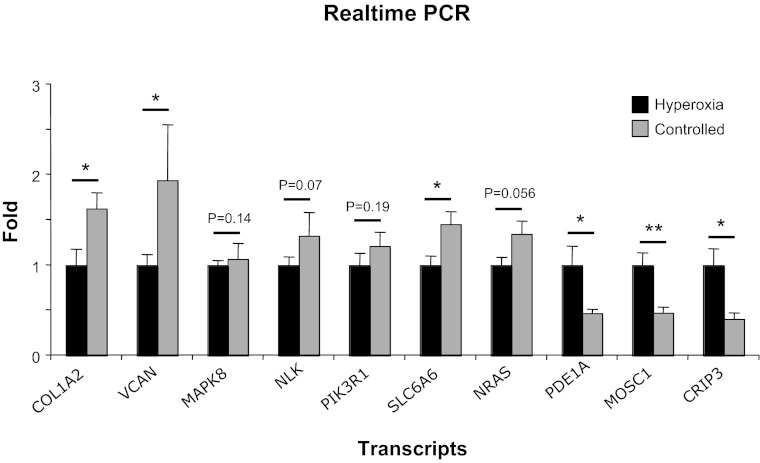
Microarray results were confirmed by real-time quantitative PCR on 10 selected genes that demonstrated high differential gene expression. The changes in expression levels of these genes showed a high degree of correlation with the microarray data (Fig. 3). Four out of the 10 examined genes showed a tendency to change similar to that of microarray experiment although not quite significant (Fig. 3).
Fig. 3.
Confirmation of the microarray results of changed genes in hyperoxic vs. controlled reoxygenation procedures. Changes in mRNA expression of COL1A2, VCAN, MAPK8, NLK, SLC6A6, NRAS1, PDE1, MOSC1, and CRIP3 were verified in 10 patients (5 using hyperoxic and 5 using controlled reoxygenation procedure) by quantitative real-time-PCR. Results are shown as means (±SE) fold-change compared with hyperoxia. *P < 0.05, **P < 0.01.
Western blotting of a selection of the identified genes' products.
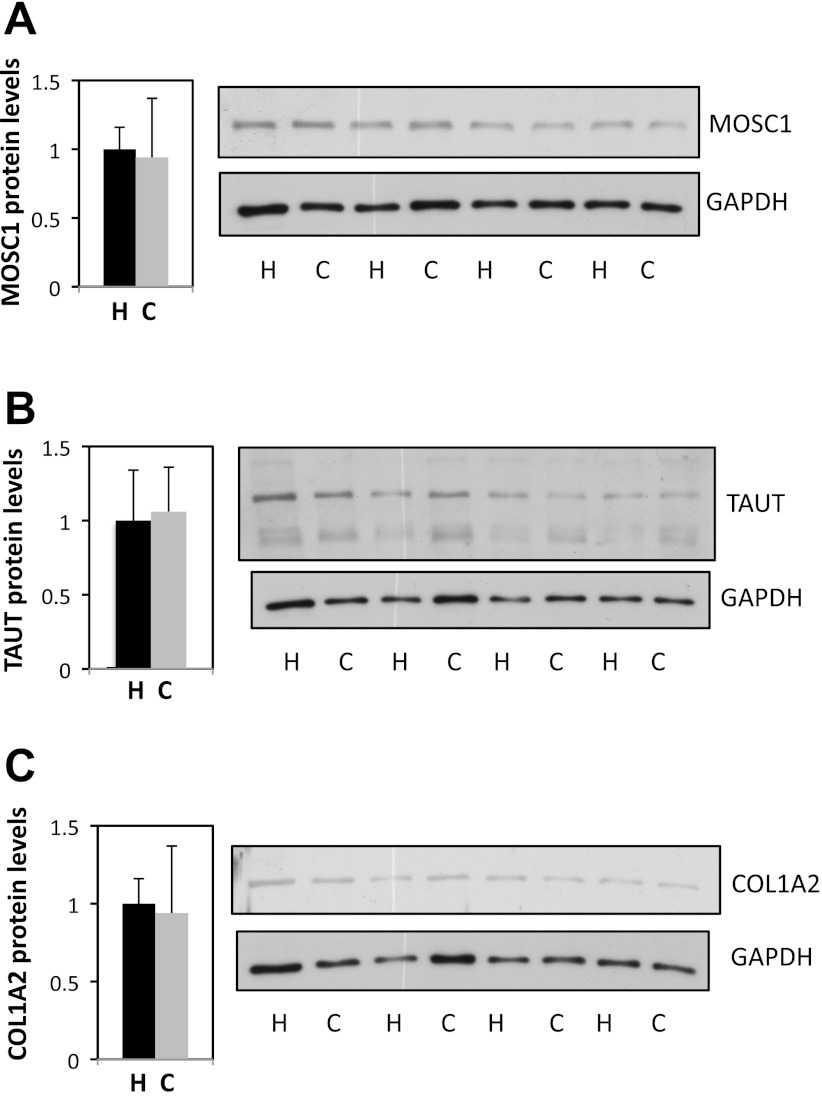
In an attempt to predict protein expression levels of some of the identified genes, we went onto assessing the protein levels of these genes' products in the myocardium of children subjected to hyperoxic/standard and controlled reoxygenation CPB using Western blotting. Protein levels of MOSC1, TAUT, and COL1A2 showed no alteration in the myocardium of the two groups of patients (Fig. 4).
Fig. 4.
Protein levels of MOSC1, TAUT, and COL1A2 in ventricular biopsies of controlled reoxygenation vs. hyperoxic patients. Tissues were lysed to isolate protein content and Western blotting analysis performed probing for MOSC1 (A), TAUT (B), COL1A2 (C), and GAPDH. No significant changes were observed in controlled reoxygenation (C) compared with hyperoxic (H) samples. MOSC1, CRIP3, and COL1A2 bands were normalized to GAPDH levels. Data are means ± SE.
DISCUSSION
To our knowledge, this study is the first attempt to examine transcriptomic changes following the use of a novel strategy of controlled reoxygenation CPB in corrective heart surgery of cyanotic children. Our study confirmed that high levels of oxygen on starting CPB are associated with significant gene expression changes that are not seen when the reoxygenation is controlled. This was also associated with higher release of 8-isoprostane in the hyperoxic/standard CPB group, indicating an overall reduction in oxidative stress when oxygen levels are controlled on starting CPB in cyanotic patients. We have recently shown that controlling reoxygenation on starting CPB in cyanotic children undergoing cardiac surgery is associated with reduced reperfusion injury and oxidative stress compared with hyperoxic/standard CPB (5). There is substantial experimental evidence for the existence of a myocardial reoxygenation injury in the cyanotic, immature heart. Studies of hypoxemia/reoxygenation in immature piglets have provided evidence for oxygen-mediated myocardial injury as a result of hyperoxemia in the setting of previous cyanosis (33). The immature heart has a higher tolerance to hypoxia, but preceding hypoxia prior to ischemic cardioplegic arrest results in poor functional recovery and is associated with derangements in several metabolites (19, 23). Hypoxia also reduces the antioxidant reserve capacity, leading to a greater susceptibility to the oxidative stress of ischemia (e.g., cross-clamping of the aorta) and reoxygenation (12, 18, 38). Re-introduction of oxygen exacerbates this situation and eventually leads to a reduction in myocardial contractility (1, 12, 13, 16, 18, 38).
One major finding in our study is that controlled reoxygenation reduces the transcriptomic alterations observed following hyperoxic CPB. Most of the genes altered by hyperoxic CPB were downregulated genes involved in intracellular signaling, metabolic process, and transport, suggesting that hyperoxic CPB can have a deleterious effect on the myocardium as shown by the reduction in expression of genes involved in important cell processes. In contrast, most of the genes altered by controlled reoxygenation CPB were upregulated genes implicated in extracellular matrix remodeling, suggesting that this strategy could improve myocardium remodeling by increasing extracellular matrix proteins.
Most of the differentially expressed genes in hyperoxic/standard versus controlled reoxygenation CPB were downregulated. These genes functionally clustered into four major classes: extracellular matrix/cell adhesion, transcription, transport, and cellular metabolic process. These findings suggest a decrease in the adaptation and remodeling capacity of cyanotic hearts subjected to hyperoxic compared with controlled reoxygenation CPB. Additionally, a number of CDNA clones seem to play a role in reoxygenation injury. However, these clones are not well characterized.
It seems that the short time (70 min) between the starting of surgery and taking the second biopsy was enough to see the alterations at the mRNA levels observed by GeneChip and real-time PCR. However, this same time was too short to see any significant changes at the protein levels of the selected proteins: MOSC1, TAUT, and COL1A2. It is known that there's usually a delay between transcription and translation that can be hours to days. Because of the limitation of working on humans, it is not possible to examine the protein levels in human myocardium after hours or days from hyperoxic/standard or controlled reoxygenation CPB.
MOSC1 has a sulfite oxidase, a xanthine dehydrogenase and an aldehyde oxidase activities. Interestingly, the aldehyde oxidase (AO) produces hydrogen peroxide and, under certain conditions, can catalyze the formation of superoxide (14, 25, 26). When molecular oxygen acts as an electron acceptor in the AO-catalyzed oxidation of aldehydes or azaheterocycles, it undergoes a two-electron reduction to produce H2O2; however, a portion undergoes one-electron reduction to produce superoxide. Our study shows an augmentation of MOSC1 mRNA levels by hyperoxic/standard CPB, suggesting a possible role of this enzyme in the increase of oxidative stress observed when this CPB procedure is used. Very early studies have already demonstrated the ability of this enzyme to generate superoxide and suggested a role for it in reperfusion injury (29). More recent investigations have shown that MOSC1 is an important source of both superoxide and H2O2 in biological tissues (28).
Another interesting gene is the taurine transporter (SLC6A6, or TAUT). It is thought that taurine play an important role in ion movement, calcium handling, osmoregulation, and cytoprotection. Taurine depletion causes cardiomyocyte atrophy, mitochondrial and myofiber damage, and cardiac dysfunction, effects likely related to the actions of taurine (22). Indeed this investigation suggests that multiple actions of taurine, including osmoregulation, regulation of mitochondrial protein expression, and inhibition of apoptosis, collectively ensure proper maintenance of cardiac and skeletal muscular structure and function (22). Our data showed a reduction in mRNA levels by hyperoxic/standard CPB, suggesting a deleterious effect of this CPB protocol to the myocardium occurring probably by reduction of taurine cytoprotective action.
Clinical implications.
One of the strategies proposed to avoid reoxygenation injury is the use of controlled reoxygenation during CPB. Allen and associates (1) demonstrated an improvement in the antioxidant reserve capacity with lower levels of oxygen in cyanotic infants undergoing cardiac surgery, and Bulutcu and coworkers (4) obtained similar findings. Our results confirm these findings and provide the first direct evidence that an unintended oxygen-mediated myocardial genomic alteration injury occurs in cyanotic patients with TOF undergoing surgical repair. Reoxygenation injury is associated with the production of reactive oxygen species by the formation of oxygen-derived free radicals such as superoxide and peroxide. This leads to cell membrane degradation through lipid peroxidation (12, 18). It is now evident that oxidative stress is a major part of the cellular mechanism of the resulting myocardial damage (3, 10, 30, 35). 8-Isoprostane has been shown to be a reliable marker for the volume of myocardium exposed to oxidant stress during acute myocardial infarction (30, 35), as well as a quantitative marker of oxidant stress during coronary reperfusion (10). The increased 8-isoprostane levels in the hyperoxic/standard CPB group associated with the profound downregulation of key genetic pathways related to myocardial function suggests a direct effect of CPB oxygen levels on the degree of reperfusion injury and can possibly explain the increased need of postoperative inotropic support observed in these patients. The perfusion strategy of keeping Po2 as close as possible to the patient's preoperative values resulted in a reduced oxidative stress and in a significant reduction in the genomic alterations observed with the hyperoxic/standard CPB group.
This novel and simple strategy of controlling reoxygenation on starting CPB does not interfere with the surgical procedure and, by limiting oxidative stress and reoxygenation injury, might lead to improvements in early clinical outcomes in the very high risk group of cyanotic infants and children undergoing cardiac surgery.
Limitations.
Our study cannot detect differences in clinical outcome between the two groups; the primary outcomes were related to differences in myocardial gene expression and biochemical markers of organ dysfunction. Recruitment to a larger trial to evaluate clinical outcomes as primary end points is ongoing at our institution.
This study investigated a single congenital pathology, and its findings cannot automatically be related to other cyanotic cardiac conditions.
GRANTS
This work was funded by the British Heart Foundation, the Garfield Weston Trust, the Bristol NIHR BRU in Cardiovascular Medicine, and the BUPA Foundation. M. T. Ghorbel was supported by an Intermediate Research Fellowship from the British Heart Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.T.G., G.D.A., and M.C. conception and design of research; M.T.G., A.M., and M.S. performed experiments; M.T.G. and M.C. analyzed data; M.T.G. and M.C. interpreted results of experiments; M.T.G. prepared figures; M.T.G. and M.C. drafted manuscript; M.T.G., G.D.A., and M.C. edited and revised manuscript; M.T.G., G.D.A., and M.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge A. Parry and S. Stoica for surgical help, Christine MacFadden for helping with data collection, and Megan Musson for technical assistance.
REFERENCES
- 1. Allen BS, Rahman S, Ilbawi MN, Kronon M, Bolling KS, Halldorsson AO, Feinberg H. Detrimental effects of cardiopulmonary bypass in cyanotic infants: preventing the reoxygenation injury. Ann Thorac Surg 64: 1381–1387, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25: 25–29, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blasig IE, Grune T, Schonheit K, Rohde E, Jakstadt M, Haseloff RF, Siems WG. 4-Hydroxynonenal, a novel indicator of lipid peroxidation for reperfusion injury of the myocardium. Am J Physiol Heart Circ Physiol 269: H14–H22, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Bulutcu FS, Bayindir O, Polat B, Yalcin Y, oZbek U, Cakali E. Does normoxemic cardiopulmonary bypass prevent myocardial reoxygenation injury in cyanotic children? J Cardiothorac Vasc Anesth 16: 330–333, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Caputo M, Mokhtari A, Rogers CA, Panayiotou N, Chen Q, Ghorbel MT, Angelini GD, Parry AJ. The effects of normoxic versus hyperoxic cardiopulmonary bypass on oxidative stress and inflammatory response in cyanotic pediatric patients undergoing open cardiac surgery: a randomized controlled trial. J Thorac Cardiovasc Surg 138: 206–214, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chi NC, Karliner JS. Molecular determinants of responses to myocardial ischemia/reperfusion injury: focus on hypoxia-inducible and heat shock factors. Cardiovasc Res 61: 437–447, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Cope LM, Irizarry RA, Jaffee HA, Wu Z, Speed TP. A benchmark for Affymetrix GeneChip expression measures. Bioinformatics 20: 323–331, 2004 [DOI] [PubMed] [Google Scholar]
- 8. del Nido PJ, Mickle DA, Wilson GJ, Benson LN, Coles JG, Trusler GA, Williams WG. Evidence of myocardial free radical injury during elective repair of tetralogy of Fallot. Circulation 76: V174–V179, 1987 [PubMed] [Google Scholar]
- 9. del Nido PJ, Mickle DA, Wilson GJ, Benson LN, Weisel RD, Coles JG, Trusler GA, Williams WG. Inadequate myocardial protection with cold cardioplegic arrest during repair of tetralogy of Fallot. J Thorac Cardiovasc Surg 95: 223–229, 1988 [PubMed] [Google Scholar]
- 10. Delanty N, Reilly MP, Pratico D, Lawson JA, McCarthy JF, Wood AE, Ohnishi ST, Fitzgerald DJ, FitzGerald GA. 8-epi PGF2 alpha generation during coronary reperfusion. A potential quantitative marker of oxidant stress in vivo. Circulation 95: 2492–2499, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 4: P3, 2003 [PubMed] [Google Scholar]
- 12. Dhaliwal H, Kirshenbaum LA, Randhawa AK, Singal PK. Correlation between antioxidant changes during hypoxia and recovery on reoxygenation. Am J Physiol Heart Circ Physiol 261: H632–H638, 1991 [DOI] [PubMed] [Google Scholar]
- 13. Freeman BA, Topolosky MK, Crapo JD. Hyperoxia increases oxygen radical production in rat lung homogenates. Arch Biochem Biophys 216: 477–484, 1982 [DOI] [PubMed] [Google Scholar]
- 14. Garattini E, Mendel R, Romao MJ, Wright R, Terao M. Mammalian molybdo-flavoenzymes, an expanding family of proteins: structure, genetics, regulation, function and pathophysiology. Biochem J 372: 15–32, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghorbel MT, Cherif M, Jenkins E, Mokhtari A, Kenny D, Angelini GD, Caputo M. Transcriptomic analysis of patients with tetralogy of Fallot reveals the effect of chronic hypoxia on myocardial gene expression. J Thorac Cardiovasc Surg 140: 337–345, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hearse DJ, Humphrey SM, Chain EB. Abrupt reoxygenation of the anoxic potassium-arrested perfused rat heart: a study of myocardial enzyme release. J Mol Cell Cardiol 5: 395–407, 1973 [DOI] [PubMed] [Google Scholar]
- 17. Ihnken K, Morita K, Buckberg GD. Delayed cardioplegic reoxygenation reduces reoxygenation injury in cyanotic immature hearts. Ann Thorac Surg 66: 177–182, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Ihnken K, Morita K, Buckberg GD, Matheis G, Sherman MP, Allen BS, Young HH. Studies of hypoxemic/reoxygenation injury: without aortic clamping. II. Evidence for reoxygenation damage. J Thorac Cardiovasc Surg 110: 1171–1181, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Ihnken K, Morita K, Buckberg GD, Sherman MP, Young HH. Studies of hypoxemic/reoxygenation injury: without aortic clamping. III. Comparison of the magnitude of damage by hypoxemia/reoxygenation versus ischemia/reperfusion. J Thorac Cardiovasc Surg 110: 1182–1189, 1995 [DOI] [PubMed] [Google Scholar]
- 20. Ihnken K, Winkler A, Schlensak C, Sarai K, Neidhart G, Unkelbach U, Mulsch A, Sewell A. Normoxic cardiopulmonary bypass reduces oxidative myocardial damage and nitric oxide during cardiac operations in the adult. J Thorac Cardiovasc Surg 116: 327–334, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Imura H, Caputo M, Parry A, Pawade A, Angelini GD, Suleiman MS. Age-dependent and hypoxia-related differences in myocardial protection during pediatric open heart surgery. Circulation 103: 1551–1556, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Ito T, Kimura Y, Uozumi Y, Takai M, Muraoka S, Matsuda T, Ueki K, Yoshiyama M, Ikawa M, Okabe M, Schaffer SW, Fujio Y, Azuma J. Taurine depletion caused by knocking out the taurine transporter gene leads to cardiomyopathy with cardiac atrophy. J Mol Cell Cardiol 44: 927–937, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Julia P, Young HH, Buckberg GD, Kofsky ER, Bugyi HI. Studies of myocardial protection in the immature heart. IV. Improved tolerance of immature myocardium to hypoxia and ischemia by intravenous metabolic support. J Thorac Cardiovasc Surg 101: 23–32, 1991 [PubMed] [Google Scholar]
- 24. Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucl Acids Res 28: 27–30, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kisker C, Schindelin H, Pacheco A, Wehbi WA, Garrett RM, Rajagopalan KV, Enemark JH, Rees DC. Molecular basis of sulfite oxidase deficiency from the structure of sulfite oxidase. Cell 91: 973–983, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Kisker C, Schindelin H, Rees DC. Molybdenum-cofactor-containing enzymes: structure and mechanism. Ann Rev Biochem 66: 233–267, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Konstantinov IE, Coles JG, Boscarino C, Takahashi M, Goncalves J, Ritter J, Van Arsdell GS. Gene expression profiles in children undergoing cardiac surgery for right heart obstructive lesions. J Thorac Cardiovasc Surg 127: 746–754, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Kundu TK, Hille R, Velayutham M, Zweier JL. Characterization of superoxide production from aldehyde oxidase: an important source of oxidants in biological tissues. Arch Biochem Biophys 460: 113–121, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 312: 159–163, 1985 [DOI] [PubMed] [Google Scholar]
- 30. Mehlhorn U, Krahwinkel A, Geissler HJ, LaRosee K, Fischer UM, Klass O, Suedkamp M, Hekmat K, Tossios P, Bloch W. Nitrotyrosine and 8-isoprostane formation indicate free radical-mediated injury in hearts of patients subjected to cardioplegia. J Thorac Cardiovasc Surg 125: 178–183, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Modi P, Imura H, Caputo M, Pawade A, Parry A, Angelini GD, Suleiman MS. Cardiopulmonary bypass-induced myocardial reoxygenation injury in pediatric patients with cyanosis. J Thorac Cardiovasc Surg 124: 1035–1036, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Modi P, Suleiman MS, Reeves B, Pawade A, Parry AJ, Angelini GD, Caputo M. Myocardial metabolic changes during pediatric cardiac surgery: a randomized study of 3 cardioplegic techniques. J Thorac Cardiovasc Surg 128: 67–75, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Morita K, Ihnken K, Buckberg GD, Sherman MP, Young HH. Studies of hypoxemic/reoxygenation injury: without aortic clamping. IX. Importance of avoiding perioperative hyperoxemia in the setting of previous cyanosis. J Thorac Cardiovasc Surg 110: 1235–1244, 1995 [DOI] [PubMed] [Google Scholar]
- 34. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucl Acids Res 29: e45, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reilly MP, Delanty N, Roy L, Rokach J, Callaghan PO, Crean P, Lawson JA, FitzGerald GA. Increased formation of the isoprostanes IPF2alpha-I and 8-epi-prostaglandin F2alpha in acute coronary angioplasty: evidence for oxidant stress during coronary reperfusion in humans. Circulation 96: 3314–3320, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Ruel M, Bianchi C, Khan TA, Xu S, Liddicoat JR, Voisine P, Araujo E, Lyon H, Kohane IS, Libermann TA, Sellke FW. Gene expression profile after cardiopulmonary bypass and cardioplegic arrest. J Thorac Cardiovasc Surg 126: 1521–1530, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Sehl PD, Tai JT, Hillan KJ, Brown LA, Goddard A, Yang R, Jin H, Lowe DG. Application of cDNA microarrays in determining molecular phenotype in cardiac growth, development, and response to injury. Circulation 101: 1990–1999, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Teoh KH, Mickle DA, Weisel RD, Li RK, Tumiati LC, Coles JG, Williams WG. Effect of oxygen tension and cardiovascular operations on the myocardial antioxidant enzyme activities in patients with tetralogy of Fallot and aorta-coronary bypass. J Thorac Cardiovasc Surg 104: 159–164, 1992 [PubMed] [Google Scholar]
- 39. Voisine P, Ruel M, Khan TA, Bianchi C, Xu SH, Kohane I, Libermann TA, Otu H, Saltiel AR, Sellke FW. Differences in gene expression profiles of diabetic and nondiabetic patients undergoing cardiopulmonary bypass and cardioplegic arrest. Circulation 110: II280–II286, 2004 [DOI] [PubMed] [Google Scholar]