Abstract
This study identifies a novel relationship between cerebrospinal fluid (CSF) stroke volume through the cerebral aqueduct and the characteristic peaks of the intracranial pulse (ICP) waveform. ICP waveform analysis has become much more advanced in recent years; however, clinical practice remains restricted to mean ICP, mainly due to the lack of physiological understanding of the ICP waveform. Therefore, the present study set out to shed some light on the physiological meaning of ICP morphological metrics derived by the morphological clustering and analysis of continuous intracranial pulse (MOCAIP) algorithm by investigating their relationships with a well defined physiological variable, i.e., the stroke volume of CSF through the cerebral aqueduct. Seven patients received both overnight ICP monitoring along with a phase-contrast MRI (PC-MRI) of the cerebral aqueduct to quantify aqueductal stroke volume (ASV). Waveform morphological analysis of the ICP signal was performed by the MOCAIP algorithm. Following extraction of morphological metrics from the ICP signal, nine temporal ICP metrics and two amplitude-based metrics were compared with the ASV via Spearman's rank correlation. Of the nine temporal metrics correlated with the ASV, only the width of the P2 region (ICP-Wi2) reached significance. Furthermore, both ICP pulse pressure amplitude and mean ICP did not reach significance. In this study, we showed the width of the second peak (ICP-Wi2) of an ICP pulse wave is positively related to the volume of CSF movement through the cerebral aqueduct. This finding is an initial step in bridging the gap between ICP waveform morphology research and clinical practice.
Keywords: intracranial pressure, normal pressure hydrocephalus, cerebrospinal fluid, intracranial dynamics, phase-contrast MRI
it is well accepted that the shape of the intracranial pressure (ICP) waveform arises as a result of the interaction between the pulsatile cerebral blood flow (CBF) pulse and complex intracranial compartment (3, 5, 8). Typically, the individual ICP waveform is triphasic (24). Early physiological studies concerning the origins of ICP pulse suggested that the first peak of ICP pulse mainly reflects the pulsations of large intracranial conductive arteries and that the third peak of an ICP pulse mainly reflects venous pulsation (2, 24). Logically, one might conclude that the second peak (P2) might represent the cerebral vascular pulsations in the capillary or brain parenchymal phase.
The dynamic, pulsatile movement of intracranial cerebrospinal fluid (CSF) is also highly coupled to CBF (40): specifically, the differential arterial vs. venous intracranial blood volumes occurring during each cardiac pulse. Using careful MRI measurements, Greitz et al. demonstrated that the caudally directed aqueductal movement of CSF is predominantly caused by the blood volume brain expansion, i.e., the capillary filling phase (21).
In the early 1990s, Bradley et al. (7) found a correlation between normal pressure hydrocephalus (NPH) shunt responsiveness and cerebral aqueduct CSF flow velocity. Although other studies revealed inconsistent results with regard to the positive predictive value of this parameter (12, 31), aqueductal stroke volume (ASV) has been used in a wide range of studies (34, 38), including recent work that shows changes in ASV with age (39) and therefore likely represents a fundamental physiological feature. The underlying physiological basis for increased aqueductal CSF flow in hydrocephalus is unknown, but its existence suggests that pulsatile CSF dynamics is key to hydrocephalus pathophysiology.
For several years, our laboratory has been seeking to understand the possible role of pulsatile CSF dynamics in the pathophysiology of hydrocephalus (27). The basic premise is that CSF volumetric buffering is essential for transmission of pulsatile CBF within the cranium. Greitz (21) established the concept that, in normal circumstances, the majority of pulsatile CSF volume buffering occurred within the basal cisterns driven by a cranial-caudal piston action of the brain in response to blood volume changes. A second type of brain movement, inward implosion of the hemispheres, results in a smaller ventricular CSF movement. We proposed that so-called “communicating” hydrocephalus arose from interference of dynamic cisternal CSF movement, a situation that would be difficult to distinguish from the traditional concept of impaired CSF absorption (33). The decreased cisternal CSF buffering capacity would be compensated by an increase in ventricular buffering. This would manifest as an increase in aqueductal CSF stroke volume, which is exactly what Bradley et al. and others found (7, 11, 34).
Here, we examined the possible relationship between ICP waveform morphology and MRI CSF flow dynamics in a group of hydrocephalus patients that had both been directly measured. We hypothesized that characteristics of the P2 peak would be more closely tied to measures of aqueductal CSF flow than the other ICP peaks (P1 and P3). The accurate measurement of ICP waveform morphology requires robust waveform extraction techniques. Our group and others have done a series of work in advancing the techniques of ICP signal analysis (10, 15, 18, 25, 30, 36). We developed the Morphological Clustering and Analysis of Continuous Intracranial Pulse (MOCAIP) algorithm (30), through which one can first robustly identify the locations of the three subpeaks and troughs of an ICP pulse and then systematically extract additional metrics on the ICP morphology. Studies have been conducted using these MOCAIP metrics to detect acute cerebral ventricular enlargement (29), predict acute ICP hypertension (23, 28), detect cerebral hypoperfusion (26), segment overnight ICP slow waves (32), and characterize acute cerebrovascular changes (4). To quantify CSF hydrodynamics in this patient population, CSF movement through the cerebral aqueduct was measured using phase-contrast MRI (PC-MRI) (19).
METHODS
Patient characteristics and data acquisition.
While under evaluation for NPH at the UCLA Adult Hydrocephalus Center, seven patients received both overnight ICP monitoring and a PC-MRI CSF flow protocol. Patients were recommended for extended lumbar drain trail (ELD) based on presence of the clinical triad (gait ataxia, urinary incontinence, and dementia); however, progressive gait ataxia was the most common symptom. All ICP sensor and data acquisition (both imaging and pressure monitoring) were approved by the local IRB committee, and all subjects signed informed consent to participate. All measurements were taken while the patient was in the supine position.
Phase-contrast imaging.
Seven patients (71.1 ± 9.8 yr) received the imaging protocol, which either preceded admission for ELD or followed the ELD by no less than 3 wk, which allowed for equilibration of the baseline hydrodynamics. For patients that had temporarily improved after the ELD trial, all had completely reverted back to their original state at least 2 wk before the MRI study. Patient manuscript ID, age, and sex characteristics are shown in Table 1, all patients showed temporary improvement from ELD along with an average Evan's ratio of 0.35 ± 0.04. All MRI scans were performed using a 3-T Siemens Trio T-class MRI (Siemens Medical Systems, Erlanger, Germany). Using a Siemens Head Matrix bird cage head coil, the participants were placed in the supine position with their neck and head in the neutral position. Phase-contrast imaging took place in the cerebral aqueduct. The imaging protocol contained both anatomical and flow quantification sequences. The standard anatomical sequences included a three-dimensional axial T1-weighted MPRage (1,900 ms/3.4 ms/900 ms/0.84 mm/0.90 mm; TR/TE/TI/resolution/slice thickness), axial T2-weighted BLADE (7,110 ms/107 ms/0.57 mm/3 mm; TR/TE/resolution/slice thickness), and a sagittal T2-weighted Turbo spin echo (TSE) sequence (750 ms/100 ms/0.34 mm/8 mm).
Table 1.
Patient characteristics
| Patient Index | Age, yr | Sex | Time Between Measurements, days |
|---|---|---|---|
| 1 | 73 | Male | −4 |
| 2 | 74 | Male | 0 |
| 3 | 79 | Male | 0 |
| 4 | 84 | Female | −6 |
| 5 | 58 | Male | +33 |
| 6 | 58 | Male | +24 |
| 7 | 72 | Male | +32 |
General patient characteristics including patient index, age, sex, and time separation between the MRI scan and overnight intracranial pulse (ICP) monitoring in days. Negative values represent the MRI preceding the ICP monitoring. Again, for MRI scans following extended lumbar drain trail and ICP monitoring (positive values), at least 3 wk separated the two to allow for baseline hydrodynamics to return.
Flow quantification was achieved using a standard phase-contrast sequence. An oblique plane was defined perpendicular to the presumed direction of CSF flow (Fig. 1). The velocity encoding parameter (Venc) was set with the aid of a flow scout to reduce aliasing. Following the definition of the Venc, the phase-contrast sequence (39.1 ms/6.01 ms/0.63 mm/3 mm) was applied. The average and standard deviation of the Venc for the seven patients was 17.4 ± 4.4, respectively with a range from 12 to 25 cm/s. Finally, a temporal resolution of 30 frames was defined with retrospective gating with either ECG or pulse oximetry signals.
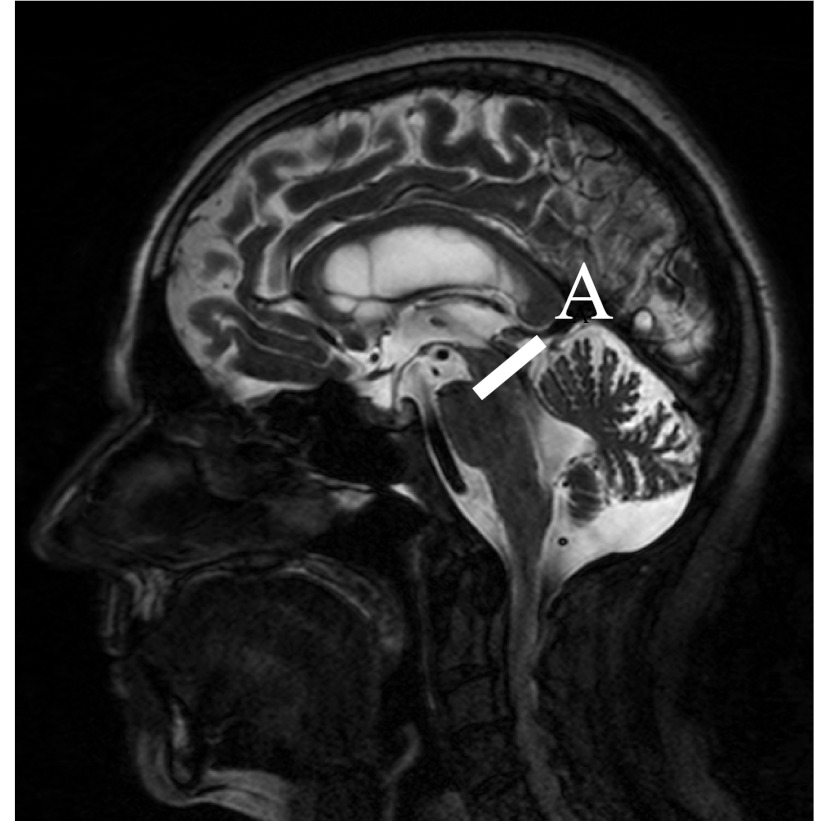
Fig. 1.
Midsagittal T2 depicting cerebral aqueduct. Shows the position of the oblique slices for the cerebral aqueduct (A). The slices are positioned perpendicular to the assumed direction of cerebrospinal fluid (CSF) flow.
Overnight ICP monitoring.
During the overnight monitoring, patients assumed a supine or near supine position. An intraparenchymal ICP microsensor (Codman and Schurtleff, Raynaud, MA) was inserted in the right frontal lobe and continuously monitored ICP one night before the placement of the lumbar drain (LD). Continuous waveform data, including ECG and ICP, was captured using the BedMaster system (Excel Medical Electronics, Jupiter, FL) with a sampling rate of 240 Hz. Following overnight monitoring, the intraparenchymal ICP monitor was removed, and the patient was transitioned into the ELD trial.
Data analysis.
For the imaging data, a semi-automated segmentation algorithm was implemented for the designation of the region of interest (ROI). The algorithm was developed using MATLAB 7.5 R2007b (The MathWorks, Natick, MA), which utilized both anatomical and dynamic information from the phase-contrast study (22). Following the extraction of the ROI, the hydrodynamic metric ASV was calculated as the average of the volume moving in the cranial-caudal and caudal-cranial direction over the cardiac cycle, which represents the net volume through the aqueduct (7).
The ICP waveform morphology characterization was done using the MOCAIP analysis toolbox. MOCAIP provides a real-time, time-domain analysis of intracranial waveform morphology (30). MOCAIP utilizes a five-step process to analyze the raw ICP and ECG inputs (if ECG is not available, pulse detection can still be done using a modified algorithm) into a series of clean valid ICP pulses (known as a dominant pulse) based on a segment of raw data. First, ECG is used to segment the raw ICP signal into a series of pulses. Following the pulse segmentation, a segment of pulses (1-min segment in this study) is clustered using a hierarchical clustering method, which defines the dominant pulse of the cluster as the mean of the largest cluster. This alone, however, could still produce an invalid ICP pulse (dominant pulse) because the entire segment could be noise; therefore, the segment's dominant pulse is checked against a reference ICP pulse library to determine the legitimacy of the pulse. Once the dominant pulse has been verified, six landmarks are determined, the three characteristic peaks and their corresponding valleys. The automated process for identifying these peaks has evolved over time, and details can be found in previous publications (37). Following the identification of the landmarks, 128 MOCAIP metrics are extracted for each dominant pulse; a detailed description of the MOCAIP algorithm can be found in Hu et al. (30).
There were 2,785 dominant ICP pulses analyzed for the patient population. The results had a mean and standard deviation of 397.9 ± 95.8 dominant pulses per patient, with a range from 209 to 503 pulses.
For the individual dominant pulses, we focused on nine temporal metrics and two common amplitude metrics. The small subset of metrics were chosen to specifically investigate the relationship of the three characteristic peaks where the amplitude metrics (mean ICP and ICP pulse pressure wave amplitude) are commonly used as the gold-standard measurement in ICP monitoring/analysis. For the temporal metrics, we investigated three physiological parameters related to each of the three characteristic pulses, giving a total of nine metrics. The three physiological parameters are the rising time of the leading edge (the time between the preceding valley and subsequent peak), the time of the falling edge (the time from the peak to the following valley), and, finally, the time between subsequent valley points (representing the width of the peak). Mean ICP (mICP) and the ICP pulse pressure amplitude (waveAmp) were chosen as the amplitude metrics for this study.
Following calculation of the metrics sets (PC-MRI and ICP), Spearman's rank correlation coefficient (ρ) was used to quantity the dependencies of the ICP metrics and the ASV. Each patient's overnight ICP data contained several dominant pulses produced by the MOCAIP algorithm. Therefore, for the statistical comparison, the median values of the overnight metric (nine temporal and two amplitude metrics) were compared with the ASV imaging data. The overnight section of ICP data was selected based on relatively low noise compared with the day time hours and patient position (supine); this allowed us to remove any subjectivity in selecting specific segments of ICP and has been used in several studies (9, 17, 32). Finally, to account for the multiple comparisons used in this study, the statistical level of 0.05 was altered based on the Bonferroni correction. All statistical tests were performed in MATLAB 7.5 R2007b.
RESULTS
The ASV for the seven patients had a mean ± SD of 0.156 ± 0.043 ml. These values compare well with previously published ASV for NPH patients (1, 5). The age of the patients did not show any significant relationship for either the ASV or ICP metrics.
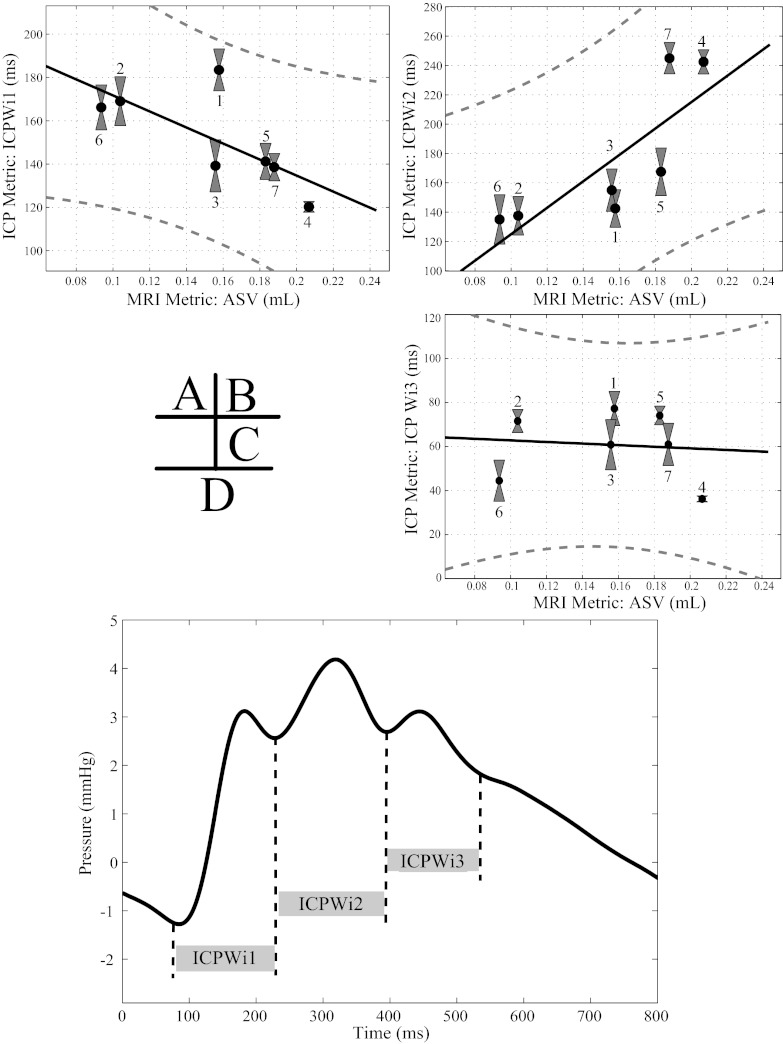
The correlation values between the ICP temporal metrics and PC-MRI ASV metrics are shown in Table 2, with significant values marked with an asterisk for P values of <0.05 corrected for multiple comparisons. Of the nine temporal metrics correlated with the ASV, only the width of P2 (ICP-Wi2) was significantly correlated (P = 0.0025); however, the rising edge of P2 (ICP-Wi2+) only trended toward significance (P = 0.0068) based off multiple comparisons. Scatter plots of selected metrics (peak widths) are shown in Fig. 2, A–C, along with a linear fit of the variables and a 95% prediction interval. Also shown in Fig. 2D is a sample ICP pulse pressure waveform that depicts the definitions of the studied temporal metrics. Both the ICP pulse pressure amplitude (P = 0.023) and the mean ICP (P = 0.6445) failed to reach the corrected significance level (Fig. 3).
Table 2.
Correlation values for ICP temporal metrics and MRI aqueductal stroke volume
| P1 |
P2 |
P3 |
||||
|---|---|---|---|---|---|---|
| Metric Type | Metric | ρ | Metric | ρ | Metric | ρ |
| Rising | ICP-Wi1+ | −0.5 | ICP-Wi2+ | 0.9 | ICP-Wi3+ | −0.1 |
| Falling | ICP-Wi1− | −0.3 | ICP-Wi2− | 0.7 | ICP-Wi3− | −0.1 |
| Pulse width | ICP-Wi1 | −0.6 | ICP-Wi2 | 0.9* | ICP-Wi3 | −0.1 |
Spearman's rank correlation coefficient (ρ) for the aqueductal stroke volume. The metrics are divided into three regions defined by the characteristic ICP pulse pressure waveform peaks (columns P1, P2, P3) and by temporal category (rising time, falling time, and peak width time).
Significant correlations (P < 0.05).
Fig. 2.
Intracranial pulse (ICP) temporal metrics correlations with MRI aqueductal stroke volume (ASV). Plots with linear fit and 95% prediction interval: ICP-Wi1 vs. ASV (A), ICP-Wi2 vs. ASV (B) ICP-Wi3 vs. ASV (C). D: a sample ICP pulse pressure waveform with the corresponding temporal width metrics are shown graphically. On A–C, gray boxes show the standard deviation of the overnight ICP metrics along with the unique patient index defined in Table 1.
Fig. 3.
ICP amplitude metric correlations with MRI ASV. Scatter plots of mean ICP vs. ASV (A) and ICP pulse pressure amplitude (waveAmp) vs. ASV (B) are shown with the unique patient index defined in Table 1.
DISCUSSION
Our study found a significant positive correlation between the width of the second peak of an ICP pulse (ICP-Wi2) and the aqueduct stroke volume (ASV) in patients with probable NPH. This was a rather specific finding in that neither mean ICP, the pulse amplitude (waveAmp), nor the width of the other two peaks achieved a significant correlation with ASV. This correlation supports our hypothesis that the P2 peak morphology would be more closely tied to measures of aqueductal CSF flow than the other ICP peaks (P1 and P3).
As shown in Table 2, the significant correlation with ASV was exclusive to the P2 region of ICP. Qualitatively the different characteristics of the three regions of an ICP pulse can be seen in Fig. 4 where two sets of ICP pulse pressure waveforms (Fig. 4, C and D) are shown for relatively different ASV (Fig. 4B). Furthermore, the waveforms in Fig. 4, C and D, have equal mean ICP values (for both sets of waveforms) since it has been well established that mean ICP impacts ICP waveform morphology (24). One interpretation for this result is that the driving pressure required to displace an increased CSF volume through the aqueduct is related to the second peak of the ICP pulse. As noted previously, early physiological studies suggested that the first peak of ICP pulse primarily reflects the pulsations of large intracranial conductive arteries and that the third peak of an ICP pulse mainly reflects venous pulsation (2, 24). We of course can only speculate as to what factors contribute to the P2 morphology. The high correlation with ASV might suggest that capillary-phase brain expansion, as proposed by Greitz (21), is a likely dominant component. There are other possible drivers of ventricular CSF pulsatile movement, including pulsations in the ventricular choroid plexus (6).
Fig. 4.
Qualitative ICP waveform and ASV comparison. A: scatter plot of ICP-Wi2 vs. ASV, where ICP-Wi2 represents the width of P2. The correlation is significant with a P value of 0.0025. B: the ASV for two selected patients from A. C: two example dominant ICP pulses with different mean ICPs for patient 2 (low ASV). D: two example dominant ICP pulses with different mean ICPs for patient 4 (high ASV). For C and D, the dominant pulses are plotted on separate axes, which are color coded (black waveforms correspond to the left y-axis and the gray waveforms are plotted on the right for both C and D) and are meant to compare waveform shapes between patients at equal mean ICP values. The mean ICP of the pressure pulse for each pair of dominant pulses (black and gray from C and D) are within 0.1 mmHg of each other (black = 8.3 mmHg; gray = 3.0 mmHg).
Physiologically, the relationship between the width of P2 and the ASV is likely related to the finite time required for capillary bed expansion after each heart beat. Since the early 1940s, CSF flow dynamics have been tied to arterial blood flow into the cranial vault (35). Greitz et al. expanded on this idea using phase-contrast MRI, where they showed distinct brain expansion and retractions phases with an estimation of CSF flow in the aqueduct delayed several hundred milliseconds after the ECG R-wave due to the capillary bed expansion (20). In our study, the PC-MRI measurement was made in the cerebral aqueduct. Incorporating the relationship between capillary bed expansion and CSF movement through the aqueduct, we believe that the proposed link between the temporal metric of ICP and ASV is justifiable based on the capillary filling time. Moreover, we believe that the temporal correlations of ICP do not end with the filling of the capillary bed, as suggested by this study. We speculate a different temporal ICP metric may be correlated if a pulsatility measurement (flow stroke volume, etc.) was made in another location (foramen of magnum, prepontine cistern, etc.). Finally, from a technical standpoint, studies investigating ICP pulse pressure temporal metrics are rare; Eide et al. reported no significant difference between NPH shunt responders and nonresponders for mean ICP rise time (time from diastolic to the systolic peak) (17). However, to our knowledge, the temporal features of the subpeaks of the ICP pulse have not been studied. MOCAIP provides the ability to reliably study subpeak temporal metrics and thus addresses an unmet need in the investigation of ICP waveform morphology investigation.
The existence of correlation between a specific local feature of ICP pulses and ASV indicates that the global mean value of ICP and the characteristics of the three individual regions of an ICP pulse may reflect different aspects of the CSF dynamics and can provide complementary information. Such a finding therefore challenges the current paradigm of monitoring only mean ICP while ignoring the shape of an ICP pulse (16). Eide's recent clinical studies have shown that ICP amplitude can be potentially more important to guide the management of hydrocephalus patients (14, 17) and subarachnoid hemorrhage patients (13) than mean ICP. The present work shows that temporal metrics, such as ICP-Wi2, may provide additional insights to CSF dynamics and play a role in managing patients with a wide variety of altered CSF dynamics.
A few tangentially related studies have shown relationships between ICP and CSF dynamics, although not directly linking ICP morphological metrics and CSF dynamics. Alperin et al. utilized the characteristic pressure volume curve to estimate ICP noninvasively using MRI to quantify CSF and blood flow into the cranial vault (3). Although this study did not address ICP waveform morphology, it did suggest an association between the two measures of pulsatility investigated in our study. Another study investigated the efficacy of ASV for predicting outcome of shunt surgery in NPH, comparing ASV to lumbar CSF pressures before and during lumbar infusion study. The results showed no significant correlation between ASV and mean baseline lumbar pressure or ASV and mean plateau pressure. However, there was a correlation with both baseline and plateau pulse amplitude and ASV (31). Again, the aforementioned studies simply suggest a possible relationship between noninvasive measures of CSF pulsatility and invasive pressure measurements, which act as reasonable background for our results.
A limitation of our study was the small sample size. In our study design, patients being evaluated for possible NPH were given the option to participate in research MRI study. Some chose not to for personal reasons (including claustrophobia), whereas, for some, the MRI studies obtained were not analyzable due to technical reasons (such as malfunctioning ECG gating software). In one respect, this small number of subjects coupled with the multiple comparisons would challenge us more in achieving a significant P value when quantifying the correlation between metrics. We also acknowledge that there was a variable time lag between the ICP measurement and the time of PC-MRI imaging. However, acute changes of either ICP pulse morphology or CSF flow dynamics are unlikely in this patient population, and simultaneous measurements of invasive ICP morphology and CSF dynamics are not currently possible. It remains to be studied whether ICP-Wi2 can be a specific and sensitive metric to provide continuous surveillance of acute changes of the ASV.
In conclusion, in this study, we showed the width of the second peak of an ICP pulse wave is positively related to the volume of pulsatile CSF movement through the cerebral aqueduct. This finding is important because it helps our understanding of the specific ICP pulse wave morphological metrics studied in this work. Given the existing evidence that increased aqueductal CSF stroke volume is a marker of impaired CSF flow dynamics, ICP monitoring equipped with a metric of second peak width can thus offer continuous assessment of CSF dynamics change that mean ICP will fail to capture.
GRANTS
The present work is partially supported by National Institute of Neurological Disorders and Stroke Grants NS-059797, NS-054881, and NS-066008.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.B.H., P.V., X.H., and M.B. conception and design of research; R.B.H., K.B., and J.F. analyzed data; R.B.H., X.H., and M.B. interpreted results of experiments; R.B.H. prepared figures; R.B.H. drafted manuscript; R.B.H., X.H., and M.B. edited and revised manuscript; R.B.H., X.H., and M.B. approved final version of manuscript; P.V. performed experiments.
ACKNOWLEDGMENTS
We thank Tatiana Orloff and Elizabeth Cattell for clinical support on the floor unit.
REFERENCES
- 1. Abbey P, Singh P, Khandelwal N, Mukherjee KK. Shunt surgery effects on cerebrospinal fluid flow across the aqueduct of Sylvius in patients with communicating hydrocephalus. J Clin Neurosci 16: 514–518, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Adolph RJ, Fukusumi H, Fowler NO. Origin of cerebrospinal fluid pulsations. Am J Physiol 212: 840–846, 1967 [DOI] [PubMed] [Google Scholar]
- 3. Alperin NJ, Lee SH, Loth F, Raksin PB, Lichtor T. MR-Intracranial pressure (ICP): a method to measure intracranial elastance and pressure noninvasively by means of MR imaging: baboon and human study. Radiology 217: 877–885, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Asgari S, Bergsneider M, Hamilton R, Vespa P, Hu X. Consistent changes in intracranial pressure waveform morphology induced by acute hypercapnic cerebral vasodilatation. Neurocrit Care 15: 55–62, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baledent O, Gondry-Jouet C, Meyer ME, De Marco G, Le Gars D, Henry-Feugeas MC, Idy-Peretti I. Relationship between cerebrospinal fluid and blood dynamics in healthy volunteers and patients with communicating hydrocephalus. Invest Radiol 39: 45–55, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Bering EA., Jr Choroid plexus and arterial pulsation of cerebrospinal fluid; demonstration of the choroid plexuses as a cerebrospinal fluid pump. AMA 73: 165–172, 1955 [DOI] [PubMed] [Google Scholar]
- 7. Bradley WG, Jr, Scalzo D, Queralt J, Nitz WN, Atkinson DJ, Wong P. Normal-pressure hydrocephalus: evaluation with cerebrospinal fluid flow measurements at MR imaging. Radiology 198: 523–529, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Carrera E, Kim DJ, Castellani G, Zweifel C, Czosnyka Z, Kasparowicz M, Smielewski P, Pickard JD, Czosnyka M. What shapes pulse amplitude of intracranial pressure? J Neurotrauma 27: 317–324, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Czosnyka M, Pickard JD. Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiatry 75: 813–821, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery 41: 11–17; discussion 17–19, 1997 [DOI] [PubMed] [Google Scholar]
- 11. de Marco G, Idy-Peretti I, Didon-Poncelet A, Baledent O, Onen F, Feugeas MC. Intracranial fluid dynamics in normal and hydrocephalic states: systems analysis with phase-contrast magnetic resonance imaging. J Comput Assist Tomogr 28: 247–254, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Dixon GR, Friedman JA, Luetmer PH, Quast LM, McClelland RL, Petersen RC, Maher CO, Ebersold MJ. Use of cerebrospinal fluid flow rates measured by phase-contrast MR to predict outcome of ventriculoperitoneal shunting for idiopathic normal-pressure hydrocephalus. Mayo Clin Proc 77: 509–514, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Eide PK, Bentsen G, Stanisic M, Stubhaug A. Association between intracranial pulse pressure levels and brain energy metabolism in a patient with an aneurysmal subarachnoid haemorrhage. Acta Anaesthesiol Scand 51: 1273–1276, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Eide PK, Brean A. Intracranial pulse pressure amplitude levels determined during preoperative assessment of subjects with possible idiopathic normal pressure hydrocephalus. Acta Neurochir (Wien) 148: 1151–1156; discussion 1156, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Eide PK, Fremming AD. A new method and software for quantitative analysis of continuous intracranial pressure recordings. Acta Neurochir (Wien) 143: 1237–1247, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Eide PK, Rapoport BI, Gormley WB, Madsen JR. A dynamic nonlinear relationship between the static and pulsatile components of intracranial pressure in patients with subarachnoid hemorrhage. J Neurosurgery 112: 616–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eide PK, Sorteberg W. Diagnostic intracranial pressure monitoring and surgical management in idiopathic normal pressure hydrocephalus: a 6-year review of 214 patients. Neurosurgery 66: 80–91, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Ellis T, McNames J, Aboy M. Pulse morphology visualization and analysis with applications in cardiovascular pressure signals. IEEE Trans Biomed Eng 54: 1552–1559, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Enzmann DR, Pelc NJ. Cerebrospinal fluid flow measured by phase-contrast cine MR. Am J Neuroradiol 14: 1301–1307; discussion 1309–1310, 1993 [PMC free article] [PubMed] [Google Scholar]
- 20. Greitz D, Franck A, Nordell B. On the pulsatile nature of intracranial and spinal CSF-circulation demonstrated by MR imaging. Acta Radiol 34: 321–328, 1993 [PubMed] [Google Scholar]
- 21. Greitz D, Wirestam R, Franck A, Nordell B, Thomsen C, Stahlberg F. Pulsatile brain movement and associated hydrodynamics studied by magnetic resonance phase imaging. The Monro-Kellie doctrine revisited. Neuroradiology 34: 370–380, 1992 [DOI] [PubMed] [Google Scholar]
- 22. Hamilton R, Dye J, Frew A, Baldwin K, Hu X, Bergsneider M. Quantification of pulsatile, cerebrospinal fluid flow within in the prepontine cistern. Acta Neurochir (Wien) 114: 191–195, 2012 [DOI] [PubMed] [Google Scholar]
- 23. Hamilton R, Xu P, Asgari S, Kasprowicz M, Vespa P, Bergsneider M, Hu X. Forecasting intracranial pressure elevation using pulse waveform morphology. Conf Proc IEEE Eng Med Biol Soc 2009: 4331–4334, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Hirai O, Handa H, Ishikawa M, Kim SH. Epidural pulse waveform as an indicator of intracranial pressure dynamics. Surgical Neurol 21: 67–74, 1984 [PubMed] [Google Scholar]
- 25. Hornero R, Aboy M, Abasolo D, McNames J, Goldstein B. Interpretation of approximate entropy: analysis of intracranial pressure approximate entropy during acute intracranial hypertension. IEEE Trans Biomed Eng 52: 1671–1680, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Hu X, Glenn T, Scalzo F, Bergsneider M, Sarkiss C, Martin N, Vespa P. Intracranial pressure pulse morphological features improved detection of decreased cerebral blood flow. Physiol Measurement 31: 679–695, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hu X, Hamilton R, Baldwin K, Vespa P, Bergsneider M. Automated extraction of decision rules for predicting lumbar drain outcome by analyzing overnight intracranial pressure. Acta Neurochir (Wien) 114: 207–212, 2012 [DOI] [PubMed] [Google Scholar]
- 28. Hu X, Xu P, Asgari S, Vespa P, Bergsneider M. Forecasting ICP elevation based on prescient changes of intracranial pressure waveform morphology. IEEE Trans Biomed Eng 57: 1070–1078, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hu X, Xu P, Lee DJ, Paul V, Bergsneider M. Morphological changes of intracranial pressure pulses are correlated with acute dilatation of ventricles. Acta Neurochir (Wien) 102: 131–136, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Hu X, Xu P, Scalzo F, Vespa P, Bergsneider M. Morphological clustering and analysis of continuous intracranial pressure. IEEE Trans Biomed Eng 56: 696–705, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kahlon B, Annertz M, Stahlberg F, Rehncrona S. Is aqueductal stroke volume, measured with cine phase-contrast magnetic resonance imaging scans useful in predicting outcome of shunt surgery in suspected normal pressure hydrocephalus? Neurosurgery 60: 124–129; discussion 129–130, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Kasprowicz M, Asgari S, Bergsneider M, Czosnyka M, Hamilton R, Hu X. Pattern recognition of overnight intracranial pressure slow waves using morphological features of intracranial pressure pulse. J Neurosci Methods 190: 310–318, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lorenzo AV, Page LK, Watters GV. Relationship between cerebrospinal fluid formation, absorption and pressure in human hydrocephalus. Brain 93: 679–692, 1970 [DOI] [PubMed] [Google Scholar]
- 34. Luetmer PH, Huston J, Friedman JA, Dixon GR, Petersen RC, Jack CR, McClelland RL, Ebersold MJ. Measurement of cerebrospinal fluid flow at the cerebral aqueduct by use of phase-contrast magnetic resonance imaging: technique validation and utility in diagnosing idiopathic normal pressure hydrocephalus. Neurosurgery 50: 534–543; discussion 543–534, 2002 [DOI] [PubMed] [Google Scholar]
- 35. O'Connell J. The vascular factor in intracranial pressure and the maintenance of the cerebrospinal fluid circulation. Brain 66: 204–228, 1943 [Google Scholar]
- 36. Santamarta D, Hornero R, Abasolo D, Martinez-Madrigal M, Fernandez J, Garcia-Cosamalon J. Complexity analysis of the cerebrospinal fluid pulse waveform during infusion studies. Childs Nerv Syst 26: 1683–1689, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Scalzo F, Asgari S, Kim S, Bergsneider M, Hu X. Bayesian tracking of intracranial pressure signal morphology. Artif Intell Med 54: 115–123, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schroeder HW, Schweim C, Schweim KH, Gaab MR. Analysis of aqueductal cerebrospinal fluid flow after endoscopic aqueductoplasty by using cine phase-contrast magnetic resonance imaging. J Neurosurgery 93: 237–244, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Stoquart-ElSankari S, Baledent O, Gondry-Jouet C, Makki M, Godefroy O, Meyer ME. Aging effects on cerebral blood and cerebrospinal fluid flows. J Cereb Blood Flow Metab 27: 1563–1572, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Ursino M, Lodi CA. A simple mathematical model of the interaction between intracranial pressure and cerebral hemodynamics. J Appl Physiol 82: 1256–1269, 1997 [DOI] [PubMed] [Google Scholar]