Abstract
Exercise capacity and performance strongly associate with metabolic and biophysical characteristics of skeletal muscle, factors that also relate to overall disease risk. Despite its importance, the exact mechanistic features that connect aerobic metabolism with health status are unknown. To explore this, we applied artificial selection of rats for intrinsic (i.e., untrained) aerobic treadmill running to generate strains of low- and high-capacity runners (LCR and HCR, respectively), subsequently shown to diverge for disease risk. Concurrent breeding of LCR and HCR per generation allows the lines to serve as reciprocal controls for unknown environmental changes. Here we provide the first direct evidence in mitochondria isolated from skeletal muscle that intrinsic mitochondrial capacity is higher in HCR rats. Maximal phosphorylating respiration was ∼40% greater in HCR mitochondria, independent of substrate and without altered proton leak or major changes in protein levels or muscle fiber type, consistent with altered control of phosphorylating respiration. Unexpectedly, H2O2 emission was ∼20% higher in HCR mitochondria, due to greater reduction of more harmful reactive oxygen species to H2O2; indeed, oxidative modification of mitochondrial proteins was lower. When the higher mitochondrial yield was considered, phosphorylating respiration and H2O2 emission were 70–80% greater in HCR muscle. Greater capacity of HCR muscle for work and H2O2 signaling may result in enhanced and more immediate cellular repair, possibly explaining lowered disease risks.
Keywords: skeletal muscle, mitochondria, reactive oxygen species, rat, selective breeding.
clinical studies reveal a strong statistical relationship between low aerobic endurance exercise capacity and all-cause morbidity and mortality (43). Indeed, for both healthy individuals and those with cardiovascular disorders, low exercise capacity is a stronger predictor of decreased survival compared with other conventional risk factors such as body mass index, smoking, hypertension, or diabetes (36, 43). Despite its importance, the mechanisms that connect aerobic metabolism with health status are unknown.
For clues, we and others have looked for patterns within our evolutionary history as a basis for generating a hypothesis wide enough to encompass these most complex of phenotypes (32, 33, 45, 67). Much attention has been given to the pivotal importance of oxygen availability in the evolutionary transition from single to multicellular complexity (49, 50). We speculated that the deeply embedded energy transfer role of oxygen in our evolutionary history translated forward such that low integrity of aerobic metabolism is the leading determinant of increased risk for complex disease (aerobic hypothesis). As a first test of this hypothesis, we used artificial selective breeding to develop two strains of genetically heterogeneous rats that differ for inborn maximal endurance running capacity (34, 69). This artificial selection strategy allowed prospective testing of the hypothesis that intrinsic capacity for aerobic metabolism segregates with disease risk factors. Importantly, the underlying mechanisms can be sought in the resulting contrasting animal models. Experimental outcomes were in accord with the hypothesis. That is, numerous studies have demonstrated that the low-capacity runner (LCR) and high-capacity runner (HCR) rats differ markedly for physiological features underlying endurance performance (18, 31, 52) and for numerous disease risk factors (22, 62, 69).
Evidence has been presented that mitochondrial content and some aspects of mitochondrial function and protein expression differ between LCR and HCR and thus may at least partially explain the divergent aerobic capacity and risk for disease (44, 52, 62, 64, 69). While mitochondrial content contributes importantly to aerobic capacity, the intrinsic function of mitochondria may be at least as significant. For example, in brown adipose tissue mitochondria, regardless of abundance, the activation of uncoupling protein 1 is required for the dramatic increases in cellular respiration and the resulting thermogenesis. Also, primary mitochondrial disease can be associated with an increase in mitochondrial mass that may only at best preserve some functional aspects (19, 42), indicating that increased mitochondrial content may in fact reflect a compensation for decreased inherent function of mitochondria. Recent studies have indicated the role of posttranslational modification in the control of oxidative phosphorylation (2), further suggesting that inherent function, and not just content, contributes importantly to overall mitochondrial function. The goal of the present study was to determine whether, in addition to mitochondrial content, intrinsic mitochondrial electron transport chain function also differed between LCR and HCR rats. In addition to assessing phosphorylation capacity, we also tested the capacity of mitochondria for reactive oxygen species (ROS) generation and detoxification because mitochondria are the primary source of ROS in many cell types and mitochondrial ROS can play important roles in cellular (dys)function including sensitivity of glucose uptake to insulin (3, 6, 23, 26, 41). Here we provide the first direct evidence in mitochondria isolated from skeletal muscle that intrinsic mitochondrial electron transport chain function is elevated in HCR rats. This result is important and novel because it supports the concept that intrinsic skeletal muscle metabolic features that underlie high intrinsic functional capacity can differ markedly from those acquired from training.
MATERIALS AND METHODS
Reagents.
Unless otherwise specified, all chemicals were purchased from Sigma (Oakville, ON, Canada).
Animals.
Rats were selected for 23 generations as described previously (34). Twenty-four female rats (12 LCR and 12 HCR) were transferred from University of Michigan to University of Ottawa and given at least 3 wk to acclimatize. Rats were housed singly, in standard caging, at 23°C (12:12-h light cycle, lights on 0600) with free access to chow (4.5% fat/weight; Harlan-Teklad) and water, without running wheels. With the exception of three bouts of treadmill running to establish LCR and HCR phenotype, rats did not run on a treadmill or perform structured exercise. Each run test was performed at a 15° incline using a speed-ramped protocol that started at 10 m/min and was increased by 1 m/min every 2 min. Animals were cared for in accordance with the principles and guidelines of the Canadian Council on Animal Care and the Institute of Laboratory Animal Resources (National Research Council). This study was approved by the Animal Care Committee of the University of Ottawa and the University Committee on Use and Care of Animals of the University of Michigan in accordance with National Institutes of Health guidelines.
Isolation of mitochondria from skeletal muscle.
On each experimental day, two isolations (from 1 leg/rat) were performed concurrently from an HCR and an LCR rat. Rats were killed under isofluorane anesthesia by cardiac puncture at ∼8:00 AM. Isolation of skeletal muscle mitochondria was performed on ice or at 4°C, according to a modified version of the procedure described by Chappell and Perry (10) and Seifert et al. (55). Skeletal muscle was dissected from one leg; the sample contained gastrocnemius, soleus, tibialis anterior, and vastus lateralis, and thus comprised both type I and type II (a, x, and b) muscle fibers. Dissected muscle was placed in ice-cold basic medium (BM: 140 mM KCl, 20 mM, HEPES, 5 mM MgCl2, and 1 mM EGTA, pH 7.0), rapidly cleaned of visible connective tissue and fat, weighed, minced, and placed in 15 vol of homogenizing medium [HM: BM with 1 mM ATP, and 1% BSA (wt/vol)] plus one unit of protease (subtilisin A) per grams muscle wet weight. Tissue was homogenized using a glass/Teflon Potter-Elvehjem tissue grinder and centrifuged at 800 g for 9 min at 4°C. The supernatant was collected and spun at 12,000 g for 9 min, 4°C. The pellet was resuspended in BM and left on ice for 5 min (myofibrillar repolymerization). Samples were then spun at 800 g for 9 min, 4°C, and the supernatant was collected and spun at 12,000 g for another 9 min at 4°C. The final pellet was resuspended in BM (∼200 μl). Protein concentration was determined by a modified Lowry method with BSA as standard.
Mitochondrial oxygen consumption.
Oxygen consumption of isolated mitochondria was measured at 37°C using a Clark-type oxygen electrode (Hansatech, Norfolk, UK). Mitochondrial protein (0.2 mg protein/ml) was added to prewarmed incubation medium [IM: 120 mM KCl, 5 mM HEPES, 5 mM MgCl2, 1 mM EGTA, 5 mM KH2PO43−, and 0.3% BSA (wt/vol), pH 7.4]. State 3 (maximal phosphorylating) and state 4 (maximal nonphosphorylating) respiration were determined for each of the following substrates: the NADH-linked substrate, pyruvate and malate (5 and 2.5 mM, respectively), the fatty acid palmitoyl-l-carnitine/malate (20 μM/1 mM), or succinate (10 mM), which enters the electron transport chain directly at complex II. ADP (200 μM) was added to induce state 3 respiration. Concentrations of substrates and ADP were saturating. The respiratory control ratio was determined as state 3/state 4. The oxygen electrode was calibrated using air-saturated IM (100% O2) followed by sodium dithionite addition (0% O2). The amount of dissolved O2 was taken as ∼409 nmol/ml (51). Oxygen consumption rates were expressed as nanomoles of oxygen per milligrams of mitochondrial protein per minute. Determinations for each substrate were made in duplicate.
Mitochondrial proton leak kinetics.
Mitochondrial protonmotive force (PMF) and O2 consumption were determined in parallel. Mitochondrial PMF was measured fluorimetrically (FLx800; BioTek, Winooski, VT), using Safranin O dye (5 μM; excitation 485 nm, emission 580 nm; Ref. 55). Oxygen consumption was measured in parallel using a Clark electrode. All determinations were performed with 0.2 mg/ml mitochondrial protein in IM prewarmed to 37°C with succinate (10 mM) as the substrate. Leak kinetics were determined by measuring O2 consumption and PMF under state 4 conditions (maximal nonphosphorylating, using oligomycin at 8 μg/mg mitochondria) and then titrating electron transport chain activity with malonate (5 × 1 mM additions). The following were also included in the reaction: rotenone (5 μM) and nigericin (0.4 μg/ml; nigericin mediates transport of K+ out of the matrix in exchange for H+, thereby dissipating the pH component of PMF). Oxygen consumption and PMF determinations were made in duplicate.
Mitochondrial H2O2 emission.
The rate of H2O2 emission was determined using the p-hydroxyphenyl acetic acid/horseradish peroxidase assay (27). The p-hydroxyphenyl acetic acid (167 μg/ml) and H2O2, in the presence of the horseradish peroxidase assay, is excited at 320 nm to form a fluorescent product at 400 nm. The rate of H2O2 emission was monitored using a temperature-controlled fluorimeter (FLx800; BioTek). Determinations were made in IM, at 37°C, using 0.3 mg/ml of mitochondrial protein, and with addition of CuZnSOD (60 U/ml) to convert all superoxide into H2O2.
The rate of H2O2 emission of isolated mitochondria was assessed in the presence of oligomycin (8 μg/mg) or with addition of either the complex III inhibitor antimycin (5 μM) or the complex I inhibitor rotenone (5 μM). Mitochondria were added to prewarmed IM and the reaction was initiated by addition of substrate [18 μM palmitoyl-l-carnitine (PCarn), 5/2.5 mM pyruvate/malate, or 10 mM succinate]. Thus ROS production from different sites of the electron transport chain, as well as with different substrates, was assessed. Experiments were carried out in duplicate. Controls were run in the absence of mitochondria to account for any background rates. Rate of change in fluorescence was calculated from the linear portion of the fluorescence vs. time relationship, then converted into rate of H2O2 emission using a calibration curve obtained with H2O2 at the end of each experimental session, and determined in the presence of mitochondria since mitochondria quench the fluorescence.
Western blot analysis.
The following primary antibodies were used at the indicated dilutions: complex I 39-kDa subunit (1:2,000; Invitrogen, Carlsbad, CA), succinate dehydrogenase subunit A (SDHA; 1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA), complex III core I subunit (1:4,000; Invitrogen), complex IV cyclooxygenase-I subunit (COX-I; 1:1,000; MitoSciences, Eugene, OR), adenine nucleotide translocase (ANT; 1:1,000; Santa Cruz); superoxide dismutase 2 (SOD2; 1:1,000; Santa Cruz), uncoupling protein 3 (UCP3; 1:1,000; Abcam, Cambridge, MA), glutathione peroxidase 4 (GPx4; 1:500; Abcam); hexokinase II (Hex II; 1:2,000; Calbiochem, San Diego, CA), and aconitase (1:5,000; a gift from Dr. Luke Szweda). After blocking for 1 h at room temperature in 5% skim milk, incubation in primary antibody was overnight at 4°C (except for aconitase, which was 1 h at room temperature). Following 3 × 10 min washes with TBS + 0.1% Tween-20, incubation in the appropriate horseradish peroxidase-conjugated secondary antibody (Santa Cruz) diluted in 5% skim milk was at room temperature for 1 h. Bands were visualized using enhanced chemiluminescence. Band intensity was quantified by densitometry (ImageJ, Scion, Frederick, MD) and expressed relative to the intensity of the complex I 39-kDa, the SDHA band, or the Ponceau-stained blot for that sample.
Oxidative stress.
Oxidative stress was determined in mitochondrial extracts by immunoblot detection of 4-hydroxynonenal (4-HNE)-modified proteins (the stable fluorophore resulting from lysine-HNE crosslinks; Ref. 63) by polyclonal rabbit primary antibody (1:1,000; a gift from Dr. Luke Szweda). Band intensity was measured by densitometry (ImageJ software; National Institutes of Health); all bands were included in the analysis (see Fig. 3D).
Fig. 3.
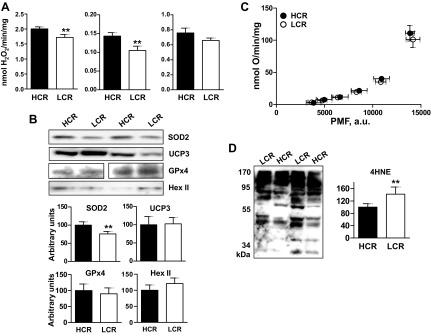
Higher H2O2 emission in skeletal muscle mitochondria from HCR. A: H2O2 emission from mitochondria oxidizing palmitoyl-l-carnitine (Pcarn; 18 μM + 5 μM antimycin), or pyruvate/malate (P/M, 5 mM/2.5 mM) + 5 μM rotenone, or succinate alone (10 mM). Values are means ± SE; n = 6 rats/group. **P = 0.03, unpaired t-test. In all cases, 0.3 mg mitochondrial protein/ml were used. Rates were calculated from the linear portion of the curves. B: expression of antioxidant proteins in mitochondria. Top: representative Western blots; bottom: values are means ± SE; n = 6 rats/group, relative to complex I (39-kDA protein). In all cases, 20 μg of mitochondrial protein/lane were used. SOD2, superoxide dismutase2, UCP3: uncoupling protein 3; Gpx4, glutathione peroxidase4; Hex II, hexokinase II; a.u., arbitrary units. **P = 0.03, unpaired t-test. C: proton leak kinetics. Values are means ± SE; n = 7 rats/group. Oxygen consumption (y-axis) and proton motive force (x-axis), measured in parallel under the same conditions (37°C; 0.2 mg protein/ml, 5 μM rotenone, 0.4 μg/ml nigericin, 10 mM succinate, and 1 mM malonate titrations). D: oxidative stress, measured as the extent of 4-hydroxynonenal (4-HNE) protein modification by Western blot. Left: representative blot; right: quantification. **P < 0.01.
Citrate synthase activity.
Citrate synthase (CS) activity was measured (58) in the same isolated skeletal muscle mitochondrial fractions that were used for the functional determinations.
Isolation of muscle for histology.
The leg muscles (whole soleus, and a piece of each of gastrocnemius, vastus lateralis, and tibialis anterior) from the leg not used for mitochondrial isolation were dissected. Care was taken to harvest approximately the same section of a given muscle in each rat. A piece of thread was tied to the ends of the harvested muscle, and the muscle was placed lengthwise in a transfer pipette cut open along its long axis and filled with optimal cutting temperature compound (Fisher Scientific). The free ends of the string were fixed to notches cut at the ends of the pipette such that the muscle adopted its approximate in vivo resting length.
Analysis of myosin heavy chain isoform composition and fiber size.
Fiber composition and size were determined essentially as described by Gerrits et al. (17). Briefly, serial cross sections (10 μm) were cut at −20°C using a cryostat, then mounted onto slides. Sections were air dried (60 min at room temperature), and then sequentially probed with antibodies against the following myosin heavy chain (MHC) isoforms: IIb [BF-F3, IgM; Developmental Studies Hybridoma Bank (DSHB)], IIa (SC-71, IgG1; DSHB), and I (A4.840 IgM; DSHB). Blocking before incubation with IgG primary antibiodies was for 1 h at room temperature, using mouse on mouse blocking reagent (M.O.M.; Vector Labs, Burlingame, CA). IgM primary antibody blocking was in 5% goat serum, for 20 min, at room temperature. For all primary antibodies, the hybridoma cell supernatant was used at a working concentration of 1:10 (diluted in PBS + 0.3% Triton X-100). Incubations in primary antibody were overnight at 4°C. Incubations with the appropriate secondary antibodies were for 1 h, room temperature. Type IIb fibers were visualized using horseradish peroxidase followed by DAB. Type I and IIa fibers were visualized using alkaline phosphatase followed by addition of substrate (Vector Red AP Kit I, SK-5100 for type I; and Vector Blue AP Kit III, SK-5300 for type IIa; both from Vector Labs). Slides were mounted in antifade medium and stored at −20°C. Controls were run as above but omitting the primary antibodies (not shown).
Analysis was done in a blinded fashion (HCR vs. LCR identity was unknown). Fiber type proportion was determined by counting stained fibers from two fields per muscle type; 722 ± 96 (SE) fibers/rat from gastrocnemius were counted (5 rats/group), 248 ± 33 fibers/rat from vastus lateralis (12 rats), 265 ± 16 fibers/rat from soleus (10 rats), and 761 ± 44 fibers/rat from tibialis anterior (12 rats). Intensity of blue stain (type IIa + x, see below) was determined by converting images to 8-bit grey scale (Scion Image software; Scion).
Statistics.
Values are presented as means ± SE. Comparisons between LCR and HCR were by unpaired Student's t-test or two-way ANOVA and Bonferroni post hoc comparisons (GraphPad Prism 4, GraphPad Software,, La Jolla, CA). Significance was taken as P < 0.05.
RESULTS
Running capacity and body compositional analyses.
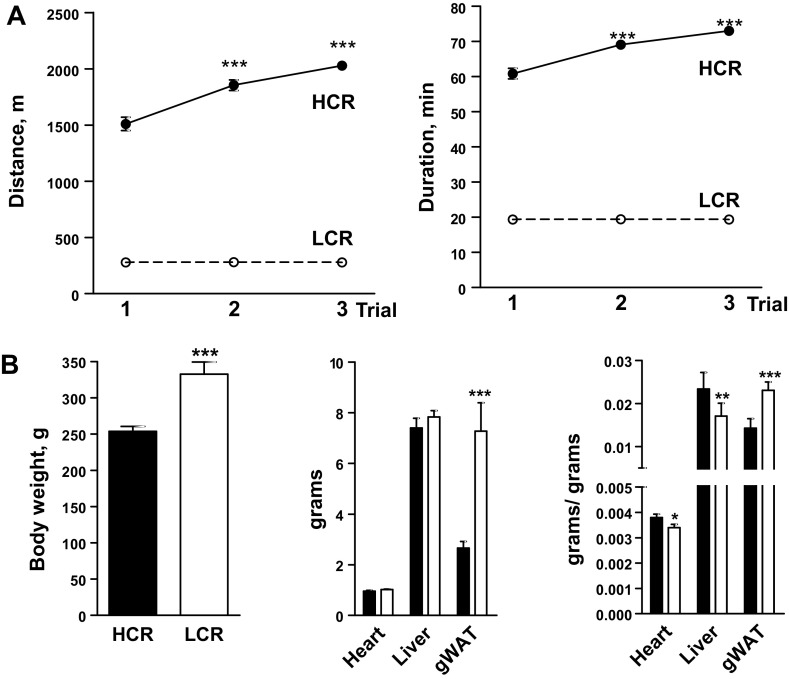
To determine aerobic capacity, rats underwent three treadmill exercise trials (1 trial/day, every 2 days). After 23 generations of artificial selection for intrinsic aerobic capacity, the best running distance and time by HCR were, respectively, 2,030 ± 32 m in 73 ± 0.7 min, whereas LCR ran 300 ± 2 m in 20 ± 0.1 min (P < 0.001 for both measures). Large differences were even apparent when naïve rats were exercised (trial 1 in Fig. 1A): HCR ran 1,510 ± 60 m in 61 ± 2 min while LCR ran 278 ± 7 m in 19 ± 4 min (P < 0.001 for both measures; Fig. 1A). In addition, HCR, but not LCR, exhibited an increased running performance over the three trials (Fig. 1A). These trials were conducted at ∼4 mo of age, after which rats did not engage in further structured physical activity. Rats were studied at ∼15 mo of age; clear differences in aerobic capacity remained between HCR and LCR at this age (35).
Fig. 1.
Whole body characteristics of low and high capacity runners (LCR and HCR, respectively). A: for phenotyping, rats ran on a treadmill 3 times until exhaustion, on 3 separate days, 1 day apart. An improvement in running performance across session was evident in HCR only; ***P < 0.001, two-way ANOVA, Bonferroni post hoc test, vs. HCR trial 1. For each trial, P < 0.001, HCR vs. LCR; n = 12/group. Some error bars are within the symbols. B: body and organ weights. gWAT, gonadal white adipose tissue. Right: organ weights/body weight. **P = 0.01; ***P < 0.001, unpaired Student's t-test; n = 12/group. ○: LCR; ●: HCR. Values are means ± SE.
At the time of study, LCR weighed ∼24% more than age-matched HCR (P < 0.001; Fig. 1B). The higher body weight was contributed by greater adiposity as determined by gonadal white adipose tissue weight, which was 63% higher in absolute value in LCR (P < 0.001; Fig. 1B) or ∼38% higher when expressed relative to body weight (P < 0.001; Fig. 1B). In contrast, weights of heart and liver were similar in LCR and HCR in absolute value or lower by 9% (P = 0.03) and 27% (P = 0.003), respectively, in LCR per body weight (Fig. 1B).
Mitochondrial content and intrinsic function of mitochondria.
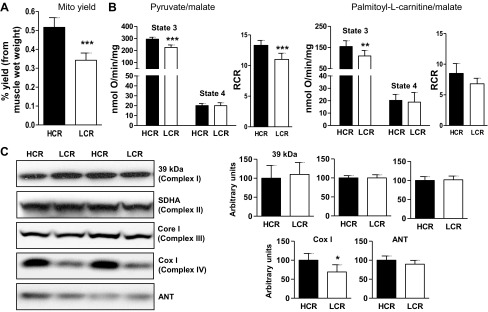
Mitochondria were extracted from a pooled sample comprising the same muscles used for MHC isoform analysis but from the opposite leg. Mitochondrial content, estimated from the yield of mitochondria from the isolation procedure, and expressed relative to wet muscle weight, was ∼43% greater in HCR compared with LCR muscle (P < 0.01; Fig. 2A).
Fig. 2.
Increased maximal phosphorylating O2 consumption in skeletal muscle mitochondria from HCR. A: mitochondrial content, as yield of mitochondria from the isolation procedure, per wet muscle weight. ***P < 0.001, unpaired Student's t-test; n = 9/group. B: maximal phosphorylating (state 3) and nonphosphorylating (state 4) respiration with pyruvate/malate (5 mM/2.5 mM; n = 6/group) or palmitoyl-l-carnitine/malate (20 μM/1 mM; n = 4/group) as substrates. RCR, respiratory control ratio (state 3/state 4); SDHA, succinate dehydrogenase subunit A; COX-I, cyclooxygenase-I.**P = 0.02; ***P = 0.005: unpaired Student's t-test. In all cases, 0.2 mg mitochondrial protein/ml were used. C: expression of electron transport chain proteins and of the adenine nucleotide translocase (ANT). In all cases, 20 μg of mitochondrial protein/lane were used. Left: representative blots; right: quantification by densitometry, relative to Ponceau stained blot or complex I (39-kDa protein). *P = 0.05, unpaired t-test; n = 6/group. Values are means ± SE.
Maximal phosphorylating (state 3; Fig. 2B) respiration was higher in mitochondria from HCR muscle, whether the substrate was pyruvate/malate (∼30% higher; P = 0.005), PCarn (∼40% higher; P = 0.02), or succinate (∼60%; P = 0.05, n = 3, not shown). When the state 3 O2 consumption values were normalized to CS activity, state 3 remained elevated in HCR mitochondria for pyruvate/malate (0.97 ± 0.1 nmol O/CS in LCR vs. 1.37 ± 0.2 nmol O/CS in HCR: P = 0.025) and PCarn (0.56 ± 0.06 nmol O/CS in LCR vs. 0.79 ± 0.08 nmol O/CS in HCR: P < 0.01). State 3 values for succinate, normalized to CS, were not significantly different, possibly due to the small sample size (0.68 ± 0.1 nmol O/CS in LCR vs. 1.03 ± 0.17 nmol O/CS in HCR: P = 0.07). Note that CS activity (per mg mitochondrial protein) did not differ between LCR and HCR (not shown). On the other hand, maximal nonphosphorylating respiration (state 4) was similar, regardless of substrate (Fig. 2B). Thus respiratory control ratios tended to be higher in HCR, reaching significance only for pyruvate/malate (P = 0.01). Electron transport chain capacity, determined as the respiration rate following carbonyl cyanide p-trifluormethoxyphenylhydrazone (FCCP) addition, could only be reliably measured in mitochondria oxidizing pyruvate/malate; differently from state 3, FCCP values were similar in HCR and LCR mitochondria (319 ± 26 and 369 ± 22 nmol O·min−1·mg−1 for LCR and HCR, respectively).
To determine whether protein abundance could explain the higher state 3 values in HCR mitochondria, we measured the protein expression levels of subunits from each holoenzyme of the electron transport chain, as well as expression of ANT (Fig. 2C). Only COX-I (complex IV) differed between HCR and LCR (∼25% higher in HCR; P = 0.059). ANT protein levels were similar between HCR and LCR, as were levels of the tricarboxylic acid cycle enzyme aconitase (data not shown).
Because mitochondria are an important source of ROS, we also compared the intrinsic capacity for ROS production in HCR and LCR mitochondria. In addition, the higher state 3 respiration in HCR mitochondria together with increased expression of COX-I could be associated with lower production of ROS by the electron transport chain in HCR mitochondria. ROS production was determined as the rate of H2O2 emission from mitochondria, using different substrates and inhibitors to provide mechanistic insight. H2O2 emission was significantly greater in HCR compared with LCR mitochondria respiring on pyruvate/malate (+rotenone; 27% increase; P = 0.025) or PCarn (+antimycin; 14% increase; P = 0.036), that is, when ROS were generated primarily at complexes I and III, respectively. It is noteworthy that PMF is minimal under these conditions (Fig. 3A). When ROS were generated with succinate alone (i.e., via backflow through complex I), a trend for higher H2O2 in HCR was evident (P = 0.2, however, HCR > LCR on each experimental day). Levels of mitochondrial antioxidant enzymes and related proteins (SOD2, UCP3, GPx4, and Hex II) were determined, and only SOD2 expression differed between HCR and LCR (∼20% higher in HCR; P = 0.01; Fig. 3B). Because ROS production is highly sensitive to proton leak, we assessed proton leak kinetics and found no differences between HCR and LCR; that is, at any given PMF, oxygen consumption was similar in HCR and LCR mitochondria (Fig. 3C). Finally, oxidative stress in mitochondria was evaluated by Western blots of 4-HNE-modified proteins (63). The extent of 4-HNE modification was greater in LCR mitochondria (Fig. 3D).
MHC isoform expression and fiber size.
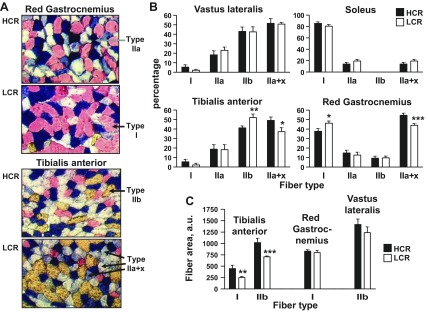
Transcriptional programs can regulate, in a coordinated fashion, mitochondrial content and function as well as MHC isoform expression, with MHC type I and IIa isoforms being expressed in conjunction with the highest aerobic capacity (4, 21). We measured MHC isoform proportion in the muscles used for analysis of mitochondrial yield and function to determine whether differences in mitochondrial yield and inherent function could be caused by differential activation of transcriptional programs that regulate mitochondria and MHC expression in parallel. MHC isoform expression was determined in four muscles: soleus, (primarily MHC I isoform), tibialis anterior and vastus lateralis (both largely mixed type II), and gastrocnemius [comprises a region rich in fibers expressing MHC I (“red gastrocnemius”) as well as regions rich in MHC II isoforms]. Monoclonal antibodies to MHC I, IIa, and IIb were used. In theory, fibers primarily expressing MHC IIx would be unstained; however, this was rarely the case. Instead, we observed a norandom gradation in the blue stain for MHC IIa, with a subset staining intensely and uniformly, which we have classified as IIa; otherwise, the fiber was designated as hybrid IIa + x. Differences between HCR and LCR were evident in tibialis anterior and gastrocnemius (Fig. 4, A and B). In both muscles, the proportion of MHC IIa + x was greater in HCR (P = 0.05 for tibialis; P = 0.007 for gastrocnemius), which was offset by a lower proportion of IIb (tibialis) or I (gastrocnemius).
Fig. 4.
Myosin heavy chain isoform proportion and fiber size in skeletal muscle from LCR and HCR. A: representative histology. Images were taken at the same magnification and illumination. B: proportion of myosin heavy chain isoforms. *P = 0.05; **P = 0.03; ***P = 0.007, unpaired Student's t-test. C: fiber size for selected fiber types and muscles. **P = 0.03; ***P = 0.008, unpaired Student's t-test. Values are means ± SE; n = 5–6 rats/group.
Fiber surface area was also determined, as a measure of fiber hypertrophy. By inspection, fibers expressing MHC I had the smallest area and MHC IIb fibers the greatest. Accordingly, measurements were made of MHC I fibers, in gastrocnemius and tibialis, and of MHC IIb fibers in tibialis and vastus lateralis. In tibialis anterior, the surface area of both MHC I and IIb fibers was higher in HCR (P = 0.03 and 0.008 for I and IIb, respectively; Fig. 4C). No differences between HCR and LCR were apparent in gastrocnemius.
DISCUSSION
This study provides the first direct evidence in mitochondria isolated from skeletal muscle that intrinsic mitochondrial function is higher in rats that have been selected for high aerobic capacity and have lowered disease risks. Our findings demonstrate higher state 3 respiration rates regardless of substrate. In the absence of altered proton leak kinetics or major changes in protein levels, our results suggest altered control of oxidative phosphorylation. The higher state 3 respiration (expressed per mg mitochondrial protein or relative to CS activity) was as great as the increase in mitochondrial yield (∼40% higher for each, compared with LCR values), suggesting that the greater intrinsic mitochondrial capacity of HCR skeletal muscle mitochondria may be just as important as increases in mitochondrial yield with respect to contributing to overall aerobic capacity of skeletal muscle. Observations from several recent studies are consistent with increased mitochondrial phosphorylating potential in HCR skeletal muscle; however, many of the observations could not clearly discriminate between higher mitochondrial content vs. intrinsic function. Thus our findings are the first to unequivocally demonstrate higher phosphorylating potential of mitochondria from HCR skeletal muscle and are consistent with the idea that HCR muscle is more inherently poised to handle higher workloads and energy demands, even at the level of mitochondrial function. Intriguingly, H2O2 emission capacity was higher in HCR mitochondria, whereas 4-HNE modification of mitochondrial proteins was lower. As discussed below, elevated H2O2 emission in HCR mitochondria may serve a signaling role to facilitate adaptation to increased work and calorie loads. Finally, analysis of MHC isoforms indicates that the higher phosphorylation capacity of HCR mitochondria is not the result of a global switch toward a slow twitch isoform; indeed, MHC I expression was higher in some of the LCR muscles.
Methodological considerations.
In the present study, we assessed inherent mitochondrial function in muscle pooled from four muscle groups. Isolated mitochondria were used, rather than a more intact tissue preparation, to best assess inherent organellar function in the absence of complexities related to normalization for mitochondrial content. Pooled muscle was used to determine average characteristics for muscle mitochondria that best represents the muscle mitochondrial properties available for systemic functions such as glucose homeostasis. Our results from the pooled samples may reflect heterogeneity in different fiber types. In particular, the study by Rivas et al. (52) lends some support to the possibility that muscle groups having predominantly type II fibers may be largely responsible for the higher state 3 rates in HCR mitochondria. However, the higher palmitate oxidation rates measured by Naples et al. (44) in isolated HCR mitochondria from red gastrocnemius suggest that a muscle group from HCR with a high proportion of type I fibers may also express higher intrinsic mitochondria function. Yet, because red gastrocnemius contains a significant fraction of type II fibers, it remains possible that it was mitochondria from those fibers that were responsible for the higher palmitate oxidation rates. Our observations from pooled muscle do not address the question of whether there is a fiber type contribution to the HCR mitochondrial phenotype; however, our approach does suggest that increases, wherever they may arise, are large enough to be detected from a pooled sample. Because the pooled sample comprises almost the entire hindlimb, the increases in phosphorylating potential may influence systemic variables such as glucose homeostasis.
The possibility that only a subpopulation of mitochondria was isolated, and that this subpopulation differs between LCR and HCR, does not seem likely. In particular, on each experimental day, parallel isolations were performed on muscle from one LCR and one HCR rat, thereby limiting possible day-to-day variations in solutions and technique. In addition, once dissected, the muscle was randomly aggregated and minced, and the minced muscle was poured into a homogenizing tube and homogenized. Thus, overall, our methods could tend to increase sample-to-sample variability rather than introduce bias that would underlie differences between LCR and HCR mitochondria. As well, use of protease would result in isolation of both subsarcolemmal and intermyofibrillar mitochondria; the high yield of mitochondria is consistent with isolation from both populations. While theoretically possible, the idea that observations from LCR mitochondria reflect greater damage due to greater fragility of the mitochondria seems unlikely given that several characteristics of LCR and HCR mitochondria are similar. In particular, identical proton leak kinetics and state 4 rates were measured for LCR and HCR mitochondria, measures that would have been especially sensitive to damage.
Intrinsic function of mitochondria.
Both mitochondrial mass and maximal phosphorylating respiration per milligram of mitochondrial protein were higher in HCR muscle by ∼40 and ∼30–40%, respectively. Thus the higher intrinsic phosphorylating capacity may be as important as the increase in mitochondrial yield with respect to increasing the aerobic capacity of HCR muscle. If these increases reflect the mitochondrial characteristics of all skeletal muscle, then maximal phosphorylation capacity would be as much as ∼80% greater in HCR muscle. Differently from the state 3 values, FCCP rates from HCR and LCR mitochondria were not clearly different (at least for P/M); thus the higher state 3 in HCR mitochondria does not reflect a greater capacity of the electron transport chain. In support, protein levels of electron transport chain subunits and of TCA cycle enzymes were largely unchanged. Rather, observations suggest differences in flux control in LCR and HCR mitochondria. Application of metabolic control analysis to isolated mitochondria suggests that control of oxidative phosphorylation is shared among several reactions and pathways and that distribution of control varies according to tissue and metabolic state (8, 13, 14, 54). Using inhibitors to probe control in mitochondria from several rat tissues, Rossignol et al. (54) determined that control of skeletal muscle mitochondria occurs mainly at the level of the respiratory chain as opposed to the ATP synthase, ANT, or phosphate and pyruvate carriers. While our study does not allow a more detailed understanding of differences in flux control between HCR and LCR mitochondria, altogether our findings indicate that change(s) in the control of phosphorylating respiration underlies the higher state 3 in HCR mitochondria. It is noteworthy that fine control of respiratory chain function by mechanisms such as protein kinase A-mediated phosphorylation of complex IV and possibly of other electron transport chain complexes is emerging as an important source of control over electron transport chain function (2).
Leptin was greater and adiponectin was lower in chow-fed LCR compared with HCR rats (9, 46, 47). TNFα was also higher in chow-fed LCR rats (35). Since some adipokines and inflammatory mediators can modulate mitochondrial content and function (12, 28, 66), it is possible that the different adipokine profiles and levels of inflammatory mediators in LCR and HCR rats drive the differences in mitochondrial yield and function. In particular, lower adiponectin and higher TNFα may contribute to the LCR skeletal muscle mitochondrial phenotype. However, the expression of adipokine receptors and capacity for signaling in LCR and HCR muscle still need to be understood before a link between these circulating factors and muscle mitochondrial phenotype can really be considered.
Our finding of intrinsic differences in mitochondrial function in HCR vs. LCR skeletal muscle is in contrast to the effects of exercise training on skeletal muscle mitochondria. In rats, strenuous exercise increased mitochondrial content but did not increase O2 consumption per mg of mitochondrial protein (24). Thus the mechanisms that drive intrinsic differences in metabolism in HCR and LCR rats differ from those that mediate the adaptive metabolic response to strenuous exercise. Our findings are, however, consistent with studies in twins that indicate that separate genetic components contribute to the variation that exists for intrinsic and acquired exercise performance (7).
Contrary to our hypothesis, H2O2 emission rates, with pyruvate/malate or PCarn as energy substrates, were higher in HCR mitochondria, and a trend was evident for higher levels with succinate. It should be noted that experimental conditions resulted in measurement of maximal or near-maximal H2O2 emission, as saturating concentrations of substrate were used, and inhibitors, rotenone and antimycin, were added to inhibit complex I and III, respectively. Thus measurements are best interpreted as capacity measurements. When the greater mitochondrial mass is considered, the increase in H2O2 emission capacity is significant (up to ∼70% increase depending on substrate). Complex I is responsible for the majority of ROS generated by NADH-linked substrates, whereas complex III contributes significantly to ROS production with fatty acid oxidation (55, 59). ROS generated at complex I are released toward the matrix side of the mitochondrial inner membrane, whereas ROS generated at complex III are released toward both matrix and intermembrane space sides of the inner membrane. That H2O2 emission was greater with both P/M + rotenone and PCarn + antimycin indicates that emission was greater on both matrix and intermembrane space sides of the inner membrane, potentially impacting matrix as well as extramitochondrial processes. Our findings generally accord with recent observations that H2O2 emission capacity in permeabilized muscle fibers from two restricted muscle groups was higher in HCR rats (64) (see Supplemental Table; Supplemental Material for this article is available online at the J Appl Physiol website). Herein, we clearly show that this increase reflects an increased intrinsic capacity of mitochondria for higher H2O2 emission.
Superoxide generation strongly depends upon electron transport chain redox state (often estimated as PMF; Refs. 37, 55), and the greater proton leak at higher membrane potentials would mitigate superoxide generation when the electron transport chain is highly reduced (state 4). Use of rotenone or antimycin minimizes membrane hyperpolarization, whereas ROS generated with succinate is strongly dependent on PMF (38). That differences between LCR and HCR in H2O2 emission were less evident with succinate is consistent with our finding of similar proton leak in LCR and HCR mitochondria.
ROS can negatively impact insulin sensitivity (1, 3, 6, 26, 39). Yet, insulin sensitivity is also positively regulated by mitochondrial H2O2 (23, 41). These apparently divergent results may be reconciled by the general concept that ROS can have beneficial or deleterious effects on cellular function depending on the amount of ROS being generated, the particular ROS species, and the environment where ROS are produced (16, 20). In this regard, it is of interest that SOD2 expression was higher in HCR mitochondria, favoring a greater reduction of superoxide to H2O2. Oxidative stress was also lower in HCR mitochondria, possibly because more harmful ROS were converted to less reactive H2O2. Because insulin sensitivity is higher in HCR muscle (44), it seems reasonable to speculate that the higher H2O2 emission by HCR mitochondria leads to greater potential for H2O2 signaling to positively impact insulin signaling. However, it remains formally possible that a higher ROS load in HCR mitochondria has deleterious effects that are more than offset by other mechanisms that lead to greater insulin sensitivity in HCR muscle. These possibilities agree with the more general hypothesis that HCR have fewer disease risks and are resistant to negative environments because their higher intrinsic work capacity translates into more immediate and complete cellular repair (32).
Another possible consequence of elevated H2O2 emission in HCR mitochondria is an alteration in the dynamics of the reticulum. In many cell types including skeletal muscle, mitochondria exist as a reticulum (48). This reticulum is dynamic (25, 57), with fusion and fission events mediated by the regulation of shaping proteins (e.g., mitofusin1 and 2, OPA1, and Drp1). In vivo models of simultaneous deletion of mitofusin1 and 2 (11) or overexpression of Drp1 (53), reveal that fused mitochondria in skeletal muscle protect against mitochondrial DNA depletion and muscle atrophy and indicate the importance of mitochondrial dynamics in skeletal muscle. In a cell-free system, oxidizing conditions promoted oligomerization of the mitofusins and increased fusion (56), whereas exogenous H2O2 applied to cells can fragment mitochondria (15, 29), which in cultured skeletal myocytes was attributed to a prior decrease in membrane potential (15). Thus redox changes can impact the mitochondrial reticulum. Mitochondria subjected to oxidative stress or other depolarizing stresses may also depend on fission to for removal of damaged units (65). Thus the lower levels of 4-HNE-modified mitochondrial proteins in HCR muscle may reflect more efficient removal of damaged mitochondria. A general prediction is that mitochondria in HCR muscle are more motile.
MHC isoforms.
Quantitative histological analysis of MHC isoforms in four muscle groups, two of which comprised mainly mixed MHC II isoforms (vastus lateralis and tibialis anterior) and the other two a high proportion of the MHC I isoform (soleus, gastrocnemius), clearly showed that HCR muscle is not characterized by a global shift to a slow-twitch isoform. Rather, a small but significant shift to a hybrid MHC IIa + x type was apparent in HCR tibialis anterior and red gastrocnemius at the expense of MHC I (gastrocnemius) and IIb (tibialis anterior); vastus lateralis showed no such differences. Consistent with our findings, Kivela et al. (32) using protein electrophoresis of soleus, extensor digitorum longus (predominantly MHC IIb), and white gastrocnemius (mixed MHC II) also observed a higher proportion of MHC IIa + x in gastrocnemius from HCR; small differences in extensor digitorum longus did not reach statistical significance, and MHC IIa proportion was not determined. Thus the present study supports and significantly expands upon this analysis to demonstrate that extremes in aerobic capacity do not necessarily depend on the expression of the MHC I or IIa isoforms.
Mechanisms determining MHC isoform expression are complex and incompletely understood (5), although evidence suggests important roles for peroxisome proliferator-activated receptor δ (PPARδ) and peroxisome proliferator-activated receptor γ coactivator 1α and β (PGC-1α and PGC-1β, respectively) as key transcriptional (co)activators. In particular, mice with a single gene modification demonstrate that PPARδ, PGC-1α, and PGC-1β can each drive transcriptional programs that coordinately shape muscle biophysical and metabolic characteristics. Specifically, PPARδ and PGC-1α can drive type I and IIa fiber formation (21, 40, 68). PGC-1β promotes expression of the IIx isoform, but suppresses that of I and IIa, whereas PGC-1α drives expression of MHC I, IIa and IIx (4). Like PGC-1α, PGC-1β also induces genes of oxidative phosphorylation, fatty acid oxidation, and antioxidant defense and is associated with increased mitochondrial content in muscle and higher aerobic performance (4, 30, 60, 61). Thus our findings of a higher proportion of mixed IIa + x (and not IIa) MHC coupled with lower MHC I expression, increased mitochondrial content, and higher SOD2 expression and activity (present study; Ref. 44) are consistent with a greater role for PGC-1β co-activation in some HCR muscles. Recently, gene set enrichment analysis revealed increased enrichment for genes of the PPAR signaling pathway in HCR muscle (32), which could explain the greater mitochondrial mass. Yet, higher PPAR signaling would predict greater MHC I expression (68), which was largely not the case (present study; Ref. 32). Thus, if PPAR signaling is indeed higher in HCR muscle, this would suggest a less important role than predicted for PPAR in determining MHC isoform. Alternatively, since MHC isoform is largely developmentally determined, the discordant MHC isoform and metabolic phenotypes could be due to a later onset of higher PPAR activity in HCR.
In conclusion, we have shown that skeletal muscle mitochondria from HCR rats, in addition to being more abundant than in HCR rat skeletal muscle, exhibit different inherent properties. The magnitude of the increase in maximal phosphorylating respiration and ROS emission in HCR mitochondria is as great as that of mitochondrial yield. Thus differences in intrinsic muscle mitochondrial function may be just as important as the difference in mitochondrial content in establishing aspects of the LCR vs. HCR phenotype.
GRANTS
This study was supported by the Natural Sciences and Engineering Research Council of Canada (to M.-E. Harper). The LCR and HCR rat resource was supported by Award R24RR017718 (to S. L. Britton and L. G. Koch) from the National Center for Research Resources (a component of the National Institutes of Health).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.L.S., L.G.K., S.L.B., and M.-E.H. conception and design of research; E.L.S., M.B., C.A., C.M., and C.E. performed experiments; E.L.S., M.B., C.A., C.M., C.E., and M.-E.H. analyzed data; E.L.S., M.B., and M.-E.H. interpreted results of experiments; E.L.S., M.B., and M.-E.H. prepared figures; E.L.S. and M.-E.H. drafted manuscript; E.L.S., M.B., C.A., L.G.K., S.L.B., and M.-E.H. edited and revised manuscript; E.L.S., M.B., C.A., C.M., C.E., L.G.K., S.L.B., and M.-E.H. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Linda Jui for exceptional fiber typing histology; Jian Xuan and Mahmoud Salkhordeh for expert technical support; and Luke Szweda (Oklahoma Medical Research Foundation) for the kind gift of aconitase and 4-HNE antibodies. We acknowledge the expert care of the rat colony provided by Molly Kalahar and Lori Gilligan.
Present address for E. L Seifert: Dept. Pathology, Anatomy, Cell Biology, Thomas Jefferson Univ., Jefferson Alumni Hall, Suite 212D, 1020 Locust St., Philadelphia, PA 19107.
REFERENCES
- 1. Abdul-Ghani MA, Jani R, Chavez A, Molina-Carrion M, Tripathy D, Defronzo RA. Mitochondrial reactive oxygen species generation in obese non-diabetic and type 2 diabetic participants. Diabetologia 52: 574–582, 2009. [DOI] [PubMed] [Google Scholar]
- 2. Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab 9: 265–276, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, III, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119: 573–581, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, Chin S, Spiegelman BM. The transcriptional coactivator PGC-1beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab 5: 35–46, 2007. [DOI] [PubMed] [Google Scholar]
- 5. Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem 75: 19–37, 2006. [DOI] [PubMed] [Google Scholar]
- 6. Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 118: 789–800, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bouchard C, An P, Rice T, Skinner JS, Wilmore JH, Gagnon J, Perusse L, Leon AS, Rao DC. Familial aggregation of V̇o2 max response to exercise training: results from the HERITAGE Family Study. J Appl Physiol 87: 1003–1008, 1999. [DOI] [PubMed] [Google Scholar]
- 8. Brown GC. Control of respiration and ATP synthesis in mammalian mitochondria and cells. Biochem J 284: 1–13, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burghardt PR, Kemmerer ES, Buck BJ, Osetek AJ, Yan C, Koch LG, Britton SL, Evans SJ. Dietary n-3:n-6 fatty acid ratios differentially influence hormonal signature in a rodent model of metabolic syndrome relative to healthy controls. Nutr Metab (Lond) 7: 53, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chappell JB, Perry SV. Biochemical and osmotic properties of skeletal muscle mitochondria. Nature 173: 1094–1095, 1954. [DOI] [PubMed] [Google Scholar]
- 11. Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141: 280–289, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Civitarese AE, Ukropcova B, Carling S, Hulver M, DeFronzo RA, Mandarino L, Ravussin E, Smith SR. Role of adiponectin in human skeletal muscle bioenergetics. Cell Metab 4: 75–87, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cortassa S, O'Rourke B, Winslow RL, Aon MA. Control and regulation of integrated mitochondrial function in metabolic and transport networks. Int J Mol Sci 10: 1500–1513, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cortassa S, O'Rourke B, Winslow RL, Aon MA. Control and regulation of mitochondrial energetics in an integrated model of cardiomyocyte function. Biophys J 96: 2466–2478, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fan X, Hussien R, Brooks GA. H2O2-induced mitochondrial fragmentation in C2C12 myocytes. Free Radic Biol Med 49: 1646–1654, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Forman HJ, Maiorino M, Ursini F. Signaling functions of reactive oxygen species. Biochemistry 49: 835–842, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gerrits MF, Ghosh S, Kavaslar N, Hill B, Tour A, Seifert EL, Beauchamp B, Gorman S, Stuart J, Dent R, McPherson R, Harper ME. Distinct skeletal muscle fiber characteristics and gene expression in diet-sensitive versus diet-resistant obesity. J Lipid Res 51: 2394–2404, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gonzalez NC, Kirkton SD, Howlett RA, Britton SL, Koch LG, Wagner HE, Wagner PD. Continued divergence in V̇o2 max of rats artificially selected for running endurance is mediated by greater convective blood O2 delivery. J Appl Physiol 101: 1288–1296, 2006. [DOI] [PubMed] [Google Scholar]
- 19. Haas RH, Parikh S, Falk MJ, Saneto RP, Wolf NI, Darin N, Wong LJ, Cohen BH, Naviaux RK. The in-depth evaluation of suspected mitochondrial disease. Mol Genet Metab 94: 16–37, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci 35: 505–513, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem 282: 30014–30021, 2007. [DOI] [PubMed] [Google Scholar]
- 22. Haram PM, Kemi OJ, Lee SJ, Bendheim MO, Al-Share QY, Waldum HL, Gilligan LJ, Koch LG, Britton SL, Najjar SM, Wisloff U. Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity. Cardiovasc Res 81: 723–732, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoehn KL, Salmon AB, Hohnen-Behrens C, Turner N, Hoy AJ, Maghzal GJ, Stocker R, Van Remmen H, Kraegen EW, Cooney GJ, Richardson AR, James DE. Insulin resistance is a cellular antioxidant defense mechanism. Proc Natl Acad Sci USA 106: 17787–17792, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 242: 2278–2282, 1967. [PubMed] [Google Scholar]
- 25. Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu Rev Biochem 76: 751–780, 2007. [DOI] [PubMed] [Google Scholar]
- 26. Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440: 944–948, 2006. [DOI] [PubMed] [Google Scholar]
- 27. Hyslop PA, Sklar LA. A quantitative fluorimetric assay for the determination of oxidant production by polymorphonuclear leukocytes: its use in the simultaneous fluorimetric assay of cellular activation processes. Anal Biochem 141: 280–286, 1984. [DOI] [PubMed] [Google Scholar]
- 28. Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, Ogata H, Kubota N, Takamoto I, Hayashi YK, Yamauchi N, Waki H, Fukayama M, Nishino I, Tokuyama K, Ueki K, Oike Y, Ishii S, Hirose K, Shimizu T, Touhara K, Kadowaki T. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature 464: 1313–1319, 2010. [DOI] [PubMed] [Google Scholar]
- 29. Jendrach M, Mai S, Pohl S, Voth M, Bereiter-Hahn J. Short- and long-term alterations of mitochondrial morphology, dynamics and mtDNA after transient oxidative stress. Mitochondrion 8: 293–304, 2008. [DOI] [PubMed] [Google Scholar]
- 30. Kamei Y, Ohizumi H, Fujitani Y, Nemoto T, Tanaka T, Takahashi N, Kawada T, Miyoshi M, Ezaki O, Kakizuka A. PPARgamma coactivator 1beta/ERR ligand 1 is an ERR protein ligand, whose expression induces a high-energy expenditure and antagonizes obesity. Proc Natl Acad Sci USA 100: 12378–12383, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kirkton SD, Howlett RA, Gonzalez NC, Giuliano PG, Britton SL, Koch LG, Wagner HE, Wagner PD. Continued artificial selection for running endurance in rats is associated with improved lung function. J Appl Physiol 106: 1810–1818, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kivela R, Silvennoinen M, Lehti M, Rinnankoski-Tuikka R, Purhonen T, Ketola T, Pullinen K, Vuento M, Mutanen N, Sartor MA, Reunanen H, Koch LG, Britton SL, Kainulainen H. Gene expression centroids that link with low intrinsic aerobic exercise capacity and complex disease risk. FASEB J 24: 4565–4574, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koch LG, Britton SL. Aerobic metabolism underlies complexity and capacity. J Physiol 586: 83–95, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics 5: 45–52, 2001. [DOI] [PubMed] [Google Scholar]
- 35. Koch LG, Kemi OJ, Qi N, Leng SX, Bijma P, Gilligan LJ, Wilkinson JE, Wisloff H, Hoydal MA, Rolim N, Abadir PM, van Grevenhof EM, Smith GL, Burant CF, Ellingsen O, Britton SL, Wisloff U. Intrinsic aerobic capacity sets a divide for aging and longevity. Circ Res 109: 1162–1172, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kokkinos P, Myers J, Kokkinos JP, Pittaras A, Narayan P, Manolis A, Karasik P, Greenberg M, Papademetriou V, Singh S. Exercise capacity and mortality in black and white men. Circulation 117: 614–622, 2008. [DOI] [PubMed] [Google Scholar]
- 37. Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 416: 15–18, 1997. [DOI] [PubMed] [Google Scholar]
- 38. Lambert AJ, Buckingham JA, Brand MD. Dissociation of superoxide production by mitochondrial complex I from NAD(P)H redox state. FEBS Lett 582: 1711–1714, 2008. [DOI] [PubMed] [Google Scholar]
- 39. Lefort N, Glancy B, Bowen B, Willis WT, Bailowitz Z, De Filippis EA, Brophy C, Meyer C, Hojlund K, Yi Z, Mandarino LJ. Increased reactive oxygen species production and lower abundance of complex I subunits and carnitine palmitoyltransferase 1B protein despite normal mitochondrial respiration in insulin-resistant human skeletal muscle. Diabetes 59: 2444–2452, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418: 797–801, 2002. [DOI] [PubMed] [Google Scholar]
- 41. Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, Bruce C, Shields BJ, Skiba B, Ooms LM, Stepto N, Wu B, Mitchell CA, Tonks NK, Watt MJ, Febbraio MA, Crack PJ, Andrikopoulos S, Tiganis T. Reactive oxygen species enhance insulin sensitivity. Cell Metab 10: 260–272, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mayr JA, Zimmermann FA, Horvath R, Schneider HC, Schoser B, Holinski-Feder E, Czermin B, Freisinger P, Sperl W. Deficiency of the mitochondrial phosphate carrier presenting as myopathy and cardiomyopathy in a family with three affected children. Neuromuscul Disord 21: 803–808, 2011. [DOI] [PubMed] [Google Scholar]
- 43. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 346: 793–801, 2002. [DOI] [PubMed] [Google Scholar]
- 44. Naples SP, Borengasser SJ, Rector RS, Uptergrove GM, Morris EM, Mikus CR, Koch LG, Britton SL, Ibdah JA, Thyfault JP. Skeletal muscle mitochondrial and metabolic responses to a high-fat diet in female rats bred for high and low aerobic capacity. Appl Physiol Nutr Metab 35: 151–162, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nesse RM, Stearns SC, Omenn GS. Medicine needs evolution. Science 311: 1071, 2006. [DOI] [PubMed] [Google Scholar]
- 46. Noland RC, Thyfault JP, Henes ST, Whitfield BR, Woodlief TL, Evans JR, Lust JA, Britton SL, Koch LG, Dudek RW, Dohm GL, Cortright RN, Lust RM. Artificial selection for high-capacity endurance running is protective against high-fat diet-induced insulin resistance. Am J Physiol Endocrinol Metab 293: E31–E41, 2007. [DOI] [PubMed] [Google Scholar]
- 47. Novak CM, Escande C, Burghardt PR, Zhang M, Barbosa MT, Chini EN, Britton SL, Koch LG, Akil H, Levine JA. Spontaneous activity, economy of activity, and resistance to diet-induced obesity in rats bred for high intrinsic aerobic capacity. Horm Behav 58: 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ogata T, Yamasaki Y. Ultra-high-resolution scanning electron microscopy of mitochondria and sarcoplasmic reticulum arrangement in human red, white, and intermediate muscle fibers. Anat Rec 248: 214–223, 1997. [DOI] [PubMed] [Google Scholar]
- 49. Payne JL, Boyer AG, Brown JH, Finnegan S, Kowalewski M, Krause RA, Jr, Lyons SK, McClain CR, McShea DW, Novack-Gottshall PM, Smith FA, Stempien JA, Wang SC. Two-phase increase in the maximum size of life over 35 billion years reflects biological innovation and environmental opportunity. Proc Natl Acad Sci USA 106: 24–27, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Raymond J, Segre D. The effect of oxygen on biochemical networks and the evolution of complex life. Science 311: 1764–1767, 2006. [DOI] [PubMed] [Google Scholar]
- 51. Reynafarje B, Costa LE, Lehninger AL. O2 solubility in aqueous media determined by a kinetic method. Anal Biochem 145: 406–418, 1985. [DOI] [PubMed] [Google Scholar]
- 52. Rivas DA, Lessard SJ, Saito M, Friedhuber AM, Koch LG, Britton SL, Yaspelkis BB, III, Hawley JA. Low intrinsic running capacity is associated with reduced skeletal muscle substrate oxidation and lower mitochondrial content in white skeletal muscle. Am J Physiol Regul Integr Comp Physiol 300: R835–R843, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Romanello V, Guadagnin E, Gomes L, Roder I, Sandri C, Petersen Y, Milan G, Masiero E, Del Piccolo P, Foretz M, Scorrano L, Rudolf R, Sandri M. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J 29: 1774–1785, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rossignol R, Letellier T, Malgat M, Rocher C, Mazat JP. Tissue variation in the control of oxidative phosphorylation: implication for mitochondrial diseases. Biochem J 347: 45–53, 2000. [PMC free article] [PubMed] [Google Scholar]
- 55. Seifert EL, Estey C, Xuan JY, Harper ME. Electron transport chain-dependent and -independent mechanisms of mitochondrial H2O2 emission during long-chain fatty acid oxidation. J Biol Chem 285: 5748–5758, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shutt T, Geoffrion M, Milne R, McBride HM. The intracellular redox state is a core determinant of mitochondrial fusion. EMBO Rep 13: 909–915, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shutt TE, McBride HM. Staying cool in difficult times: mitochondrial dynamics, quality control and the stress response. Biochim Biophys Acta 2012. June 7 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 58. Srere PA, Brooks GC. The circular dichroism of glucagon solutions. Arch Biochem Biophys 129: 708–710, 1969. [DOI] [PubMed] [Google Scholar]
- 59. St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277: 44784–44790, 2002. [DOI] [PubMed] [Google Scholar]
- 60. St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127: 397–408, 2006. [DOI] [PubMed] [Google Scholar]
- 61. St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB, Spiegelman BM. Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators 1alpha and 1beta (PGC-1alpha and PGC-1beta) in muscle cells. J Biol Chem 278: 26597–26603, 2003. [DOI] [PubMed] [Google Scholar]
- 62. Thyfault JP, Rector RS, Uptergrove GM, Borengasser SJ, Morris EM, Wei Y, Laye MJ, Burant CF, Qi NR, Ridenhour SE, Koch LG, Britton SL, Ibdah JA. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol 587: 1805–1816, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tsai L, Szweda PA, Vinogradova O, Szweda LI. Structural characterization and immunochemical detection of a fluorophore derived from 4-hydroxy-2-nonenal and lysine. Proc Natl Acad Sci USA 95: 7975–7980, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tweedie C, Romestaing C, Burelle Y, Safdar A, Tarnopolsky MA, Seadon S, Britton SL, Koch LG, Hepple RT. Lower oxidative DNA damage despite greater ROS production in muscles from rats selectively bred for high running capacity. Am J Physiol Regul Integr Comp Physiol 300: R544–R553, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Twig G, Shirihai OS. The interplay between mitochondrial dynamics and mitophagy. Antioxid Redox Signal 14: 1939–1951, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Valerio A, Cardile A, Cozzi V, Bracale R, Tedesco L, Pisconti A, Palomba L, Cantoni O, Clementi E, Moncada S, Carruba MO, Nisoli E. TNF-alpha downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Invest 116: 2791–2798, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39: 359–407, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol 2: e294, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernstrom M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science 307: 418–420, 2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.