Abstract
Sympathetic circulatory control is key to the rapid cardiovascular adjustments that occur within seconds of standing upright (orthostasis) and which are required for bipedal stance. Indeed, patients with ineffective sympathetic adrenergic vasoconstriction rapidly develop orthostatic hypotension, prohibiting effective upright activities. One speaks of orthostatic intolerance (OI) when signs, such as hypotension, and symptoms, such as lightheadedness, occur when upright and are relieved by recumbence. The experience of transient mild OI is part of daily life. However, many people experience episodic acute OI as postural faint or chronic OI in the form of orthostatic tachycardia and orthostatic hypotension that significantly reduce the quality of life. Potential mechanisms for OI are discussed including forms of sympathetic hypofunction, forms of sympathetic hyperfunction, and OI that results from regional blood volume redistribution attributable to regional adrenergic hypofunction.
Keywords: autonomic, syncope, orthostatic tachycardia, orthostatic hypotension, hyperpnea
the overall purpose of this review is to discuss the effects of the sympathetic nervous system on cardiovascular homeostasis during orthostasis (upright posture). The main focus is on the role of the sympathetic nervous system in orthostatic intolerance (OI). For the most part OI is related to changes in the regulation of blood pressure, heart rate, and, ultimately, cerebral blood flow that make remaining upright impossible. I will include discussion of sympathetic mechanisms within a wider context of autonomic control systems, comprising both sympathetic and parasympathetic arms, and in relation to other vascular control mechanisms that modulate sympathetic activity. I will first discuss orthostatic regulation and the normal orthostatic response as they relate to sympathetic and parasympathetic activity. I will move on to a definition of orthostatic intolerance, how OI can be measured in the laboratory and in real life, and how problems with sympathetic adrenergic vasoconstriction produce distinct forms of OI.
NORMAL STRESSORS AND THE AUTONOMIC REGULATORY FRAMEWORK
According to Rowell (74) there are two quotidian physical stressors: upright posture and dynamic exercise that “demand the full capabilities of the reflexes that govern cardiovascular function.” Optimum orthostasis and exercise performance depend on intact intrinsic vascular structure and function, intact control of vasomotor function, adequate central blood volume and oxygen carrying capacity, and intact physical compensatory mechanisms including the integrity of skeletal and respiratory muscle pumps (59, 102). Compensatory mechanisms are often multiply redundant to offset inadequacy of any one system. Thus, for example, a small to moderate change in blood volume is well tolerated.
Short-time adjustments in hemodynamics depend most on the autonomic nervous system, although the kinetics of the myogenic response (54) and flow mediated dilation (81) are comparable. It may be fair to state that the autonomic nervous system comprises the framework in which rapid adjustments of the circulation produced by heart rate changes, arterial vasoconstriction, reflex venoconstriction, adrenal secretion, renovascular adjustments, and cardiac contractility maintain blood pressure. Apart from parasympathetic contributions to heart rate changes, these are efferent actions of the sympathetic nervous system, although recent work indicates strong vagal influences on sympathoexcitation (5). These rapid autonomic adjustments also depend on a “tonic milieu” produced by slower endocrine, paracrine, and autocrine regulatory mechanisms that may exert both direct effects on the circulation and also modulate autonomic function. Notable examples include the effects of nitric oxide and angiotensin II acting at both central (52) and peripheral (56) levels. Although parasympathetic mechanisms can play an important complementary role in the beat to beat maintenance of blood pressure, the sympathetic nervous system and its primary vascular neurotransmitter norepinephrine (101), and cotransmitters neuropeptides Y and ATP (56) are of paramount importance. Sympathetic control is provided by diverse regulatory subsystems—the arterial and cardiopulmonary baroreflexes and muscle mechanoreceptor and chemoreceptor networks—that are specifically charged with blood pressure homeostasis during orthostasis.
NORMAL ORTHOSTATIC RESPONSE
Standing up reduces venous return by translocating a large fraction of central blood volume, in excess of 500 ml in the adult human, to the dependent body parts. There is an initial transient dynamic state during which mechanical equilibrium must be re-established causing a decrease in blood pressure dependent on initial vascular tone (85); a further delay, on the order of 10–15 s, occurs in the onset of active compensatory responses. The delay coincides with the gravitationally driven redistribution of blood from the central circulation to the periphery, predominantly into the venous vasculature of the lower limbs and splanchnic circulation (79). The initial response denoted “initial orthostatic hypotension” (103) is complete within 30–60 s and blood pressure is restored. Tonically active adrenergic sympathetic activity contributes to resting vasoconstriction (3) and can alter the time to recovery. However, major interindividual variability in sympathetic activity exists (9) and could alter both baseline vascular resistance and the extent of vasoconstriction during standing. That being said, it appears that either differences in adrenergic transduction at the smooth muscle neurovascular synapse or alterations in blood volume ensure a measure of blood pressure uniformity across subjects (40). Thus peripheral resistance varies much less than muscle sympathetic nerve activity (8).
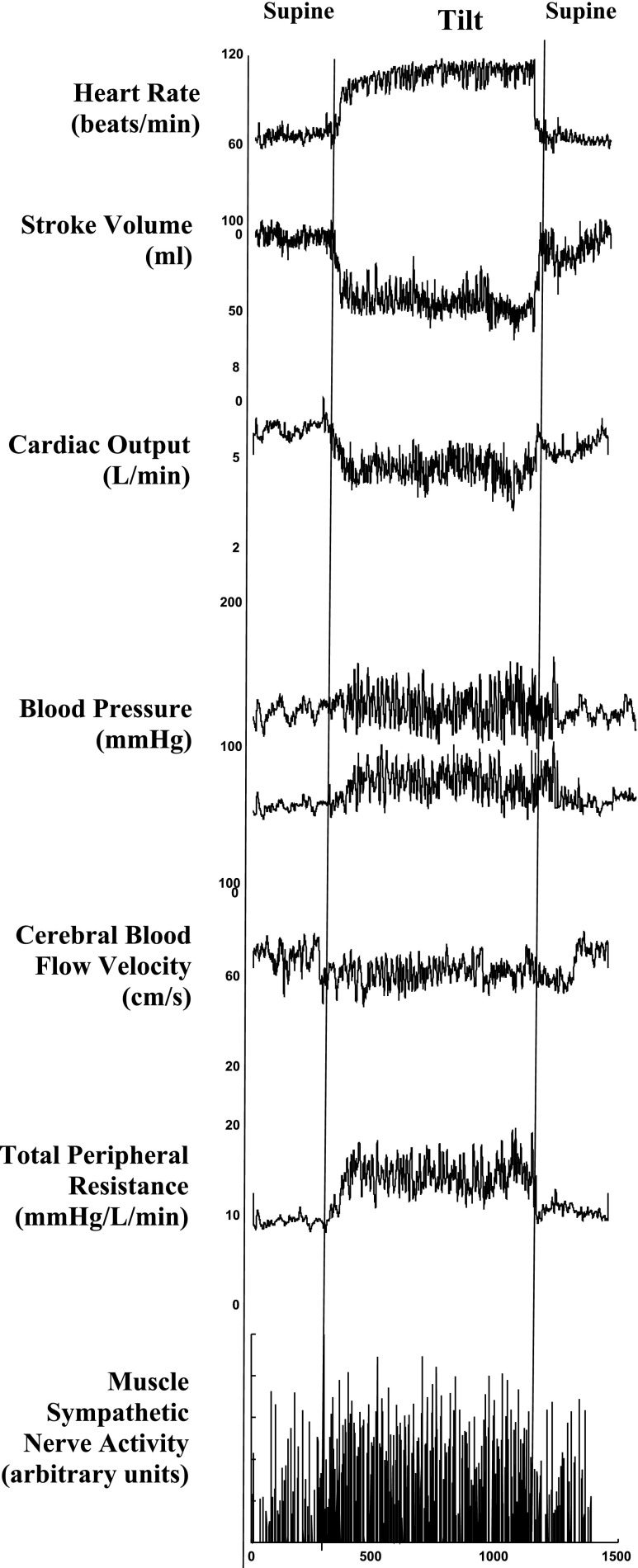
Even after mechanical equilibrium is re-established during continued standing, microvascular filtration from plasma to interstitium continues to reduce blood volume (49). Partial restitution of blood volume depends on lymphatic activity and reabsorption of interstitial fluid into the blood volume (35). Nevertheless, there is a net reduction in blood volume and venous return and thus a net reduction in cardiac output, cerebral blood flow, central blood volume, and stroke volume during quiet standing. Total peripheral resistance (TPR), sympathetic nervous activity, and blood pressure are increased (Fig. 1). Diastolic BP increases more than systolic blood pressure, and the resultant decrease in pulse pressure coincides with the reduction in stroke volume when upright.
Fig. 1.
Top to bottom: heart rate, stroke volume, cardiac output, systolic and diastolic blood pressure, cerebral blood flow velocity by transcranial Doppler ultrasound, total peripheral vascular resistance (TPR), and muscle sympathetic nerve activity (MSNA) from the peroneal nerve from a representative healthy volunteer. During upright tilt heart rate progressively increases, stroke volume decreases ∼40%, whereas cardiac output only decreases ∼20% because of the increase in heart rate. Systolic and diastolic blood pressure increase slightly, diastole more than systole. Cerebral blood flow decreases by 5–10%, while both TPR and MSNA are increased.
Common wisdom dictates that the restoration of blood pressure and venous return during standing is attributable in large part to the reduced stretch and inactivation (unloading) of the inhibitory arterial baroreflexes. These cause adrenergic vasoconstriction, active venoconstriction within the splanchnic circulation (33), and passive elastic recoil of pooled blood within the lower extremities and splanchnic vasculature, which partially counteract the loss of central blood volume (16). The cardiopulmonary baroreflexes are simultaneously unloaded when upright and markedly potentiate the actions of the arterial reflexes (100). A reduction in BP typically occurs only during the transient mechanical dysequilibrium of initial hypotension. Afterward both systolic and diastolic blood pressures are usually slightly increased compared with the supine position. Despite unchanged or even increased BP, increased sympathetic activity continues (Fig. 1), which again speaks to the importance of cardiopulmonary reflexes. Because diastolic blood pressure correlates best with muscle sympathetic nerve activity (MSNA) in humans (94) and is increased at the level of the carotid sinus, a reduction of diastolic arterial baroreflex stretch does not occur while upright. Studies using lower body negative pressure (LBNP) as an orthostatic stress emphasize this apparent paradox in the absence of any hemostatic pressure difference between heart and carotid sinus. Both cardiovagal and sympathetic vasomotor baroreflex reflex curves are reset when upright, as occurs during exercise (20), presumably through the influence of cardiopulmonary receptors. This resetting enables a sustained increase in heart rate through vagal withdrawal and sympathoexcitation and increase in sympathetic nerve activity and vasoconstriction characteristic of the normal compensatory response to orthostasis (13).
DEFINITION OF ORTHOSTATIC INTOLERANCE
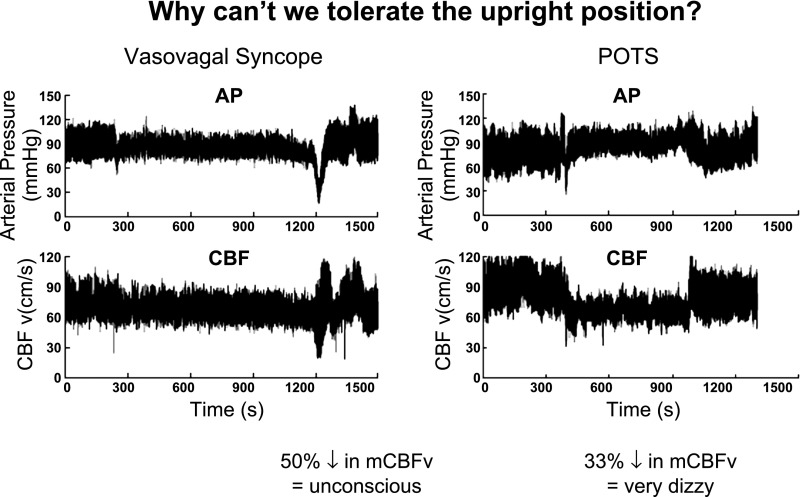
Orthostasis means standing up. Orthostatic intolerance (OI) can be defined by the inability to tolerate upright posture relieved by recumbence (72). Typical signs and symptoms include loss of consciousness or lesser cognitive deficits, visual difficulties, lightheadedness-dizziness, headache, fatigue, orthostatic hypotension and sometimes hypertension, weakness, nausea and abdominal pain, sweating, tremulousness, and exercise intolerance. Of these, loss of consciousness or severe lightheadedness and neurocognitive loss, “CNS symptoms,” are most likely to directly provoke recumbence whereas the other findings are more directly related to increased adrenergic activity. The CNS symptoms are related to reduced perfusion of the brain (67, 68) as illustrated in Fig. 2 during orthostatic stress for two common forms of OI, vasovagal syncope (simple faint), and postural tachycardia syndrome (POTS). Cerebral blood flow (CBF) is autoregulated and thus CBF should remain nearly constant within a range of perfusion pressure. Reductions of CBF indicate impaired cerebral autoregulation: thus CBF is no longer independent of perfusion pressure (67, 68). However, a well-defined quantitative relationship between lightheadedness and CBF has not been established. Most people experience some degree of episodic OI during their lives, if only transiently during infectious diseases or during dehydration (36). Abnormally reduced CBF is not explained by postural hydrostatic decreases in cerebral perfusion pressure because CBF is independent of changes in mean arterial blood pressure (MAP) within a range of ∼60 to 150 mmHg (48). Rather, cerebral blood flow is reduced by hypocapnia, which can accompany OI (45, 88). and is also dependent on parasympathetic (nitrergic) withdrawal (96) primarily at the level of pial resistance vessels. CBF is relatively independent of sympathetic influences except during very rapid changes and extremes of blood pressure (30).
Fig. 2.
Top: arterial pressure (AP); bottom: cerebral blood flow (CBF). Left: data from a representative vasovagal syncope patient; Right: data from a postural tachycardia syndrome (POTS) patient. AP and CBF are at first stable (Stage 1), fall slowly (Stage 2), and then abruptly decrease by >50% in the syncope patient at which time consciousness is lost. This compares with the POTS patient who has no decrease in AP but has a >20% reduction in CBF throughout tilt.
ORTHOSTATIC STRESS TEST AND TOOLS TO STUDY OI
As exercise stress tests are designed to test aerobic exercise capacity, so orthostatic stress tests test orthostatic capability. Approaches to standardize orthostatic testing vary. The most physiological approach is simply to have subjects stand without restriction, although exercising in place is avoided. However, OI patients can ameliorate symptoms by means of increased skeletal muscle pump activity (11). Thus many investigators use devices such as the motorized tilt table (42), which passively places the patient upright and reduces movement. More dramatic results can be obtained by upright suspension (70). Also, LBNP or suction has been used to duplicate some findings of orthostasis even while remaining supine, but it more closely simulates hemorrhage. Indeed, large negative pressures or combination of LBNP with upright tilt can evoke a fainting response in everyone.
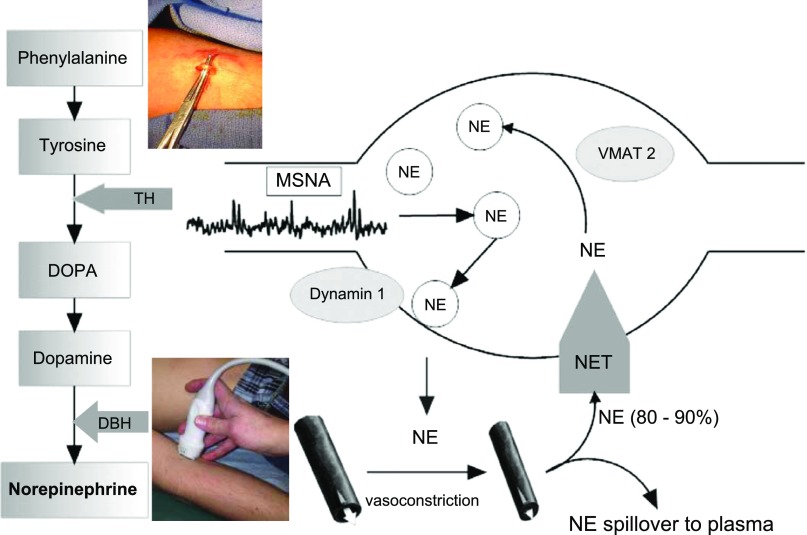
Physiological measurements made supine and during orthostasis employ a variety of instrumentation that measure BP, heart rate and cardiac rhythm, cardiac output (e.g., indicator dilution, inert gas rebreathing), regional blood flow (e.g., ultrasound, venous occlusion plethysmography, impedance plethysmography), blood volume, and blood chemistry, protein and genetic analyses. However, human in vivo studies of sympathetic adrenergic activity began in earnest with the advent of specific methods to measure sympathetic nerve activity with microneurography (94), to measure the resultant spillover of norepinephrine from the adrenergic synapse (19), to measure the effect of adrenergic vasoconstriction on local blood flow (15), and most recently to directly assess the integrity of norepinephrine synthesis and metabolic products by vascular biopsy (18, 46) (Fig. 3).
Fig. 3.
Synthetic pathway for norepinephrine (NE) and a cartoon of a sympathetic nerve ending. NE is stored in vesicles and released into neurovascular synapses in response to MSNA bursting. Postsynaptic binding results in vasoconstriction, which can be assessed by measuring local blood flow with Doppler ultrasound and other methods. Some of the released NE spills over into the plasma. However, the NE transporter (NET) takes up and conserves the large majority of released NE. A specific vesicular monoamine transporter (VMAT2) is responsible for translocating NE from the cytoplasm into the vesicles. A recent technique of venous biopsy has been successfully used to detect changes in synthetic proteins (46). [Modified with permission from (98); inset courtesy of Dr. Elisabeth Lambert of the Baker IDI Heart and Diabetes Institute.]
INEFFECTIVE SYMPATHETIC VASOCONSTRICTION PRODUCES NEUROGENIC ORTHOSTATIC HYPOTENSION
Orthostatic hypotension (OH) is defined as a sustained reduction of systolic BP >20 mmHg or diastolic BP >10 mmHg within 3 min of standing or head-up tilt to ≥60° (22). Nonneurogenic OH can be caused by drugs, age, and illnesses that secondarily cause acute or chronic hypovolemia. Neurogenic OH is identified with autonomic failure attributable to inadequate release of norepinephrine from sympathetic vasomotor neurons leading to vasoconstrictor failure (22). Autonomic failure can be primary with preganglionic, postganglionic, or both (e.g., Parkinson disease) forms of sympathetic failure (80); it can be genetic as in dopamine beta-hydroxylase deficiency (73); it can be autoimmune (43); and it can be acquired as a secondary aspect of systemic disease such as diabetes (63). Sympathetic cardiac denervation is a central aspect of Parkinson's disease (38) and may be found in other forms of autonomic failure. Cardiac parasympathetic innervation also is often defective, resulting in a steady fall in BP with little reflex tachycardia during orthostatic challenge.
Treatment of the underlying illness is essential. General therapy focuses on decreasing symptomatic orthostatic hypotension and syncope. Such therapy would include physical countermeasures including compression garments, dietary changes (increased salt, rapid water drinking), as well as pharmacotherapy. Pharmacotherapy is aimed at increasing blood volume by promoting salt and water retention (fludrocortisone) or by increasing red blood cell mass (recombinant erythropoietin). Short-acting pressor drugs such as midodrine or Droxidopa or drugs that enhance autonomic activity (atomoxetine, yohimbine, pyridostigmine) are also used (80).
COMMON VARIANT OI: CHRONIC ORTHOSTATIC INTOLERANCE (AKA POSTURAL TACHYCARDIA SYNDROME OR POTS) AND REFLEX VASOVAGAL SYNCOPE
POTS
POTS can be defined by day-to-day symptoms of OI coincident with excessive upright tachycardia but not hypotension that is improved by recumbence (25, 76). Excessive tachycardia is defined in adults by an increase exceeding 30 beats/min or to a heart rate exceeding 120 beats/min when upright. Higher heart rate changes are expected in the young with POTS (82). Tachycardia and concurrent symptoms are observed during orthostatic testing. POTS has often loosely been partitioned into patients with neuropathic POTS, in which often selective or partial dysautonomic de facto sympathetic adrenergic denervation occurs, and hyperadrenergic POTS, in which upright sympathetic overactivity dominates the picture.
As originally described, neuropathic POTS is caused by decreased sympathetic adrenergic vasoconstriction in the lower limbs, associated with reduced leg norepinephrine spillover (37) and lower extremity vasodilation (84). This results in increased blood flow (high flow) in the lower extremities even while supine. A recently described neuropathic variant has normal lower extremity hemodynamics (normal flow) but decreased splanchnic resistance when upright caused by impaired regional sympathetic vasoconstriction (89). Autonomic autoimmune neuropathy (43), when presenting as POTS, may have a similar mechanism of action. When neuropathic POTS patients are upright, a redistributive central hypovolemia causes baroreflex mediated tachycardia; indeed, baroreflex inhibition with intravenous phenylephrine eliminates the POTS response (90). This is complicated by known defects in the cardiovagal and sympathetic baroreflex in similar POTS patients (21), by the central effects of unexplained hyperpnea and hypocapnia in 50% of patients (88), and by observations of increased circulating catecholamines during orthostasis (37) even in these neuropathic patients.
The tachycardia of hyperadrenergic POTS is presumably driven by increased presynaptic or postsynaptic adrenergic potentiation. This might include central sympathoexcitation causing an increase in sympathetic nerve activity at the adrenergic synapse. Although increased sympathetic supine activity has been reported by some (25), it has not been reported by others (4). To date my laboratory has only observed increased muscle sympathetic activity in POTS when upright. Alternatively, synaptic NE may be increased: as epitomized by the norepinephrine transporter deficiency heterozygote (77), an autosomal mutation, found so far in only one pedigree with variable penetrance. Non-Mendelian NET deficiency with a smaller reduction in the transporter has been recently described and has wider prevalence (46).
Sympathetic nerve activity, and norepinephrine synthesis, release, and binding are also modulated by endocrine, paracrine, and autocrine mediators perhaps epitomized by the reciprocal actions of nitric oxide (NO) and angiotensin II. Data support a role for NO as an inhibitory neurotransmitter (105). Nitrergic NO, in particular, can act at prejunctional and postjunctional sites to reduce sympathetic transduction (93). This includes reduction of the release and binding of norepinephrine from the neurovascular junction (44) and postjunctional interference with neurotransmission (31). Downregulation of adrenergic receptors (34) and chemically denaturing of norepinephrine (55) have also been reported. Such mechanisms may contribute to the reduction of norepinephrine spillover in neuropathic POTS. Conversely, studies of sympathoexcited states show that ANG II acts via AT1R and reactive oxygen and nitrogen species (ROS) as an excitatory neurotransmitter within the brain at presynaptic sympathetic neurons (32) and in the periphery, where it exerts pre- and postjunctional modulation of sympathetic transduction, upregulation of adrenergic receptors (34), the release and binding of norepinephrine from the neuromuscular junction (44), and facilitation of the effects of norepinephrine. As in the CNS, this depends critically on the formation of ROS (7), which decrease NO (104), often uncoupling NOS (47), thus further enhancing superoxide production. This mechanism occurs in an important variant of hyperadrenergic POTS associated with a phenotype of pallor, supine tachycardia and vasoconstriction (low flow), and absolute hypovolemia (71). Bioavailable NO, plasma renin, and serum aldosterone are decreased (58), while plasma ANG II (86) is increased by a defect in ACE 2 (91).
Therapy for POTS to date is much like the treatment for neurogenic orthostatic hypotension in the use of physical countermeasures, salt and water intake, and even pharmacotherapy. Innovative treatment with ARBs and Droxidopa are under investigation. Exercise has always been a mainstay of rehabilitation in these patients. Recent work indicates that gravitational deconditioning (e.g., bedrest) is a frequent concomitant of the illness and that a graded exercise program can be very effective in improving overall patient well being (24).
Postural Syncope (Vasovagal Syncope, Acute OI, Simple Faint)
Syncope (fainting) may be defined as “complete loss of consciousness (and postural tone) attributable to transient global cerebral hypoperfusion characterized by rapid onset, short duration, and spontaneous complete recovery” (61). During a lifetime, ∼40% of people will faint, half of these presenting during adolescence with a maximum incidence at 15 yr old (26). Most syncope is caused by systemic hypotension. Syncope may be attributable to sympathetic adrenergic failure and orthostatic hypotension, which we have already discussed and which is easily ruled out by a 3-min standing test. Otherwise syncope is partitioned among cardiovascular syncope, frequently attributable to arrhythmic or structural heart disease and reflex or neurally mediated syncope. Cardiovascular syncope has a poor prognosis unless successful steps are taken to treat specific cardiac pathophysiology. Reflex syncope has a good prognosis (83). Orthostatic stress syncope and emotional stress syncope together comprise vasovagal syncope (VVS) (27), which is the largest subgroup within reflex syncope group. Regional or system-wide loss of sympathetic adrenergic vasoconstriction is an element in all vasovagal syncope, at least as a terminal event, and will be discussed in greater detail below. Orthostatic or postural syncope may be thought of as acute OI. Indeed, loss of consciousness is most often preceded by a prodrome of OI symptoms, particularly lightheadedness, nausea, sweating, weakness, and visual disturbance (e.g., black-out). Until recently postural syncope was thought to be caused by reflexes from a hypercontractile underfilled heart analogous to the Bezold-Jarisch reflex (1). This mechanism was favored despite evidence to the contrary: thus any such stimulus could only be short lived because baroreceptors would immediately be unloaded (28); few afferent nerves were excited in the original Oberg and Thoren (66) hemorrhaged cat model; VVS can occur in a ventricular denervated transplant recipient given the sodium nitroprusside (75); and the heart before syncope is neither empty nor hypercontractile (51). Thus to date, the pathophysiology of simple faint remains elusive (60) and findings are largely descriptive without necessarily informing on specific molecular mechanism(s).
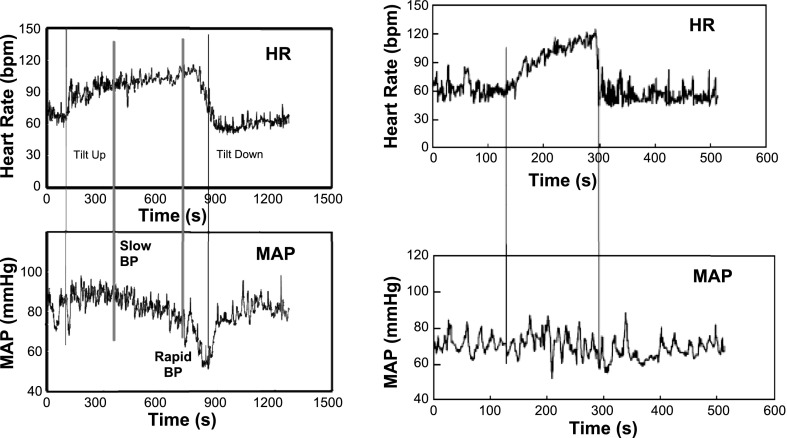
In the most common variant of postural faint that occurs in young patients, postural faint often comprises three stages (Fig. 4) that closely emulate the circulatory changes that occur during hemorrhage (2). Following initial orthostatic hypotension, mechanical and neurovascular equilibrium are reestablished, and BP stabilizes while HR increases in Stage 1. This stability distinguishes postural faint from true OH, in which BP falls early and remains low. BP is often highly oscillatory during this stage. These oscillations are sometimes referred to as Mayer waves (41) and correspond to approximately sinusoidal fluctuations in BP with an approximate 10-s period (0.1 Hz). Similar periodicity is shared by fluctuations in MSNA. The oscillations represent the time it takes for the closed loop sympathetic baroreflex to sense and compensate for a change in BP (29). Similar oscillations can be observed to a lesser extent in HR transduced by the cardiovagal baroreflex. Oscillations are accentuated during baroreflex unloading as occurs with orthostasis, in part attributable to resetting of the sympathetic baroreflex with orthostasis (23) and in part the result of thoracic hypovolemia.
Fig. 4.
Representative tracings during upright tilt for a postural syncope patient (left) and for a POTS patient (right). Heart rate (HR) is shown at top and mean arterial pressure (MAP) at bottom. HR increases in syncope, and POTS and is more excessively increased in POTS. MAP is stable throughout tilt in POTS. MAP is stable at first, decreases gradually in a second stage, and falls abruptly and rapidly in the third stage as loss of consciousness supervenes.
During Stage 2 BP slowly declines as HR reflexively increases further. This decrease in BP is often related to a reduction in cardiac output (99) despite sustained and even increased MSNA (12), although peripheral arterial resistance (95) and Mayer wave activity (65) are sustained. Both resistance and pressure oscillations subsequently diminish despite sustained sympathoexcitation. Hyperpnea and hypocapnia is observed at this point (45). In some patients Stage 2 is abbreviated. This is especially true for patients with convulsive syncope in whom episodes occur abruptly in association with asystole. Some explanations offered for the early phases of postural faint include reduced tyrosine hydroxylase and NE synthesis in patients with supine low BP, excess NET (98), or selective deficit of splanchnic adrenergic vasoconstriction/venoconstriction (89). Prodromal OI symptoms often begin during this second stage; combined with tachycardia that may lead one to diagnose POTS in the laboratory setting. However, a history of episodic faints interspersed with long periods free of signs and symptoms of OI distinguishes postural syncope from POTS, in which symptoms are chronically present. Medical history is paramount. Admittedly, the prodrome of simple faint and the signs and symptoms of neuropathic POTS are similar because they can have similar pathophysiology, namely reflex tachycardia from excessive reduction in central blood volume (84, 87, 89). On the other hand, postural fainters corresponding to the pale and vasoconstricted hyperadrenergic POTS patients are rarae aves. In our experience, POTS patients typically have day-to-day symptoms but do not faint, whereas fainters do not have daily symptoms; however, this distinction has blurred and there are some POTS patients who faint and a few fainters with daily or nearly daily symptoms of OI. Nevertheless, fainting in POTS is relatively uncommon outside the laboratory where POTS patients can be made to faint.
In the final Stage 3, CBF, BP, and HR fall precipitously in that order, seemingly defying the expected causal relationship between BP and CBF (14). Recent data suggest loss of cardiovagal and sympathetic baroreflex integrity and loss of cerebral autoregulation with entrainment of CBF, BP, and HR by an extrinsic oscillator that may be hyperpneic hyperventilation (67, 69). Why baroreflex integrity is lost is unknown. Thus, instead of the usual reciprocal BP-HR and BP-MSNA functional relationships (BP decreases, HR and MSNA increase), HR, BP, and MSNA decrease synchronously. This may result in asystole and sympathetic silence (39). Typically the faint is associated with marked systemic vasodilation while CBF becomes strictly dependent on declining BP. The requirement of sympathetic nerve withdrawal as the precipitant of final hypotension has recently been challenged (97). Although vasodilation always occurs, the sympathetic baroreflex can fail with or without MSNA silence. Similar findings occur in patients with vasodepressor syncope where vasodilation without bradycardia occurs along with loss of the sympathetic efferent baroreflex causing progressive loss of compensatory vasoconstriction. The vagal baroreflex remains intact.
Therapy for vasovagal syncope associated with a lengthy prodrome is largely avoidance and physical countermeasures; the most efficacious of these is to lie down or squat. Other countermeasures include those that enhance the skeletal muscle pump (e.g., leg crossing) or activate the exercise pressor reflex (isometric hand grip). Enhanced salt and water intake is often encouraged and has shown some efficacy in small studies employing large amounts of salt loading (10). In older patients, confounding use of antihypertensives or diuretics need to be considered. Pharmacotherapy has not been shown to be particularly effective in large multicenter studies (78). Asystolic faints can be improved by pacemaker insertion (6).
Respiration and Postural Hyperpnea
Both POTS and postural faint are associated with hyperventilation, more specifically hyperpnea (45, 64, 88). Hyperpnea and hypocapnia precede loss of consciousness in virtually every vasovagal syncope patient. Hypotension and bradycardia might be explained by the pulmonary stretch reflex unfettered by compensatory baroreflex effects (53, 69). The cause of hyperpnea is unclear. However, a ventilatory efferent arm of the arterial baroreflex has recently been found in humans that is independent of respiratory chemoreflexes (92). Thus unloaded baroreflexes in Stage 2 of fainting cause an increase in tidal volume but not in respiratory rate, resulting in markedly hyperpneic respirations. Similar findings of hyperpnea are found in POTS patients with central hypovolemia who do not faint. Measurements indicate that while cardiovagal baroreflex gain is reduced in POTS (21), the sympathetic nerve response is augmented (62).
POSTURAL HYPERPNEA AS A SEPARATE VARIANT OF OI
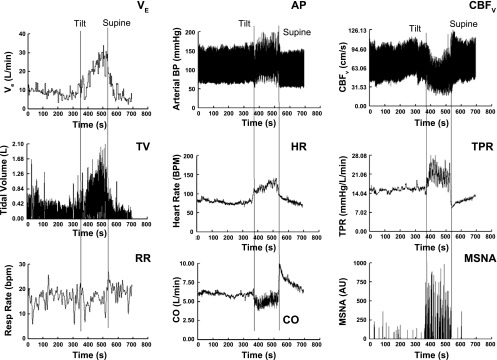
The final figure (Fig. 5) shows the results of a representative patient with involuntary hyperpnea in the upright position. Similar hemodynamic findings can be induced in healthy volunteers during upright voluntary hyperpnea. Findings include marked increase in MSNA and peripheral resistance, decreased cardiac output (CO), and decreased cerebral blood flow as a result of hypocapnia. A large initial reduction in central blood volume, an extraordinary hyperpneic breath, and rapid reduction of cerebral blood flow start a self-perpetuated process. Respiratory chemoreflex assessment is normal. During subsequent upright experiments infusion of phenylephrine or inhalation of supplemental CO2 to correct end-tidal carbon dioxide from 24 to 38 Torr decreased upright HR from 130 to 100, reduced MSNA, and normalized cerebral blood flow. There were no findings consistent with anxiety including low resting MSNA. Once upright, sympathoexcitation occurred and preceded obvious anxiety. Withdrawal of supplemental CO2 increased MSNA followed thereafter by hyperpnea, suggesting a causal relation. Similar findings were reported previously (17). Postural hyperventilation has been observed for years and often attributed to panic disorder (57). A more complex pathophysiology involves sympathetic stimulation of ventilation and cerebral alkalosis (92).
Fig. 5.
Response to upright tilt for a representative patient with postural hyperpnea. From top to bottom: ventilatory parameters: left, expiratory minute volume (VE), tidal volume (TV), and respiratory rate (RR); middle, arterial pressure (AP), HR, and cardiac output; right, cerebral blood flow CBFv, total peripheral resistance (TPR), and muscle sympathetic nerve activity (MSNA). VE rapidly increases on tilt attributable to an increase in TV. Increase in VE is progressive and preceded by an increase MSNA and TPR and decrease in CO and CBFv. Note that HR may reach levels commensurate with POTS.
Perspective
Once true neurogenic orthostatic hypotension is ruled out, orthostatic intolerance comprises non-life threatening phenomena that occur in large numbers of people and relate to inappropriate sympathetic adrenergic function. Although most of us have at least experienced mild OI as the transient initial orthostatic hypotension of rapid standing and its associated light-headedness, other forms of OI can have a serious impact on quality of life. Postural vasovagal faint and postural tachycardia syndrome are two well described common forms of OI. Other forms of OI, such as postural hyperpnea, remain to be investigated.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grants RO1-HL074873 and RO1-HL087803.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
Author contributions: J.M.S. conception and design of research; J.M.S. performed experiments; J.M.S. analyzed data; J.M.S. interpreted results of experiments; J.M.S. prepared figures; J.M.S. drafted manuscript; J.M.S. edited and revised manuscript; J.M.S. approved final version of manuscript.
REFERENCES
- 1. Aviado DM, Guevara AD. The Bezold-Jarisch reflex. A historical perspective of cardiopulmonary reflexes. Ann NY Acad Sci 940: 48–58, 2001. [PubMed] [Google Scholar]
- 2. Barcroft H, McMichael JE, Scarpey-Schafer EP. Posthaemorrhagic fainting study by cardiac output and forearm flow. Lancet 1: 489–491, 1944. [Google Scholar]
- 3. Bernard C. Sur les effets de la section de la portion cephalique du grand sympathique. C R Soc Biol Paris 4: 168–170, 1852. [Google Scholar]
- 4. Bonyhay I, Freeman R. Sympathetic nerve activity in response to hypotensive stress in the postural tachycardia syndrome. Circulation 110: 3193–3198, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Brack KE, Coote JH, Ng GA. Vagus nerve stimulation inhibits the increase in Ca2+ transient and left ventricular force caused by sympathetic nerve stimulation but has no direct effects alone—epicardial Ca2+ fluorescence studies using fura-2 AM in the isolated innervated beating rabbit heart. Exp Physiol 95: 80–92, 2010. [DOI] [PubMed] [Google Scholar]
- 6. Brignole M, Menozzi C, Moya A, Andresen D, Blanc JJ, Krahn AD, Wieling W, Beiras X, Deharo JC, Russo V, Tomaino M, Sutton R. Pacemaker therapy in patients with neurally-mediated syncope and documented asystole. Third International Study on Syncope of Uncertain Etiology (ISSUE-3): A randomized trial. Circulation 125: 2566–2571, 2012. [DOI] [PubMed] [Google Scholar]
- 7. Campese VM, Ye S, Zhong H, Yanamadala V, Ye Z, Chiu J. Reactive oxygen species stimulate central and peripheral sympathetic nervous system activity. Am J Physiol Heart Circ Physiol 287: H695–H703, 2004. [DOI] [PubMed] [Google Scholar]
- 8. Charkoudian N, Joyner MJ, Sokolnicki LA, Johnson CP, Eisenach JH, Dietz NM, Curry TB, Wallin BG. Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans. J Physiol 572: 821–827, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Charkoudian N, Rabbitts JA. Sympathetic neural mechanisms in human cardiovascular health and disease. Mayo Clin Proc 84: 822–830, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Claydon VE, Hainsworth R. Salt supplementation improves orthostatic cerebral and peripheral vascular control in patients with syncope. Hypertension 43: 809–813, 2004. [DOI] [PubMed] [Google Scholar]
- 11. Claydon VE, Hainsworth R. Increased postural sway in control subjects with poor orthostatic tolerance. J Am Coll Cardiol 46: 1309–1313, 2005. [DOI] [PubMed] [Google Scholar]
- 12. Cooke WH, Rickards CA, Ryan KL, Kuusela TA, Convertino VA. Muscle sympathetic nerve activity during intense lower body negative pressure to presyncope in humans. J Physiol 587: 4987–4999, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cooper VL, Hainsworth R. Carotid baroreceptor reflexes in humans during orthostatic stress. Exp Physiol 86: 677–681, 2001. [DOI] [PubMed] [Google Scholar]
- 14. Dan D, Hoag JB, Ellenbogen KA, Wood MA, Eckberg DL, Gilligan DM. Cerebral blood flow velocity declines before arterial pressure in patients with orthostatic vasovagal presyncope. J Am Coll Cardiol 39: 1039–1045, 2002. [DOI] [PubMed] [Google Scholar]
- 15. Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments alpha-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol 287: H2576–H2584, 2004. [DOI] [PubMed] [Google Scholar]
- 16. Donegan JF. The physiology of veins. J Physiol 55: 226–245, 1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Donnelly J, Lucas SJ, Thomas KN, Galvin SD, Ainslie PN. Profound hyperventilation and development of periodic breathing during exceptional orthostatic stress in a 21-year-old man. Respir Physiol Neurobiol 177: 66–70, 2011. [DOI] [PubMed] [Google Scholar]
- 18. Esler M. The 2009 Carl Ludwig Lecture: Pathophysiology of the human sympathetic nervous system in cardiovascular diseases: the transition from mechanisms to medical management. J Appl Physiol 108: 227–237, 2010. [DOI] [PubMed] [Google Scholar]
- 19. Esler M, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate, and functions. Physiol Rev 70: 963–985, 1990. [DOI] [PubMed] [Google Scholar]
- 20. Fadel PJ, Raven PB. Human investigations into the arterial and cardiopulmonary baroreflexes during exercise. Exp Physiol 97: 39–50, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Farquhar WB, Taylor JA, Darling SE, Chase KP, Freeman R. Abnormal baroreflex responses in patients with idiopathic orthostatic intolerance. Circulation 102: 3086–3091, 2000. [DOI] [PubMed] [Google Scholar]
- 22. Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz I, Schondorff R, Stewart JM, van Dijk JG. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 21: 69–72, 2011. [DOI] [PubMed] [Google Scholar]
- 23. Fu Q, Shook RP, Okazaki K, Hastings JL, Shibata S, Conner CL, Palmer MD, Levine BD. Vasomotor sympathetic neural control is maintained during sustained upright posture in humans. J Physiol 577: 679–687, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fu Q, Vangundy TB, Shibata S, Auchus RJ, Williams GH, Levine BD. Exercise training versus propranolol in the treatment of the postural orthostatic tachycardia syndrome. Hypertension 58: 167–175, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Furlan R, Jacob G, Snell M, Robertson D, Porta A, Harris P, Mosqueda-Garcia R. Chronic orthostatic intolerance: a disorder with discordant cardiac and vascular sympathetic control. Circulation 98: 2154–2159, 1998. [DOI] [PubMed] [Google Scholar]
- 26. Ganzeboom KS, Colman N, Reitsma JB, Shen WK, Wieling W. Prevalence and triggers of syncope in medical students. Am J Cardiol 91: 1006–1008, 2003. [DOI] [PubMed] [Google Scholar]
- 27. Gowers WR. A lecture on vagal and vasovagal attacks. Lancet 173: 716–724, 1907. [Google Scholar]
- 28. Hainsworth R. Syncope: what is the trigger? Heart 89: 123–124, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hammer PE, Saul JP. Resonance in a mathematical model of baroreflex control: arterial blood pressure waves accompanying postural stress. Am J Physiol Regul Integr Comp Physiol 288: R1637–R1648, 2005. [DOI] [PubMed] [Google Scholar]
- 30. Hamner JW, Tan CO, Lee K, Cohen MA, Taylor JA. Sympathetic control of the cerebral vasculature in humans. Stroke 41: 102–109, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hatanaka Y, Hobara N, Honghua J, Akiyama S, Nawa H, Kobayashi Y, Takayama F, Gomita Y, Kawasaki H. Neuronal nitric-oxide synthase inhibition facilitates adrenergic neurotransmission in rat mesenteric resistance arteries. J Pharmacol Exp Ther 316: 490–497, 2006. [DOI] [PubMed] [Google Scholar]
- 32. Head GA. Role of AT1 receptors in the central control of sympathetic vasomotor function. Clin Exp Pharmacol Physiol Suppl 3: S93–S98, 1996. [PubMed] [Google Scholar]
- 33. Hill L. The influences of the force of gravity on the circulation of the blood. J Physiol 18: 15–53, 1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hu ZW, Shi XY, Okazaki M, Hoffman BB. Angiotensin II induces transcription and expression of alpha 1-adrenergic receptors in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 268: H1006–H1014, 1995. [DOI] [PubMed] [Google Scholar]
- 35. Huxley VH, Scallan J. Lymphatic fluid: exchange mechanisms and regulation. J Physiol 589: 2935–2943, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iwasaki KI, Zhang R, Zuckerman JH, Pawelczyk JA, Levine BD. Effect of head-down-tilt bed rest and hypovolemia on dynamic regulation of heart rate and blood pressure. Am J Physiol Regul Integr Comp Physiol 279: R2189–R2199, 2000. [DOI] [PubMed] [Google Scholar]
- 37. Jacob G, Costa F, Shannon JR, Robertson RM, Wathen M, Stein M, Biaggioni I, Ertl A, Black B, Robertson D. The neuropathic postural tachycardia syndrome. N Engl J Med 343: 1008–1014, 2000. [DOI] [PubMed] [Google Scholar]
- 38. Jain S, Goldstein DS. Cardiovascular dysautonomia in Parkinson disease: from pathophysiology to pathogenesis. Neurobiol Dis 46: 572–580, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jardine DL, Melton IC, Crozier IG, English S, Bennett SI, Frampton CM, Ikram H. Decrease in cardiac output and muscle sympathetic activity during vasovagal syncope. Am J Physiol Heart Circ Physiol 282: H1804–H1809, 2002. [DOI] [PubMed] [Google Scholar]
- 40. Joyner MJ, Charkoudian N, Wallin BG. A sympathetic view of the sympathetic nervous system and human blood pressure regulation. Exp Physiol 93: 715–724, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Julien C. The enigma of Mayer waves: facts and models. Cardiovasc Res 70: 12–21, 2006. [DOI] [PubMed] [Google Scholar]
- 42. Kenny RA, Ingram A, Bayliss J, Sutton R. Head-up tilt: a useful test for investigating unexplained syncope. Lancet 1: 1352–1355, 1986. [DOI] [PubMed] [Google Scholar]
- 43. Klein CM, Vernino S, Lennon VA, Sandroni P, Fealey RD, Benrud-Larson L, Sletten D, Low PA. The spectrum of autoimmune autonomic neuropathies. Ann Neurol 53: 752–758, 2003. [DOI] [PubMed] [Google Scholar]
- 44. Kolo LL, Westfall TC, Macarthur H. Nitric oxide decreases the biological activity of norepinephrine resulting in altered vascular tone in the rat mesenteric arterial bed. Am J Physiol Heart Circ Physiol 286: H296–H303, 2004. [DOI] [PubMed] [Google Scholar]
- 45. Lagi A, Cencetti S, Corsoni V, Georgiadis D, Bacalli S. Cerebral vasoconstriction in vasovagal syncope: any link with symptoms? A transcranial Doppler study. Circulation 104: 2694–2698, 2001. [DOI] [PubMed] [Google Scholar]
- 46. Lambert E, Eikelis N, Esler M, Dawood T, Schlaich M, Bayles R, Socratous F, Agrotis A, Jennings G, Lambert G, Vaddadi G. Altered sympathetic nervous reactivity and norepinephrine transporter expression in patients with postural tachycardia syndrome. Circ Arrhythm Electrophysiol 1: 103–109, 2008. [DOI] [PubMed] [Google Scholar]
- 47. Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev 39: 183–238, 1959. [DOI] [PubMed] [Google Scholar]
- 49. Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res 87: 198–210, 2010. [DOI] [PubMed] [Google Scholar]
- 50. Lewis T. A lecture on vasovagal syncope and the carotid sinus mechanism: with comments on Gower's and Nothnagel's syndrome. Br Med J 1: 873–876, 1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu JE, Hahn RT, Stein KM, Markowitz SM, Okin PM, Devereux RB, Lerman BB. Left ventricular geometry and function preceding neurally mediated syncope. Circulation 101: 777–783, 2000. [DOI] [PubMed] [Google Scholar]
- 52. Liu JL, Murakami H, Zucker IH. Angiotensin II-nitric oxide interaction on sympathetic outflow in conscious rabbits. Circ Res 82: 496–502, 1998. [DOI] [PubMed] [Google Scholar]
- 53. Looga R. Reflex cardiovascular responses to lung inflation: a review. Respir Physiol 109: 95–106, 1997. [DOI] [PubMed] [Google Scholar]
- 54. Loutzenhiser R, Bidani A, Chilton L. Renal myogenic response: kinetic attributes and physiological role. Circ Res 90: 1316–1324, 2002. [DOI] [PubMed] [Google Scholar]
- 55. Macarthur H, Mattammal MB, Westfall TC. A new perspective on the inhibitory role of nitric oxide in sympathetic neurotransmission. Biochem Biophys Res Commun 216: 686–692, 1995. [DOI] [PubMed] [Google Scholar]
- 56. Macarthur H, Wilken GH, Westfall TC, Kolo LL. Neuronal and non-neuronal modulation of sympathetic neurovascular transmission. Acta Physiol (Oxf) 203: 37–45, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Malmberg LP, Tamminen K, Sovijarvi AR. Orthostatic increase of respiratory gas exchange in hyperventilation syndrome. Thorax 55: 295–301, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Medow MS, Bamji N, Clarke D, Ocon AJ, Stewart JM. Reactive oxygen species (ROS) from NADPH and xanthine oxidase modulate the cutaneous local heating response in healthy humans. J Appl Physiol 111: 20–26, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Miller JD, Pegelow DF, Jacques AJ, Dempsey JA. Skeletal muscle pump versus respiratory muscle pump: modulation of venous return from the locomotor limb in humans. J Physiol 563: 925–943, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mosqueda-Garcia R, Furlan R, Tank J, Fernandez-Violante R. The elusive pathophysiology of neurally mediated syncope. Circulation 102: 2898–2906, 2000. [DOI] [PubMed] [Google Scholar]
- 61. Moya A, Sutton R, Ammirati F, Blanc JJ, Brignole M, Dahm JB, Deharo JC, Gajek J, Gjesdal K, Krahn A, Massin M, Pepi M, Pezawas T, Ruiz GR, Sarasin F, Ungar A, van Dijk JG, Walma EP, Wieling W. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J 30: 2631–2671, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Muenter SN, Charkoudian N, Dotson RM, Suarez GA, Low PA. Baroreflex control of muscle sympathetic nerve activity in postural orthostatic tachycardia syndrome. Am J Physiol Heart Circ Physiol 289: H1226–H1233, 2005. [DOI] [PubMed] [Google Scholar]
- 63. Mukai S, Lipsitz LA. Orthostatic hypotension. Clin Geriatr Med 18: 253–268, 2002. [DOI] [PubMed] [Google Scholar]
- 64. Novak V, Spies JM, Novak P, McPhee BR, Rummans TA, Low PA. Hypocapnia and cerebral hypoperfusion in orthostatic intolerance. Stroke 29: 1876–1881, 1998. [DOI] [PubMed] [Google Scholar]
- 65. Nowak JA, Ocon A, Taneja I, Medow MS, Stewart JM. Multiresolution wavelet analysis of time-dependent physiological responses in syncopal youths. Am J Physiol Heart Circ Physiol 296: H171–H179, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Oberg B, Thoren P. Increased activity in left ventricular receptors during hemorrhage or occlusion of caval veins in the cat. A possible cause of the vaso-vagal reaction. Acta Physiol Scand 85: 164–173, 1972. [DOI] [PubMed] [Google Scholar]
- 67. Ocon AJ, Kulesa J, Clarke D, Taneja I, Medow MS, Stewart JM. Increased phase synchronization and decreased cerebral autoregulation during fainting in the young. Am J Physiol Heart Circ Physiol 297: H2084–H2095, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ocon AJ, Medow MS, Taneja I, Clarke D, Stewart JM. Decreased upright cerebral blood flow and cerebral autoregulation in normocapnic postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 297: H664–H673, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ocon AJ, Medow MS, Taneja I, Stewart JM. Respiration drives phase synchronization between blood pressure and RR interval following loss of cardiovagal baroreflex during vasovagal syncope. Am J Physiol Heart Circ Physiol 300: H527–H540, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pasquier M, Yersin B, Vallotton L, Carron PN. Clinical update: suspension trauma. Wilderness Environ Med 22: 167–171, 2011. [DOI] [PubMed] [Google Scholar]
- 71. Raj SR, Biaggioni I, Yamhure PC, Black BK, Paranjape SY, Byrne DW, Robertson D. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation 111: 1574–1582, 2005. [DOI] [PubMed] [Google Scholar]
- 72. Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci 317: 75–77, 1999. [DOI] [PubMed] [Google Scholar]
- 73. Robertson D, Haile V, Perry SE, Robertson RM, Phillips JA, III, Biaggioni I. Dopamine beta-hydroxylase deficiency. A genetic disorder of cardiovascular regulation. Hypertension 18: 1–8, 1991. [DOI] [PubMed] [Google Scholar]
- 74. Rowell LB. Human cardiovascular control. New York: Oxford University Press, 1993. [Google Scholar]
- 75. Scherrer U, Vissing S, Morgan BJ, Hanson P, Victor RG. Vasovagal syncope after infusion of a vasodilator in a heart-transplant recipient. N Engl J Med 322: 602–604, 1990. [DOI] [PubMed] [Google Scholar]
- 76. Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology 43: 132–137, 1993. [DOI] [PubMed] [Google Scholar]
- 77. Shannon JR, Flattem NL, Jordan J, Jacob G, Black BK, Biaggioni I, Blakely RD, Robertson D. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med 342: 541–549, 2000. [DOI] [PubMed] [Google Scholar]
- 78. Sheldon RS, Amuah JE, Connolly SJ, Rose S, Morillo CA, Talajic M, Kus T, Fouad-Tarazi F, Klingenheben T, Krahn AD, Koshman ML, Ritchie D. Effect of metoprolol on quality of life in the Prevention of Syncope Trial. J Cardiovasc Electrophysiol 20: 1083–1088, 2009. [DOI] [PubMed] [Google Scholar]
- 79. Sheriff DD, Nadland IH, Toska K. Role of sympathetic responses on the hemodynamic consequences of rapid changes in posture in humans. J Appl Physiol 108: 523–532, 2010. [DOI] [PubMed] [Google Scholar]
- 80. Shibao C, Okamoto L, Biaggioni I. Pharmacotherapy of autonomic failure. Pharmacol Ther 134: 279–286, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shipley RD, Kim SJ, Muller-Delp JM. Time course of flow-induced vasodilation in skeletal muscle: contributions of dilator and constrictor mechanisms. Am J Physiol Heart Circ Physiol 288: H1499–H1507, 2005. [DOI] [PubMed] [Google Scholar]
- 82. Singer W, Sletten DM, Opfer-Gehrking TL, Brands CK, Fischer PR, Low PA. Postural tachycardia in children and adolescents: what is abnormal? J Pediatr 160: 222–226, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Soteriades ES, Evans JC, Larson MG, Chen MH, Chen L, Benjamin EJ, Levy D. Incidence and prognosis of syncope. N Engl J Med 347: 878–885, 2002. [DOI] [PubMed] [Google Scholar]
- 84. Stewart JM. Pooling in chronic orthostatic intolerance: arterial vasoconstrictive but not venous compliance defects. Circulation 105: 2274–2281, 2002. [DOI] [PubMed] [Google Scholar]
- 85. Stewart JM. Transient orthostatic hypotension is common in adolescents. J Pediatr 140: 418–424, 2002. [DOI] [PubMed] [Google Scholar]
- 86. Stewart JM, Glover JL, Medow MS. Increased plasma angiotensin II in postural tachycardia syndrome (POTS) is related to reduced blood flow and blood volume. Clin Sci (Lond) 110: 255–263, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Stewart JM, McLeod KJ, Sanyal S, Herzberg G, Montgomery LD. Relation of postural vasovagal syncope to splanchnic hypervolemia in adolescents. Circulation 110: 2575–2581, 2004. [DOI] [PubMed] [Google Scholar]
- 88. Stewart JM, Medow MS, Cherniack NS, Natelson BH. Postural hypocapnic hyperventilation is associated with enhanced peripheral vasoconstriction in postural tachycardia syndrome with normal supine blood flow. Am J Physiol Heart Circ Physiol 291: H904–H913, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Stewart JM, Medow MS, Glover JL, Montgomery LD. Persistent splanchnic hyperemia during upright tilt in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 290: H665–H673, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Stewart JM, Munoz J, Weldon A. Clinical and physiological effects of an acute alpha-1 adrenergic agonist and a beta-1 adrenergic antagonist in chronic orthostatic intolerance. Circulation 106: 2946–2954, 2002. [DOI] [PubMed] [Google Scholar]
- 91. Stewart JM, Ocon AJ, Clarke D, Taneja I, Medow MS. Defects in cutaneous angiotensin-converting enzyme 2 and angiotensin-(1–7) production in postural tachycardia syndrome. Hypertension 53: 767–774, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Stewart JM, Rivera E, Clarke DA, Baugham IL, Ocon AJ, Taneja I, Terilli C, Medow MS. Ventilatory baroreflex sensitivity in humans is not modulated by chemoreflex activation. Am J Physiol Heart Circ Physiol 300: H1492–H1500, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Storgaard T, Nedergaard OA. Prejunctional modulation by angiotensins of noradrenaline release from sympathetic neurons in isolated rabbit aorta. Naunyn Schmiedebergs Arch Pharmacol 356: 706–711, 1997. [DOI] [PubMed] [Google Scholar]
- 94. Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol 274: 621–637, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Taneja I, Medow MS, Glover JL, Raghunath NK, Stewart JM. Increased vasoconstriction predisposes to hyperpnea and postural faint. Am J Physiol Heart Circ Physiol 295: H372–H381, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Toda N, Ayajiki K, Okamura T. Cerebral blood flow regulation by nitric oxide: recent advances. Pharmacol Rev 61: 62–97, 2009. [DOI] [PubMed] [Google Scholar]
- 97. Vaddadi G, Esler MD, Dawood T, Lambert E. Persistence of muscle sympathetic nerve activity during vasovagal syncope. Eur Heart J 31: 2027–2033, 2010. [DOI] [PubMed] [Google Scholar]
- 98. Vaddadi G, Guo L, Esler M, Socratous F, Schlaich M, Chopra R, Eikelis N, Lambert G, Trauer T, Lambert E. Recurrent postural vasovagal syncope: sympathetic nervous system phenotypes. Circ Arrhythm Electrophysiol 4: 711–718, 2011. [DOI] [PubMed] [Google Scholar]
- 99. Verheyden B, Liu J, van Dijk N, Westerhof BE, Reybrouck T, Aubert AE, Wieling W. Steep fall in cardiac output is main determinant of hypotension during drug-free and nitroglycerine-induced orthostatic vasovagal syncope. Heart 5: 1695–1701, 2008. [DOI] [PubMed] [Google Scholar]
- 100. Victor RG, Mark AL. Interaction of cardiopulmonary and carotid baroreflex control of vascular resistance in humans. J Clin Invest 76: 1592–1598, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Von Euler US. Identification of the sympathomimetic ergone in adrenergic nerves of cattle (sympathin N) with levo-noradrenaline. Acta Physiol Scand 16: 63–74, 1948. [Google Scholar]
- 102. Wang Y, Marsgall RJ, Shepherd JT. The effect of changes in posture and graded exercise on stroke volume in man. J Clin Invest 39: 1051–1061, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wieling W, Krediet CT, van Dijk N, Linzer M, Tschakovsky ME. Initial orthostatic hypotension: review of a forgotten condition. Clin Sci (Lond) 112: 157–165, 2007. [DOI] [PubMed] [Google Scholar]
- 104. Wolin MS. Interactions of oxidants with vascular signaling systems. Arterioscler Thromb Vasc Biol 20: 1430–1442, 2000. [DOI] [PubMed] [Google Scholar]
- 105. Zanzinger J, Czachurski J, Seller H. Inhibition of sympathetic vasoconstriction is a major principle of vasodilation by nitric oxide in vivo. Circ Res 75: 1073–1077, 1994. [DOI] [PubMed] [Google Scholar]