Abstract
The effect of exercise training on hematopoietic stem cells (HSC) is largely unknown. The aim of the present investigation was to determine whether exercise training could expand the bone marrow HSC pool and influence various aspects of HSC function. Mice were either exercise trained (EX; 1 h/day, 3 days/wk, for 8 wk) or remained sedentary (SED). Bone marrow (BM) from SED or EX mice was extracted from different HSC niches for cell cycle analysis, HSC (lineage−, Sca-1+, c-Kit+) quantification, and differentiation along various hematopoietic lineages via flow cytometry. Serum was collected for evaluation of cytokines known to regulate HSC. To determine HSC function, BM from EX and SED mice was transplanted into primary and secondary recipients in a BM transplant assay. EX increased HSC quantity in the vascular BM niche 20% vs. SED (P < 0.05) and increased the proportion of whole BM cells in G2/M phase of cell cycle (P < 0.05). The number of spleen colonies was 48% greater (P < 0.05) in recipients transplanted with BM from EX. Serum IL-6 levels were decreased 38% in EX, and differentiation along the lineage trended to increase (16%, P = 0.053 and 16%, P = 0.061, respectively). Short- or long-term engraftment and homing in primary recipients were not altered in EX. HSC self-renewal as analyzed by hematopoietic regeneration in secondary recipients was also unaffected by EX. Here we demonstrate that HSC quantity is increased in the BM niche associated with more activated, differentiated HSC, and that this expansion does not improve or impair HSC function.
Keywords: interleukin-6, niche, bone marrow transplant, cell cycle, engraftment
hematopoietic stem cells (HSC) are the most primitive cells in the hematopoietic lineage and act as a reserve cell population responsible for maintenance and production of circulating blood cells (18). HSC were first discovered in 1961 as the donor cell population responsible for survival and regeneration of all blood cell lineages in myeloablated hosts (32). HSC reside in two distinct but related niches within the bone marrow: the endosteal and vascular niche. HSC in the endosteal niche lining the bone in the bone marrow cavity are maintained in a state of quiescence via their association with osteoblasts (19) and have been shown to have greater long-term repopulating potential (13). HSC from the endosteal niche can migrate to the vascular niche where they associate with endothelial cells of the bone marrow sinusoids (19), become more mitotically active, and are primed for release into circulation or differentiation (15). Phenotypically, HSC are identified by their lack of expression of mature hematopoietic lineage markers and positive expression of c-Kit and Sca-1, collectively the so-called LSK (lineage−, Sca-1+, c-Kit+) population (11, 23). Functionally, HSC are characterized by their ability to regenerate the hematopoietic system of myeloablated hosts in the bone marrow transplantation (BMT) assay (24). For successful hematopoietic regeneration to occur, HSC must be in sufficient quantity, be able to successfully home to their niche in the bone marrow, be able to proliferate and differentiate to reconstitute all blood cell lineages, while maintaining self-renewal to ensure long-term engraftment (3, 19). HSC expansion and functional regulation by ex vivo manipulation of HSC in culture has been the focus of numerous investigations to optimize the donor cell source for BMT. For example, treatment with cytokine cocktails in vitro has revealed key paracrine mediators of HSC survival, self-renewal, and proliferation (12). Conversely, data from other studies have demonstrated that HSC expanded in vitro may result in impaired HSC function, manifesting as decreased levels of engraftment on transplantation (12, 17, 33). These data suggest that ex vivo expansion and manipulation of HSC may not be optimal. It remains unknown if physiological stress in vivo, such as exercise, may provide the appropriate stimulus to induce HSC expansion and maintenance of function.
Exercise is a potent physiological stress associated with increased red blood cell content (9) and enhanced disease resistance via improved immune function (25). Whether these beneficial effects of exercise in the most mature cells of the hematopoietic lineage extend to more primitive hematopoietic cells, like the HSC, is unknown. The majority of studies in this field have focused on the mobilization of HSC into peripheral blood following an acute exercise stimulus in humans using flow cytometry and cell surface staining to identify HSC. These studies have demonstrated that acute exercise is a potent stimulus for the mobilization of the general HSC population into peripheral blood (20, 31, 34, 36); however, the most primitive HSC population appears unresponsive to an acute exercise stimulus (5, 20). These acute changes are thought to be mediated by alterations in cytokines and growth factors involved in HSC mobilization or in proliferation with acute exercise (30, 34, 36). Studies examining the effects of exercise training on HSC quantity are few, and the data are equivocal, with both increases (5) and no effects of training on circulating HSC quantity being found (31, 34). The paucity of data regarding the effects of exercise training on HSC highlights the need for more research in this area.
Previous work from our laboratory (16) and others (6, 8, 37) has demonstrated an increase in protective mechanisms in the heterogeneous population of bone marrow cells in response to exercise training. Furthermore, we (1) have demonstrated that exercise training promoted medullary hematopoiesis, and that these effects may have been mediated through an improved cytokine milieu in the serum of exercise-trained mice or beneficial niche adaptations. The aim of the present investigation was to quantify HSC in their different niches within the marrow (i.e., endosteal and vascular) and examine their function using the BMT assay, where marrow from exercise-trained or sedentary donors was used to reconstitute myeloablated recipients. In addition to allowing us to examine basic aspects of HSC function, the BMT assay also has the advantage of being clinically relevant, as it is directly related to BMT in humans and could help determine whether using exercise-trained donors can improve recipient hematopoietic regeneration. We hypothesized that exercise training would increase HSC quantity and improve their function.
MATERIALS AND METHODS
Mice.
Animal protocols were approved by the McMaster Animal Research Ethics Board and conformed to Canadian Council for Animal Care Guidelines. Mice, housed at no more than five per cage, were maintained on a 12:12-h light-dark schedule and provided food and water ad libitum. Male C57Bl/6-eGFP (22) (kind gift from Dr. B. Trigatti, McMaster University) or C57Bl/6 were bred in-house. Female C57Bl/6 mice (Jackson Laboratories, Bar Harbor, ME), 8–10 wk of age when used as recipient or 6 wk of age when used for training studies, were allowed to acclimatize to their new surroundings for 1 wk before use in the experiments.
Exercise protocol.
At 6 wk of age, male or female C57Bl/6 mice were randomly assigned to either an endurance exercise-trained (EX) or sedentary (SED) group. EX mice followed a standard training protocol, with minor modifications, previously shown to induce adaptations in the bone marrow compartment (1, 16). Mice were trained 3 days/wk (Monday, Wednesday, Friday), 1 h/day, for 8 wk. The exercise protocol consisted of a 10-min warm-up at 12 m/min, followed by a 45-min training period that began at 14 m/min (week 1), progressed to a maximum speed of 22 m/min (week 8), and concluded with a 5-min cool-down period at 10 m/min. Speed during the training portion was adjusted to allow all mice to complete the full duration of exercise. EX mice were trained at approximately the same time each day to avoid any diurnal variation and were encouraged to run by mild electric shock. SED mice were place on the treadmill at the end of each training session to control for the stress of mouse handling and treadmill exposure without running.
LSK quantification in central vs. endosteal niche and cell cycle analysis.
Female mice were euthanized via cervical dislocation, and their femurs and tibiae were quickly removed and cleaned of surrounding muscle, fat, and connective tissue. Marrow from the vascular niche in the central marrow region was harvested, as previously described (1). Flushed bones were then used to harvest HSC from the endosteal region, as previously described (1), with minor modifications. Bones were digested mechanically with scissors and enzymatically with collagenase (0.25% collagenase/20% FBS/PBS) for 10 min at 37°C with shaking. The collagenase solution was diluted with buffer (2% FBS/1 mM EDTA/PBS), and cells were filtered twice through a 70-μm filter to remove bone fragments. Cell suspensions were immediately processed for quantification of the LSK population via flow cytometry, as previously described (1, 11). The following antibodies were used: biotinylated Lineage panel (BD Biosciences, Mississauga, Canada), anti-mouse Sca-1 (1:10; BD Biosciences), anti-mouse c-Kit (1:10; eBiosciences, San Diego, CA), and FITC anti-streptavidin (1:800; Biosource, Camarillo, CA). Cells were analyzed immediately using the Epics XL flow cytometer (Beckman Coulter, Mississauga, Canada) with unstained and single-stained samples used as controls for compensation and gating. Data were analyzed and expressed as the percentage of c-Kit and Sca-1-positive cells from the lineage-negative population.
A subset of cells from the vascular niche was used for cell cycle analysis. After counting, cells were washed, fixed in 70% ethanol, and stored at −20°C until analysis. For flow cytometric analysis of cell cycle status, fixed cells were centrifuged at 2,000 rpm for 5 min, washed with PBS, resuspended in 4,6-diamidino-2-phenylindole (Sigma, 1 μg/ml) and incubated on ice for 30 min. Single-cell suspensions were immediately analyzed via the Partec Cyflow Space flow cytometer (Partec, Swedesboro, NJ). Debris was excluded, and doublet discrimination was applied. Cell cycle analysis was based on samples with at least 2,500 cells contributing to the main cell cycle display.
Lineage panel analysis.
Previously, frozen EX and SED samples were divided into five separate aliquots for staining, with each individual antibody in the lineage panel (BD Biosciences) consisting of the following mature blood cell markers: Mac-1, TER-119, Gr-1, B220, and CD3ϵ. After incubation with biotinylated primary antibodies, cells were treated with FITC anti-streptavidin secondary antibody (1:800, Biosource), washed, and immediately analyzed by flow cytometry. Unstained and secondary-only samples were used to establish gates.
Serum cytokine analysis.
Blood was collected from the submandibular vein. Serum was allowed to separate, and samples were centrifuged at 4,500 g for 10 min. Serum was removed and stored at −80°C until analysis. Levels of the various cytokines were determined using the Bio-Plex Pro Assay (Bio-Rad, Mississauga, Canada), according to manufacturer's instructions. If a sample had undetectable levels of a cytokine, it was excluded from analysis.
Bone marrow transplants.
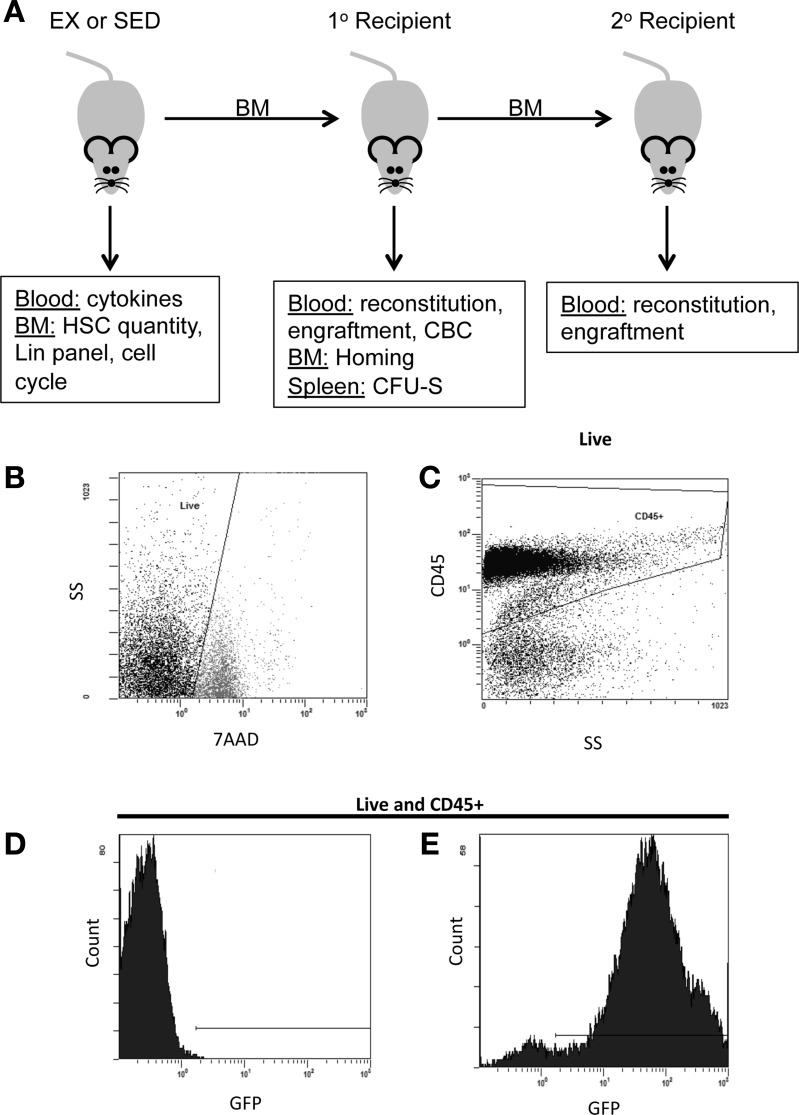
BMT was conducted as previously described (10). Briefly, 3 days after the final exercise session, male donor mice were killed by cervical dislocation. Female wild-type recipient mice, myeloablated with a fractionated dose of ∼9 Gy irradiation (137Cs; GammaCell 3000) were reconstituted with 1 × 106 whole marrow cells isolated from both femurs and tibias of male C57Bl/6-eGFP or male C57Bl/6 donors, immediately following the second irradiation dose via retroorbital injection. Marrow from a single donor mouse was used to reconstitute two to four recipients. Females were chosen as recipients in accordance with common practices for the BMT assay (10), and males were used as donors for the potential to evaluate engraftment via Y chromosome expression, if necessary. For secondary BMT, one primary recipient that originally received marrow from a SED mouse and two primary recipients that originally received marrow from EX mice were euthanized at least 7 mo following the initial BMT, and their marrow was used as the source of donor cells for transplantation into secondary recipients. The experimental design is depicted in Fig. 1A.
Fig. 1.
Experimental design and green fluorescent protein (GFP) detection in peripheral blood. A: experimental design outlining the tissues collected and their various uses. BM, bone marrow; HSC, hematopoietic stem cells; CBC, complete blood count; CFU-S, colony-forming unit spleen; Lin, lineage; EX, exercise trained; SED, sedentary; 1°, primary; 2°, secondary. B–E: representative gating strategy for analysis of donor-cell contribution to recipient hematopoiesis. B: live cells were selected based on negative staining for the viability dye 7-amino-actinomycin D (7AAD). C: next, CD45 cells were selected to exclude all nonhematopoietic cells. Gates to select for GFP-positive cells were based on blood collected from wild-type (WT; non-GFP) mice (D) and maintained constant for analysis of short- and long-term donor-derived engraftment in primary and secondary recipients (E). SS, side scatter.
Colony-forming unit spleen.
Seven days following BMT, the colony-forming unit spleen (CFU-S) assay was conducted as described (21, 32). Briefly, mice were euthanized via cervical dislocation; spleens were quickly excised and fixed in Bouin's solution for 24 h. Spleens were weighed, and splenic colonies visualized as raised lumps with yellowish tinge were counted by an investigator who was blinded to the conditions. Overlapping colonies were defined by a central nodular region of origin.
Treadmill test.
One month following the BMT, return to function was analyzed in recipient mice via a treadmill test to exhaustion. Mice were placed on a motorized treadmill (Exer6, Columbus Instruments, Columbus, OH), beginning at 11 m/min. Speed was increased 1 m/min every 2 min, and exhaustion was defined as when the mice did not respond to the shocker at the end of the treadmill for greater than 5 s continuously and were not responsive to manual encouragement. Exhaustion was evaluated by a researcher blinded to the group of each mouse.
Recipient reconstitution and donor-derived engraftment.
Blood was collected into heparinized tubes via facial bleed, and red blood cells were lysed with Tris-NH4Cl (17 mM Tris/0.75% NH4Cl/ddH2O) lysis buffer. Blood samples were then incubated with CD45 antibody (1:80, Invitrogen, Burlington, Canada) and 7-amino-actinomycin D (7AAD) (Beckman Coulter). Samples were analyzed on an Epics XL flow cytometer (Beckman Coulter). Single stained and unstained controls were used to establish gates and for compensation. The percentage of CD45+ from the 7AAD− population was identified as living leukocytes to evaluate total hematopoietic reconstitution, and the percentage of GFP+ (green fluorescent protein) cells from this population was identified as donor-derived living leukocytes to evaluate donor-derived engraftment (Fig. 1, B–E). Flow plots were analyzed with Expo32 analysis software (Beckman Coulter). Analysis was conducted in a blinded fashion with at least three to six different donor mice used to reconstitute multiple recipients at each time point for the short- and long-term repopulating assays. To allow for combination of data from separate days and experiments, peripheral blood from a nontransplanted wild-type (non-GFP) mouse and nontransplanted GFP mouse were harvested and prepared in parallel with experimental samples on each experimental day for analysis of the percentage of CD45 cells and construction of a GFP standard curve in unmanipulated mice. The GFP standard curve was constructed by mixing blood from unmanipulated wild-type and GFP mice in known percentages (0, 20, 40, 60, 80, and 100% by volume) and analyzing via flow cytometry to determine the predicted percentages on each experimental day. Recipient reconstitution was expressed relative to the average percentage of CD45 cells from one nontransplanted wild-type mouse and one nontransplanted GFP mouse, and engraftment was normalized to the GFP standard curve. Any visibly ill mice, or with skin wounds from excessive grooming, were excluded from analysis due to the potential for an inflammatory response to the wound and potential activation of hematopoiesis for reasons unrelated to the study.
Blood from recipients reconstituted with wild type (non-GFP) marrow was used for complete blood count analysis at 1 mo post-BMT. Approximately 300 μl of blood were collected, via facial bleed, into EDTA-coated microtainer tubes for analysis by the Core Facility at McMaster University Hospital.
Acute homing to bone marrow.
Bone marrow was collected as described above from both femurs and tibias of recipient mice 5 days following the BMT. An aliquot of 5 × 106 cells was used for homing analysis, and remaining unused marrow cells were frozen in 10% DMSO/20% FBS/PBS, followed by long-term storage at −80°C. Whole marrow was tagged with 7AAD and CD45, as described above, and analyzed via flow cytometry (Epics XL, Beckman Coulter). Flow cytometry gates and compensation were established based on unstained and single-stained controls. Dead cells staining positively for 7AAD were excluded, and analysis of CD45 cells expressing GFP, representing donor-derived blood cells, was conducted. Data is based on a number of recipients reconstituted with at least two donor mice. Data are normalized to standard curves developed from marrow harvested from nonmanipulated wild-type (non-GFP) and GFP mice harvested and processed in parallel with experimental samples on different analysis days. Analysis using the Expo32 analysis software (Beckman Coulter) was conducted in a blinded fashion.
Statistical analysis.
LSK, cell cycle, cytokine, lineage panel, CFU-S, and homing data were analyzed using an unpaired t-test in Excel. Since we were interested in the differences between groups and not the changes over time, early, late, and secondary reconstitution and engraftment were also analyzed with an unpaired t-test in Excel. Data are presented as means ± SE with P ≤ 0.05 considered significant. For experiments involving the BMT assay, statistical analysis was based on the number of recipients.
RESULTS
Exercise increases the percentage of HSC as well as bone marrow cell proliferation in the vascular niche.
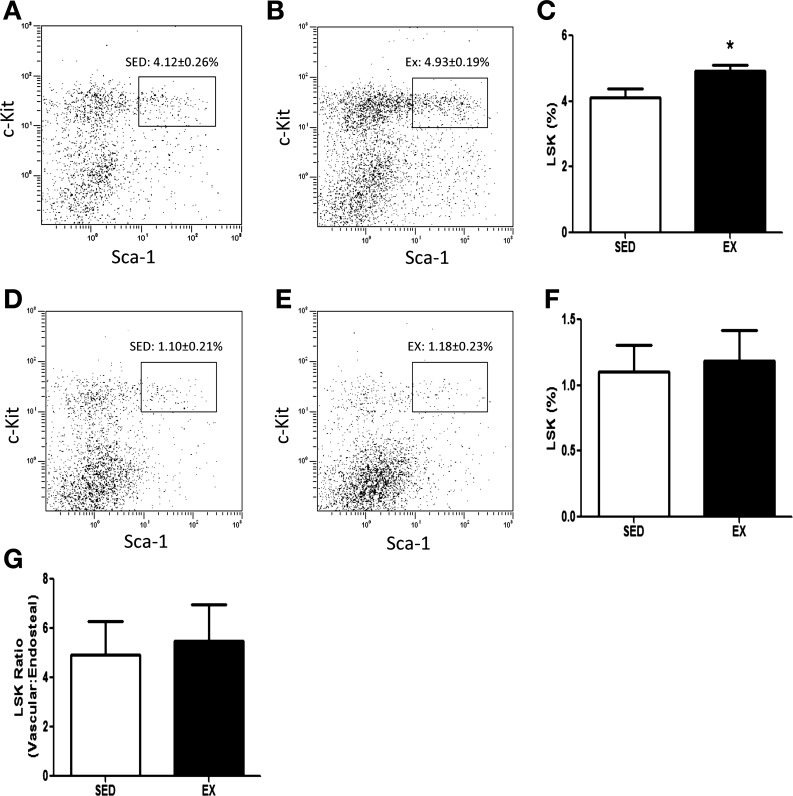
Exercise training significantly increased the percentage of HSC, defined as c-kit and Sca-1 positive within the lineage-negative population (7) (LSK), in the vascular niche by 20% (Fig. 2, A–C, P < 0.05). The percentage of HSC in the endosteal niche was not affected by exercise training (Fig. 2, D–F). The ratio of vascular vs. endosteal HSC was not changed with exercise (Fig. 2G), supporting the notion that exercise increased proliferation of HSC specifically within the vascular niche without loss of more quiescent cells in the endosteal niche.
Fig. 2.
HSC quantification. HSCs were isolated from the vascular and endosteal niche from SED and EX mice and quantified using the LSK (lineage−, Sca-1+, c-Kit+) markers via flow cytometry. Representative flow plots of the LSK population isolated from the vascular niche from SED (A) and EX (B) mice are shown. C: the percentage of Sca-1- and c-Kit-positive cells in the lineage-negative population (LSK cells) isolated from the vascular niche. Representative flow plots of the LSK population isolated from the endosteal niche from SED (D) and EX (E) mice are shown. F: the percentage of Sca-1- and c-Kit-positive cells in the lineage-negative population (LSK cells) isolated from the endosteal niche. G: the ratio of LSK cells in the vascular vs. the endosteal niche. Data are presented and analyzed as the percentage of Sca-1- and c-Kit-positive cells in the lineage-negative population. Graphs represent means ± SE (n = 6 mice/group). *P < 0.05 with t-test.
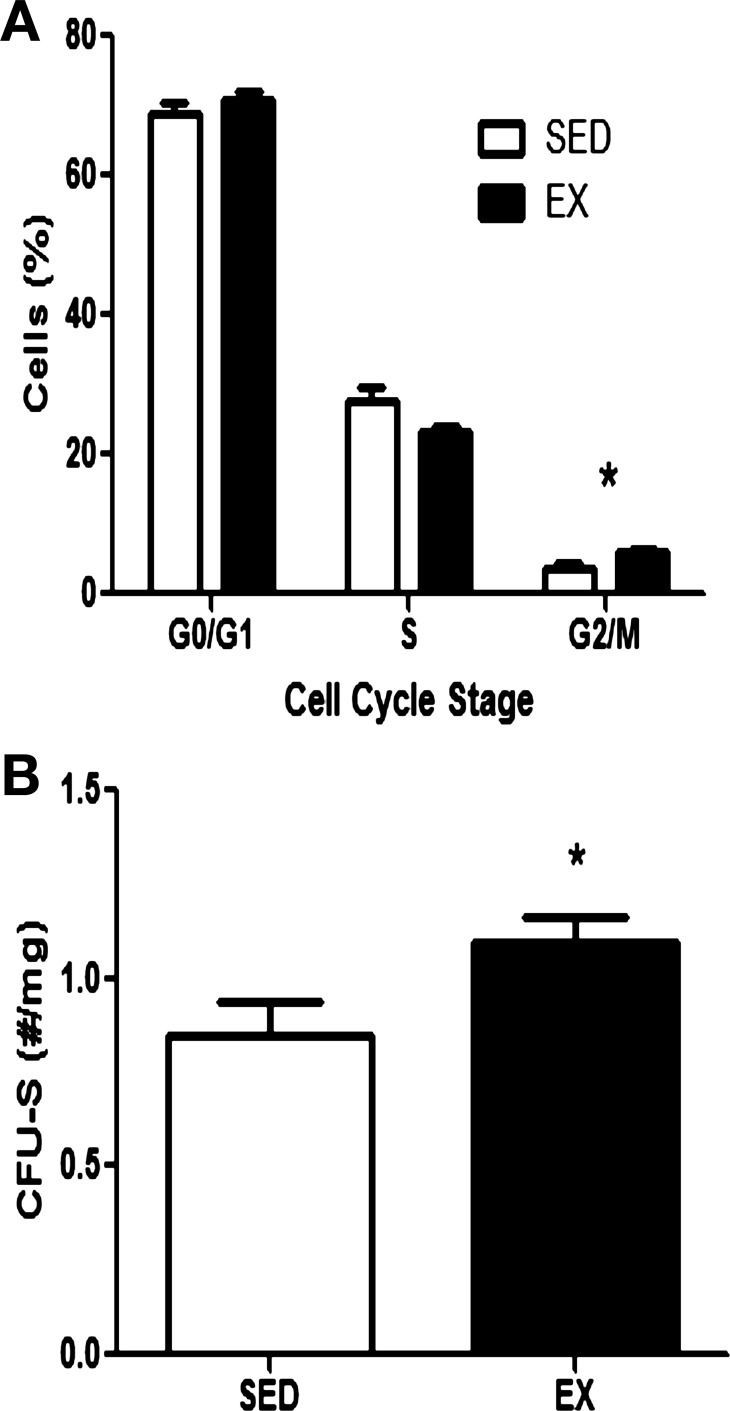
The percentage of bone marrow cells in the vascular niche in the G2/M phase of the cell cycle was significantly increased with exercise training as EX mice had 6.09 ± 0.44% cells in G2/M, while SED had 3.53 ± 1.03 cells in G2/M (Fig. 3A, P < 0.05). It has been shown that colony-forming cells in the spleens of irradiated mice 7 days posttransplant represent a proliferative progenitor population from the transplanted cell source (21); therefore, we quantified spleen colonies 7 days post-BMT. Mice transplanted with bone marrow from EX donors had significantly more spleen colonies than did mice transplanted with bone marrow from SED donors (Fig. 3B, 48%, P < 0.05).
Fig. 3.
Cell cycle analysis and CFU-S. A: analysis of cell cycle status of whole marrow cells isolated 3 days following the final exercise bout from the vascular niche from SED and EX mice. Values are means ± SE of the percentage of cells in each phase of the cell cycle (n = 6 mice/group). B: spleen colonies were quantified in recipients transplanted with BM from SED or EX mice 7 days following BMT. Values are means ± SE of spleen colonies normalized to total spleen weight of n = 7–8 recipients per group. *P < 0.05 with t-test.
No significant differences were observed between EX and SED mice in any of the blood cell lineages analyzed; however, strong trends were observed in the Mac-1 lineage (Table 1; P = 0.053) and Gr-1 lineage (Table 1; P = 0.061), both increasing by ∼15%. All cytokines related to HSC regulation were downregulated (Table 2), with a significant 38% decrease in IL-6 (P = 0.01) and a trend for a decrease observed in granulocyte-colony-stimulating factor (G-CSF) (16%, P = 0.08).
Table 1.
Lineage panel
| Lineage Marker | SED | EX | P Value |
|---|---|---|---|
| Mac-1 | 54.58 ± 3.39 | 63.12 ± 1.62 | 0.053 |
| TER-119 | 9.11 ± 1.66 | 7.37 ± 0.81 | 0.374 |
| Gr-1 | 53.28 ± 3.48 | 61.53 ± 1.49 | 0.061 |
| B220 | 44.17 ± 3.84 | 42.01 ± 1.77 | 0.623 |
| CD3ε | 3.54 ± 0.26 | 3.85 ± 0.21 | 0.388 |
Values are means ± SE, with units of percentage (n = 5 mice/group). Bone marrow isolated from the central marrow region from sedentary (SED) and exercise-trained (EX) mice was evaluated for the expression of various mature hematopoietic lineage panel markers.
Table 2.
Serum cytokine levels
| Cytokine | SED | EX | P Value |
|---|---|---|---|
| IL-3 | 18.67 ± 1.9 | 15.47 ± 1.7 | 0.231 |
| IL-6 | 24.86 ± 2.3 | 16.73 ± 2.2 | 0.02 |
| G-CSF | 165.6 ± 11.2 | 138.33 ± 9.8 | 0.08 |
| GM-CSF | 270.26 ± 20.5 | 223.44 ± 19.7 | 0.113 |
Values are mean ± SE, with units of pg/ml. Cytokine levels in SED (n = 12) and EX (n = 11–12) mice are shown. G-CSF, granulocyte-colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor.
Increased HSC in the vascular niche does not result in increased recipient reconstitution or engraftment.
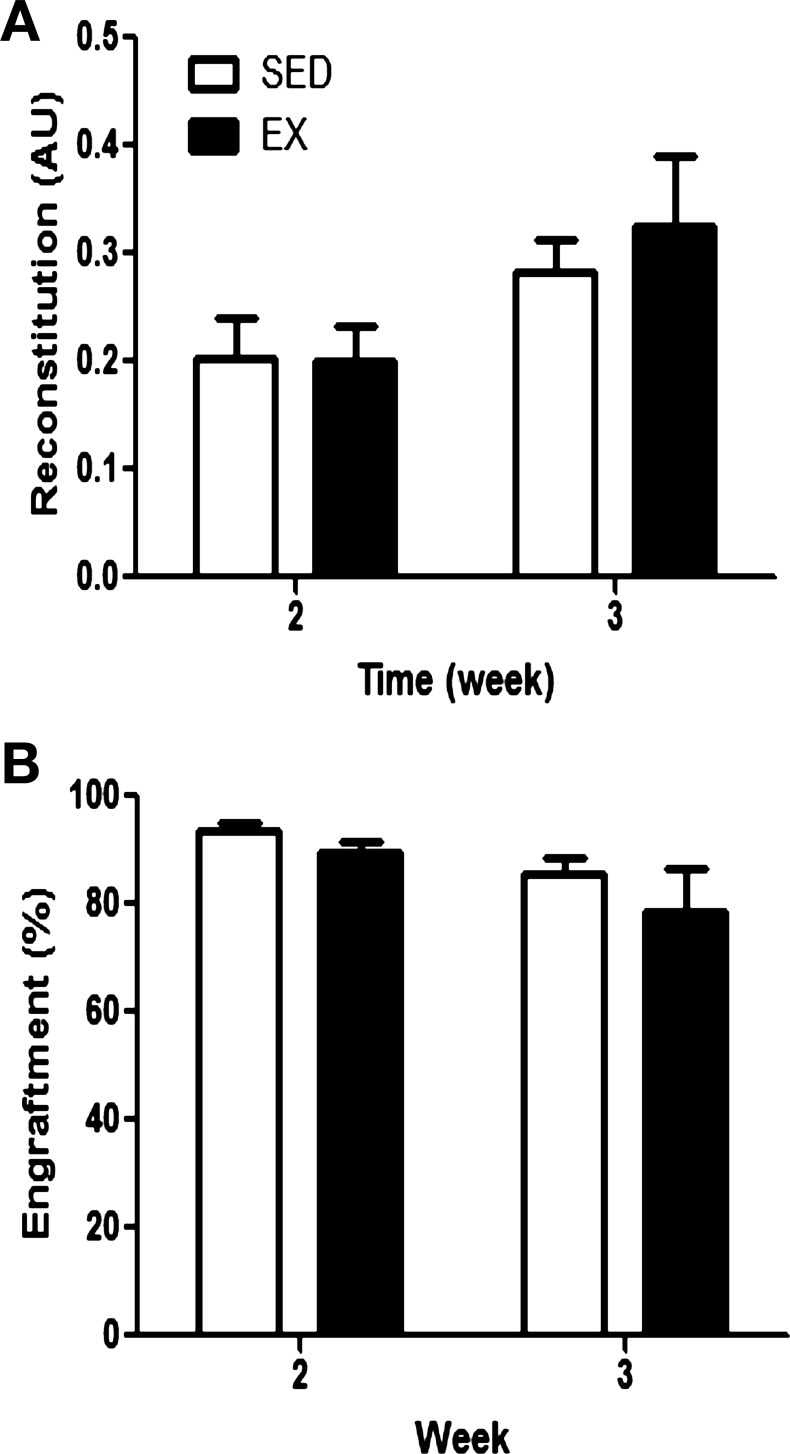
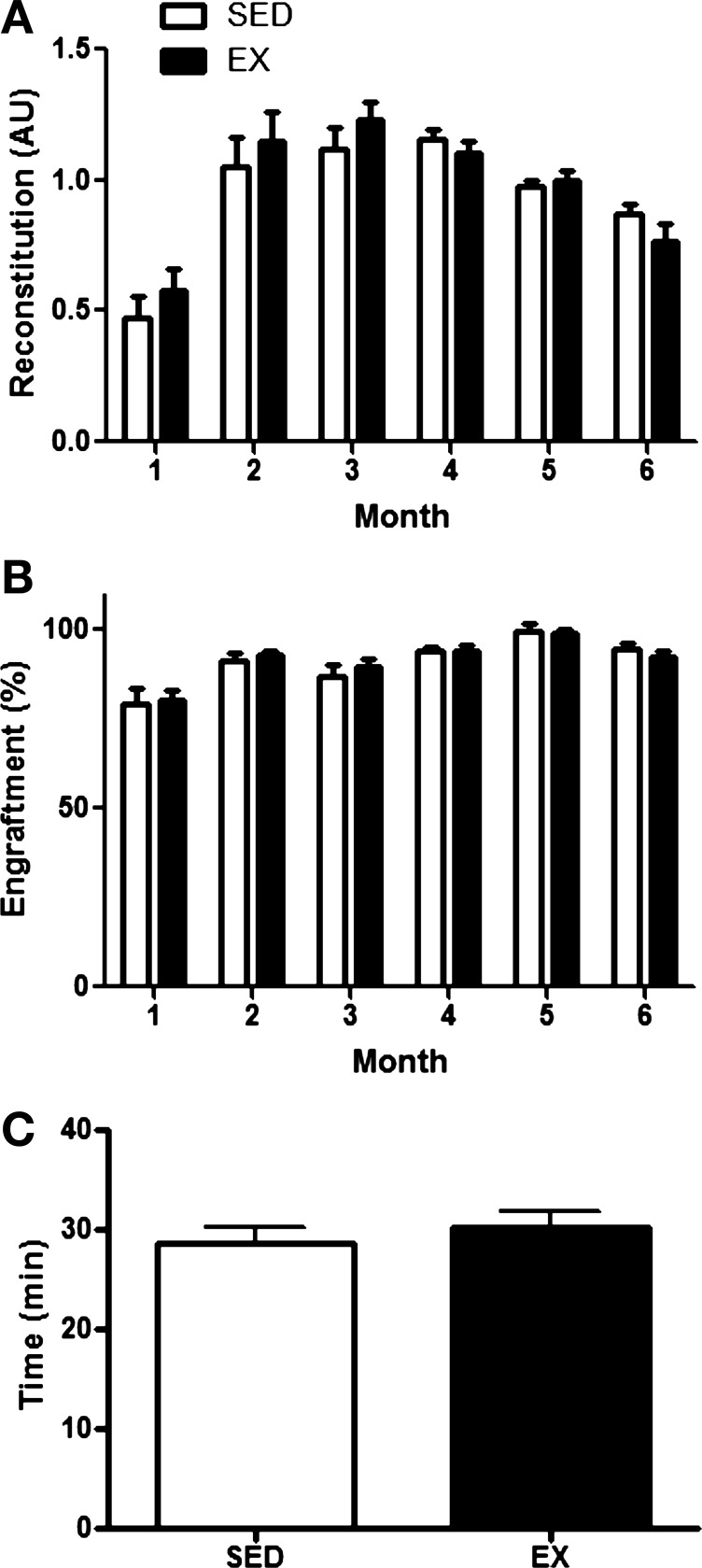
Within the first month post-BMT, no differences in leukocyte reconstitution (Fig. 4A) and donor-derived engraftment (Fig. 4B) were observed between recipient mice that received bone marrow from EX or SED donors. At later time points post-BMT, marrow from EX donors did not enhance total leukocyte reconstitution (Fig. 5A) or engraftment in recipients (Fig. 5B) at any time point analyzed up to 6 mo post-BMT. Furthermore, whole blood counts determined by complete blood cell count 1 mo post-BMT did not differ between recipients that received marrow from SED or EX mice (Table 3). Similarly, recipient recovery, as determined by functional test of treadmill endurance, did not differ (Fig. 5C). Overall health and survival were not different between recipients receiving bone marrow from EX or SED recipients, with nearly 100% survival in both groups.
Fig. 4.
Early recipient reconstitution post-BMT. Peripheral blood was harvested from recipient mice in the BMT assay 2 and 3 wk following transplantation. Analysis of peripheral blood reconstitution (donor and recipient derived; A) and donor-derived engraftment (B) in peripheral blood of recipients at each time point following BMT is shown. Reconstitution levels are presented relative to nontransplanted mice in arbitrary units (AU), while engraftment levels are normalized to a GFP standard curve, and values are means ± SE (n = 7–12 recipients/group/time point).
Fig. 5.
Long-term recipient reconstitution and functional recovery post-BMT. Peripheral blood was harvested from recipient mice in the BMT assay monthly between 1 and 6 mo following transplantation. Analysis of peripheral blood reconstitution (donor and recipient derived; A) and donor-derived engraftment (B) in peripheral blood of recipients at each time point following BMT is shown. Reconstitution levels are presented relative to nontransplanted mice, while engraftment levels are normalized to a GFP standard curve, and values are means ± SE (n = 9–17 recipients/group/time point). C: functional recovery post-BMT, evaluated by a treadmill test to exhaustion, was evaluated one mo post-BMT. Values are means ± SE of total time (minutes) to exhaustion (n = 18–19 recipients/group).
Table 3.
Complete blood counts
| Parameter | SED | EX | P Value |
|---|---|---|---|
| Leukocytes, ×109/l | 5.32 ± 0.79 | 4.73 ± 0.79 | 0.606 |
| Platelets, g/l | 622.80 ± 57.9 | 627.00 ± 66.43 | 0.963 |
| Erythrocytes, ×1012/l | 9.10 ± 0.13 | 8.92 ± 0.11 | 0.305 |
| Hemoglobin, g/l | 144.70 ± 1.98 | 141.90 ± 1.77 | 0.307 |
| Hematocrit, AU | 0.43 ± 0.01 | 0.43 ± 0.01 | 0.654 |
| MCV, fl | 47.14 ± 0.14 | 47.77 ± 0.26 | 0.054 |
| MCH, pg | 15.91 ± 0.12 | 15.92 ± 0.04 | 0.938 |
| MCHC, g/l | 336.90 ± 2.56 | 333.60 ± 1.11 | 0.252 |
Values are means ± SE based on n = 9–10 SED and Ex mice per measure. Peripheral blood was harvested from primary recipients that received marrow from SED or EX mice 1 mo following BMT, and complete blood count analysis was performed. MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration.
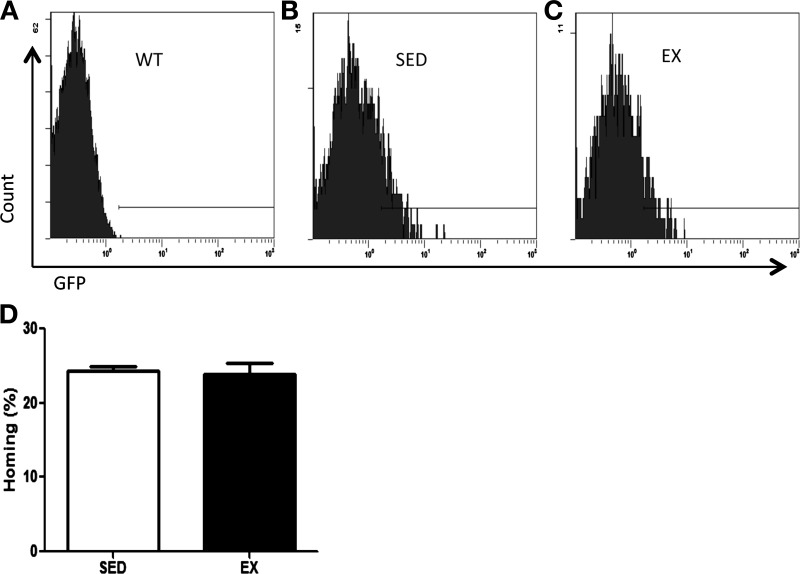
Exercise training does not impair donor cell homing or affect self-renewal capacity relative to marrow from SED donors.
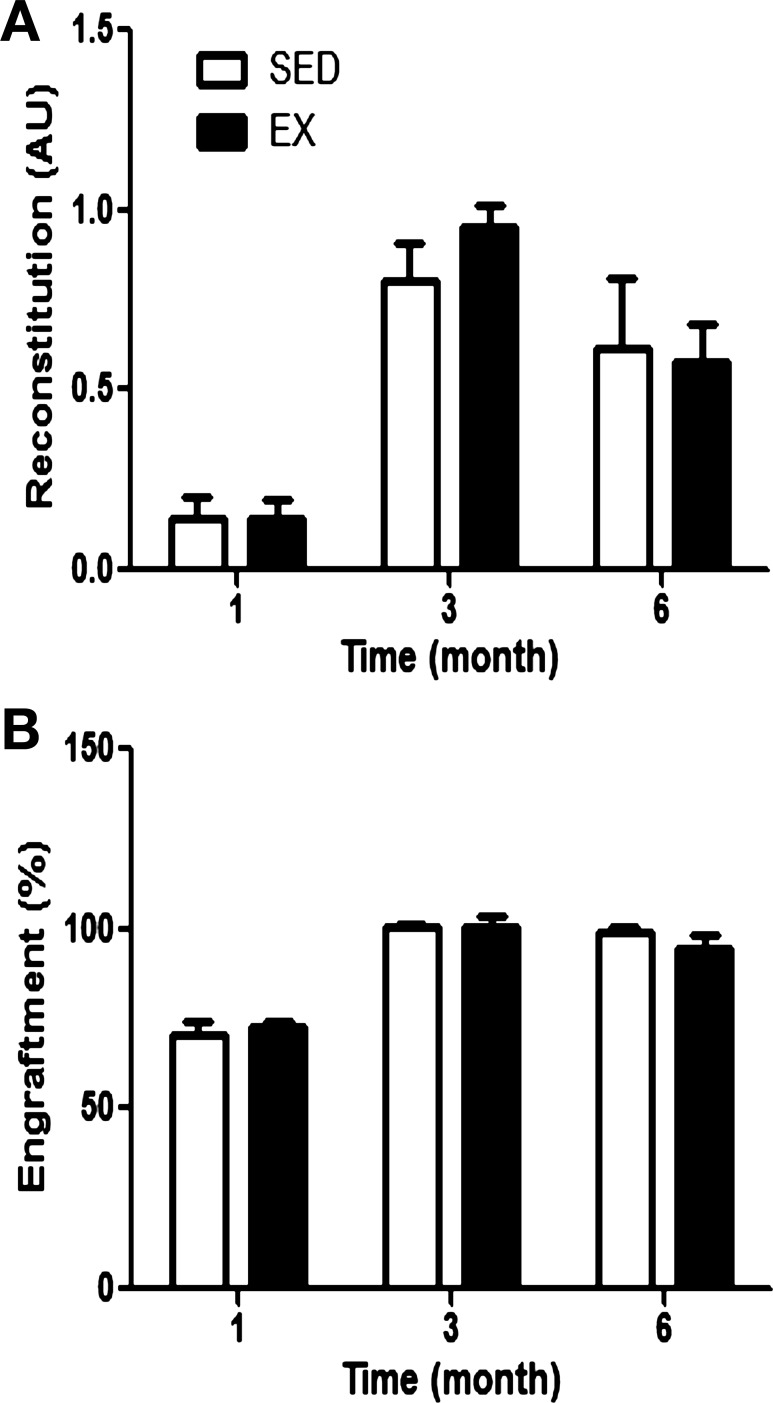
Exercise training had no effect on the donor cell homing (Fig. 6) 5 days following BMT. In the secondary reconstitution assay, a measure of HSC self-renewal, recipient reconstitution (Fig. 7A), and donor-derived engraftment (Fig. 7B) were not different between secondary recipients that received bone marrow from primary recipients previously transplanted with bone marrow from SED or EX mice.
Fig. 6.
Donor cell homing. Recipient marrow was harvested 5 days following BMT for analysis of donor cell homing. Representative flow plots are shown of non-GFP marrow (WT; A) and marrow from recipients transplanted with marrow from SED (B) or EX (C) mice. D: homing of donor hematopoietic cells to recipient BM is normalized to a GFP standard curve and presented as means ± (n = 4–6 recipients/group).
Fig. 7.
Secondary recipient reconstitution. Marrow from primary recipients reconstituted with whole marrow cells from SED (n = 3) or EX (n = 6–8) was harvested at least 7 mo following the initial transplant and was used as the donor cell population for transplantation into secondary recipients. Analysis of peripheral blood reconstitution (donor and recipient derived; A) and donor-derived engraftment (B) in peripheral blood of secondary recipients at each time point following BMT is shown. Reconstitution levels are presented relative to nontransplanted mice, while engraftment levels are normalized to a GFP standard curve, and values are means ± SE.
DISCUSSION
Previous studies have focused on the quantification of HSC in peripheral blood with training or acute exercise (5, 20, 28, 31, 34, 36), or evaluation of HSC quantity and proliferation using in vitro assays (1); however, the effects of exercise training on HSC in bone marrow in their natural environment, in vivo, has never been examined. The present investigation evaluated both the quantity of HSC in two distinct niches within the bone marrow (i.e., endosteal and vascular), as well as HSC function using the BMT model (24, 26). The primary findings from this study were that exercise training increases the quantity of HSCs in the vascular niche, without altering the functional properties of HSC, such as homing or long-term engraftment.
HSC quantity.
It has been established that HSC residing in the endosteal niche are a quiescent reserve population of HSC, while those in the vascular niche are a more activated population primed for release into peripheral blood (19, 35). In the present study, we observed a significant increase in HSC quantity only in the vascular niche, which was supported by our cell cycle, CFU-S, and lineage marker analysis. Exercise training increased the percentage of whole bone marrow cells in G2/M phase of the cell cycle in the vascular niche. Although these data collected in whole bone marrow cells may not be directly applicable to HSC in the vascular niche, they do indicate that exercise training is a proliferative stimulus for bone marrow cells. At 7 days post-BMT, recipient spleens are transiently colonized with donor-derived mature progenitor cells that are replaced later by more immature progenitor cells (21). The increase in spleen colonies at 7 days post-BMT in mice transplanted with bone marrow from EX donors provides further support for their increased HSC quantity in the vascular niche with exercise training. Additionally, we observed a strong trend for an increase myeloid lineage positive markers, Mac-1 (15%, P = 0.053) and Gr-1 (15%, P = 0.061) in EX mice. Since the vascular niche is associated with myeloid differentiation of HSC (15), the trend for an increase in cells positive for myeloid markers provides further support for an increase in HSC quantity in the vascular niche. Taken together, the CFU-S, cell cycle, and lineage marker data support the conclusions from the LSK analysis that exercise training increases the quantity of HSC in the vascular niche of the bone marrow. It is believed that HSC mature and migrate from the endosteal niche to the vascular niche to prepare for release into circulation in response to hematological stress (19, 35). Since acute exercise has repeatedly been shown to mobilize more mature HSC into peripheral blood (20, 31, 34, 36), we speculate that, with training, HSC are increased in the vascular niche in preparation for release in response to exercise.
Studies quantifying HSC with training are few and have mostly focused on HSC circulating in human peripheral blood. Most studies demonstrate that basal levels of circulating HSC are not altered with training (31, 34); however, Bonsignore and colleagues (5) did detect a significant increase in a specific population of more differentiated peripheral blood HSC in trained individuals. In a recent study from our laboratory (1), exercise training increased the number of hematopoietic progenitors of various lineages and of different stages of differentiation identified by a variety of colony-forming cell assays in vitro. In our previous investigation (1), we also quantified the HSC population, identified by the LSK panel of markers, but failed to see a significant increase with exercise training. Importantly, the magnitude of increase, 20%, was identical between our previous (1) and the present investigation, suggesting that increased statistical variability in our previous study (1), perhaps due to variability in the animal's response to training, can account for the differences in statistical outcomes. Taken together, these data suggest that exercise training increases HSC quantity in the marrow cavity and may increase certain more mature populations of HSC in peripheral blood. We considered the possibility that the increased HSC quantity in the vascular niche was due to a redistribution of HSC from the endosteal to the vascular niche. The quantity of HSC in the endosteal niche was unchanged, and the ratio of vascular to endosteal HSC was not affected by exercise training, suggesting that redistribution was not occurring. In further support of this notion, we did not detect any defects in long-term engraftment potential of marrow from EX donors, which would have been expected if HSC quantity in the endosteal niche was reduced. Therefore, we speculate that exercise training expands a specific subpopulation of HSC that is activated and primed for release into circulation, but does not influence more primitive cells responsible for long-term and short-term repopulation. This hypothesis is supported by data from previous studies, where exercise increased the quantity of circulating hematopoietic stem and progenitor cells, but not those identified by their cell surface phenotype to be the most primitive population (5, 34). At present, we can only speculate as to which specific population this may represent; however, human data have suggested that acute exercise can increase the quantity of angiogenic progenitors, derived from HSC, in peripheral blood (4), and this population may be contributing to the increase observed with exercise.
It has been widely speculated that exercise likely induces its effects on HSC through the modulation of circulating levels of growth factors and cytokines. HSC in the vascular niche are highly sensitive and responsive. In the present study, IL-6, involved in HSC self-renewal (12), and G-CSF, involved in HSC mobilization (29), were downregulated with exercise training, although the decrease in G-CSF levels did not reach statistical significance (16%, P = 0.08). Few previous studies have compared levels of IL-6 and G-CSF in trained and untrained individuals. One previous study in humans suggested that circulating levels of IL-6 and G-CSF are not affected by training status (5). Additionally, a study in rats demonstrated a nonsignificant 31% decrease in IL-6 with exercise training (27), which was similar to the decrease observed in the present study. Previous studies have demonstrated acute increases in IL-6 and G-CSF following a single exercise bout (5, 30, 34, 36); however, these were either not correlated to circulating HSC quantity (5), or correlations were not conducted (30, 34, 36). We speculate that the observed downregulation of these cytokines in the present study may represent a compensatory response to maintain homeostasis in response to the acute increases in these factors with exercise. We further speculate that the short-term pulsed increases in IL-6 and G-CSF with each exercise bout stimulate HSC proliferation, especially in the cells of the vascular niche, which are highly responsive to systemic factors due to their close proximity to the vasculature (24). This may explain the increase in HSC quantity with exercise training only in the vascular niche. The basal decrease in IL-6 levels with exercise prevents a hyper-proliferative stimulus to HSC, thereby maintaining their long-term reconstituting potential, as evidenced by the similar engraftment levels in secondary recipients of both EX and SED donors. In support of this hypothesis, HSC grown on stromal feeder layers in culture were maximally expanded when media changes occurred every other day (14). This alternating day media change schedule would be similar to conditions in the present study, where pulsed increases in hematopoietic growth factors may have occurred in mice exercised every other day. While interesting, further research will be required to test this theory.
HSC function.
HSC expansion via ex vivo manipulation has long been attempted to overcome the small quantity of HSC available in most tissue samples and increase their clinical applicability. HSC expansion in vitro has been limited by defects in HSC function, such as impaired homing (17) and differentiation without self-renewal (33) on transplantation. Unlike in vitro models, exercise training would maintain HSC in their natural microenvironment under physiological conditions. Given the importance of the niche in regulating HSC function (35), this approach may prevent functional defects often seen with ex vivo expanded HSC. Furthermore, the comprehensive time course of our analysis allowed us to evaluate the contribution of different subpopulations of HSC to recipient reconstitution. Short-term repopulating HSC (ST-HSC) are responsible for reconstitution of recipients in the early weeks and up to 3–4 mo post-BMT, after which long-term repopulating HSC (LT-HSC) with a higher capacity for self-renewal become more relevant in governing reconstitution (2, 7). Overall, recipient reconstitution was not improved by exercise training, with no differences at any time point, indicating no effect of exercise training on the function of ST- or LT-HSC. Unlike HSC expanded in vitro (12, 17, 33), pretreatment of donors with exercise did not impair donor cell hematopoietic reconstitution compared with SED donors, suggesting a maintenance of HSC function with the observed HSC expansion.
We considered a number of possibilities for the lack of improvement in hematopoietic regeneration when marrow from EX donors was used. We speculated that defects in HSC homing to the bone marrow niche, as has been reported upon transplantation of culture-expanded HSC (17), might also be occurring with marrow from EX donors. In the present study, however, HSC homing to the bone marrow upon transplantation was preserved with exercise training relative to SED mice. We next considered that HSC expansion with exercise training may result in a decrease in LT-HSC or impaired self-renewal, as has been demonstrated with in vitro expanded HSC (12, 33). Results from our secondary transplantation assay where HSC were forced to undergo multiple rounds of proliferative stress to reconstitute multiple hosts indicate that a decrease in LT-HSC quantity was not occurring with exercise training, and self-renewal capacity was maintained relative to SED mice. These conclusions are further supported by our HSC quantification analysis, where we did not observe any differences between EX and SED mice in HSC quantity in the endosteal niche, which is home to LT-HSC (13). These data lend further support to our suggestion that exercise training affects a population of more differentiated HSC not necessary for long-term engraftment and has no positive or negative effects on HSC function.
Data presented herein represent the first characterization of the effects of exercise training on HSC in their natural in vivo environment. Here we show that exercise training increases HSC quantity in the vascular niche while maintaining the population of HSC in the endosteal niche. We speculate that the mechanisms responsible for the observed increase may have been pulsed increases in hematopoietic growth factors associated with each exercise bout with maintained HSC function by basal downregulation of these factors. Increased HSC quantity was not associated with improved or impaired functional characteristics, such as regeneration of the hematopoietic system in myeloablated recipients, homing to the bone marrow cavity, or self-renewal, suggesting that the effects of exercise training may be specific to a subpopulation of HSC not directly responsible for recipient reconstitution. Whether these adaptations to exercise training induced in HSC are clinically relevant specifically to BMT will need to be determined by future studies. Furthermore, previous work has demonstrated that intensity and duration can affect the HSC response to exercise (4), and future studies should examine these parameters in the context of exercise, HSC, and BMT.
GRANTS
This research was supported by a Canadian Institutes of Health Research (CIHR) operating grant held by G. Parise and a CIHR Canadian Graduate Scholarship held by M. De Lisio.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.D.L. and G.P. conception and design of research; M.D.L. performed experiments; M.D.L. analyzed data; M.D.L. and G.P. interpreted results of experiments; M.D.L. prepared figures; M.D.L. drafted manuscript; M.D.L. and G.P. approved final version of manuscript; G.P. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Dr. B. Trigatti for generously providing the original breeding pair of GFP mice and technical assistance with the BMT assay, Dr. D. R. Boreham, Nicole McFarlane, and Lisa Laframboise for technical assistance with flow cytometry, and Dr. M. Larche and Lesley Wiltshire for technical assistance with the Luminex assay.
REFERENCES
- 1. Baker JM, De Lisio M, Parise G. Endurance exercise training promotes medullary hematopoiesis. FASEB J 25: 4348–4357, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Benveniste P. Intermediate-term hematopoietic stem cells with extended but time-limited reconstitution potential. Cell Stem Cell 6: 48–58, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Blank U, Karlsson G, Karlsson S. Signaling pathways governing stem-cell fate. Blood 111: 492–503, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Bonsignore MR, Morici G, Riccioni R, Huertas A, Petrucci E, Veca M, Mariani G, Bonanno A, Chimenti L, Gioia M, Palange P, Testa U. Hemopoietic and angiogenetic progenitors in healthy athletes: different responses to endurance and maximal exercise. J Appl Physiol 109: 60–67, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Bonsignore MR, Morici G, Santoro A, Pagano M, Cascio L, Bonanno A, Abate P, Mirabella F, Profita M, Insalaco G, Gioia M, Vignola AM, Majolino I, Testa U, Hogg JC. Circulating hematopoietic progenitor cells in runners. J Appl Physiol 93: 1691–1697, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Büttner P, Mosig S, Lechtermann A, Funke H, Mooren FC. Exercise affects the gene expression profiles of human white blood cells. J Appl Physiol 102: 26–36, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Challen GA, Boles N, Lin KYK, Goodell MA. Mouse hematopoietic stem cell identification and analysis. Cytometry A 75: 14–24, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Connolly PH, Caiozzo VJ, Zaldivar F, Nemet D, Larson J, Hung S, Heck JD, Hatfield GW, Cooper DM. Effects of exercise on gene expression in human peripheral blood mononuclear cells. J Appl Physiol 97: 1461–1469, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Convertino VA. Blood volume: its adaptation to endurance training. Med Sci Sports Exerc 23: 1338–1348, 1991 [PubMed] [Google Scholar]
- 10. Covey SD, Krieger M, Wang W, Penman M, Trigatti BL. Scavenger receptor class B type I-mediated protection against atherosclerosis in LDL receptor-negative mice involves its expression in bone marrow-derived cells. Arterioscler Thromb Vasc Biol 23: 1589–1594, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Ema H, Morita Y, Yamazaki S, Matsubara A, Seita J, Tadokoro Y, Kondo H, Takano H, Nakauchi H. Adult mouse hematopoietic stem cells: purification and single-cell assays. Nat Protoc 1: 2979–2987, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Gammaitoni L, Bruno S, Sanavio F, Gunetti M, Kollet O, Cavalloni G, Falda M, Fagioli F, Lapidot T, Aglietta M, Piacibello W. Ex vivo expansion of human adult stem cells capable of primary and secondary hemopoietic reconstitution. Exp Hematol 31: 261–270, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Haylock DN, Williams B, Johnston HM, Liu MCP, Rutherford KE, Whitty GA, Simmons PJ, Bertoncello I, Nilsson SK. Hemopoietic stem cells with higher hemopoietic potential reside at the bone marrow endosteum. Stem Cells 25: 1062–1069, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Koller MR, Palsson MA, Manchel I, Palsson BO. Long-term culture-initiating cell expansion is dependent on frequent medium exchange combined with stromal and other accessory cell effects. Blood 86: 1784–1793, 1995 [PubMed] [Google Scholar]
- 15. Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology 20: 349–356, 2005 [DOI] [PubMed] [Google Scholar]
- 16. De Lisio M, Phan N, Boreham DR, Parise G. Exercise-induced protection of bone marrow cells following exposure to radiation. Appl Physiol Nutr Metab 36: 80–87, 2011 [DOI] [PubMed] [Google Scholar]
- 17. Liu B, Buckley SM, Lewis ID, Goldman AI, Wagner JE, van der Loo JC. Homing defect of cultured human hematopoietic cells in the NOD/SCID mouse is mediated by Fas/CD95. Exp Hematol 31: 824–832, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Metcalf D. Concise review: hematopoietic stem cells and tissue stem cells: current concepts and unanswered questions. Stem Cells 25: 2390–2395, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Miller A, Van Zant G. Advances in hematopoietic stem cell research through mouse genetics. Curr Opin Hematol 13: 209–215, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Morici G, Zangla D, Santoro A, Pelosi E, Petrucci E, Gioia M, Bonanno A, Profita M, Bellia V, Testa U, Bonsignore MR. Supramaximal exercise mobilizes hematopoietic progenitors and reticulocytes in athletes. Am J Physiol Regul Integr Comp Physiol 289: R1496–R1503, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Necas E, Znojil V. A comparison of stem cell assays using early or late spleen colonies. Cell Tissue Kinet 22: 111–121, 1989 [DOI] [PubMed] [Google Scholar]
- 22. Okabe M. “Green mice” as a source of ubiquitous green cells. FEBS Lett 407: 313–319, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Okada S, Nakauchi H, Nagayoshi K, Nishikawa S, Miura Y, Suda T. In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells. Blood 80: 3044–3050, 1992 [PubMed] [Google Scholar]
- 24. van Os R, Kamminga LM, de Haan G. Stem cell assays: something old, something new, something borrowed. Stem Cells 22: 1181–1190, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Pedersen BK, Bruunsgaard H, Jensen M, Toft AD, Hansen H, Ostrowski K. Exercise and the immune system–influence of nutrition and ageing. J Sci Med Sport 2: 234–252, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Purton LE, Scadden DT. Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell 1: 263–270, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Rowsey PJ, Metzger BL, Carlson J, Gordon CJ. Long-term exercise training selectively alters serum cytokines involved in fever. Biol Res Nurs 10: 374–380, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Sandri M, Adams V, Gielen S, Linke A, Lenk K, Kränkel N, Lenz D, Erbs S, Scheinert D, Mohr FW, Schuler G, Hambrecht R. Effects of exercise and ischemia on mobilization and functional activation of blood-derived progenitor cells in patients with ischemic syndromes. Circulation 111: 3391–3399, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Schroeder MA, DiPersio JF. Mobilization of hematopoietic stem and leukemia cells. J Leukoc Biol 91: 47–57, 2012 [DOI] [PubMed] [Google Scholar]
- 30. Suzuki K, Yamada M, Kurakake S, Okamura N, Yamaya K, Liu Q, Kudoh S, Kowatari K, Nakaji S, Sugawara K. Circulating cytokines and hormones with immunosuppressive but neutrophil-priming potentials rise after endurance exercise in humans. Eur J Appl Physiol 81: 281–287, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Thijssen DHJ, Vos JB, Verseyden C, Van Zonneveld AJ, Smits P, Sweep FCGJ, Hopman MTE, De Boer HC. Haematopoietic stem cells and endothelial progenitor cells in healthy men: effect of aging and training. Aging Cell 5: 495–503, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res 14: 213–222, 1961 [PubMed] [Google Scholar]
- 33. Tisdale FJ, Hanazono Y, Sellers ES, Agricola AB, Metzger EM, Donahue ER, Dunbar EC. Ex vivo expansion of genetically marked rhesus peripheral blood progenitor cells results in diminished long-term repopulating ability. Blood 92: 1131–1141, 1998 [PubMed] [Google Scholar]
- 34. Wardyn GG, Rennard SI, Brusnahan SK, McGuire TR, Carlson ML, Smith LM, McGranaghan S, Sharp JG. Effects of exercise on hematological parameters, circulating side population cells, and cytokines. Exp Hematol 36: 216–223, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol 6: 93–106, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Zaldivar F, Eliakim A, Radom-Aizik S, Leu SY, Cooper DM. The effect of brief exercise on circulating CD34+ stem cells in early and late pubertal boys. Pediatr Res 61: 491–495, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Zieker D, Fehrenbach E, Dietzsch J, Fliegner J, Waidmann M, Nieselt K, Gebicke-Haerter P, Spanagel R, Simon P, Niess AM, Northoff H. cDNA microarray analysis reveals novel candidate genes expressed in human peripheral blood following exhaustive exercise. Physiol Genomics 23: 287–294, 2005 [DOI] [PubMed] [Google Scholar]