Abstract
Device-based therapy for resistant hypertension by electrical activation of the carotid baroreflex is currently undergoing active clinical investigation, and initial findings from clinical trials have been published. The purpose of this mini-review is to summarize the experimental studies that have provided a conceptual understanding of the mechanisms that account for the long-term lowering of arterial pressure with baroreflex activation. The well established mechanisms mediating the role of the baroreflex in short-term regulation of arterial pressure by rapid changes in peripheral resistance and cardiac function are often extended to long-term pressure control, and the more sluggish actions of the baroreflex on renal excretory function are often not taken into consideration. However, because clinical, experimental, and theoretical evidence indicates that the kidneys play a dominant role in long-term control of arterial pressure, this review focuses on the mechanisms that link baroreflex-mediated reductions in central sympathetic outflow with increases in renal excretory function that lead to sustained reductions in arterial pressure.
Keywords: baroreflex, blood pressure, norepinephrine, sympathetic nervous system, renin-angiotensin system, renal function
there has been a long-standing interest in the mechanisms that mediate the acute and the long-term effects of the sympathetic nervous system on arterial pressure and the mechanisms that chronically alter sympathetic activity. In many instances, these mechanisms have been difficult to ascertain, particularly in studies dealing with long-term blood pressure regulation and in studies conducted in the absence of anesthesia, which alters neural control of the circulation. Among the mechanisms involved in the neural control of blood pressure, perhaps the greatest attention has been given to the baroreflex, because of its powerful and well established role in the acute regulation of cardiovascular dynamics. However, because the baroreflex apparently resets to the ambient pressure, its role in long-term control of arterial pressure has been questioned (5, 16, 25). The argument put forth is that if baroreflex resetting is complete, then it could not possibly have long-term functional effects on sympathetic activity and consequently arterial pressure. Because of the paucity and inadequacy of available techniques to assess the chronic effects of the baroreflex on sympathetic activity and arterial pressure, the time course and degree of resetting has not been established with certainty and, therefore, it is the authors' contention that the role of the baroreflex in long-term control of arterial pressure is unclear. However, the goal of this mini-review is not to approach this controversy because the salient issues have been discussed more fully in recent articles (16, 25). Rather, the objective of this review is to highlight recent findings from studies using a unique approach to chronically suppress central sympathetic outflow by electrical activation of the carotid baroreflex. By avoiding the confounding effects of drug administration, these studies have provided novel insight into the neurohormonal mechanisms that are important in long-term neural control of arterial pressure.
As indicated below, experimental studies have clearly demonstrated that chronic electrical stimulation of the carotid baroreflex has impressive sustained effects to lower arterial pressure, but the specific mechanisms that account for this response have not been completely defined. Because there is extensive evidence that the kidneys dominate in long-term control of arterial pressure by altering body fluid volume through pressure natriuresis, the authors favor the viewpoint that long-term reductions in arterial pressure during baroreflex activation can only be achieved by mechanisms that increase renal excretory function. On the other hand, some investigators have challenged the concept of the renal body fluid feedback mechanism for long-term control of arterial pressure (1, 26, 30). If these latter viewpoints were correct, this would discard the primacy of pressure natriuresis in long-term control of arterial pressure and suggest that chronic neurally mediated changes in arterial pressure are not necessarily mediated by the renal body-fluid feedback mechanism but rather by chronic maintenance of acute changes in peripheral resistance and cardiac function.
It is appropriate to indicate that the impetus for development of modern technology for chronic electrical activation of the carotid baroreflex activation was to provide a device-based approach for the treatment of selective cardiovascular diseases, including resistant hypertension, which cannot be treated adequately by available pharmacological intervention. This technology was developed by CVRx. The American Heart Association definition of resistant hypertension is “blood pressure that remains above goal despite the concurrent use of 3 antihypertensive agents of different drug classes.” Ideally, one of the agents should be a diuretic and all agents should be given at an optimized dose (3). Current clinical trials using baroreflex activation for hypertension therapy in this drug-resistant population are promising (2, 20, 27), but these studies will not be discussed in this mini-review. However, the mechanistic insight from the experimental studies summarized below may help to identify subsets of this heterogeneous population who stand to benefit the most from baroreflex activation therapy and also to suggest new therapeutic targets beyond reduction of blood pressure.
EXPERIMENTAL MODEL
All of the experimental studies discussed below have been conducted in dogs chronically instrumented for continuous 24-h monitoring of arterial pressure and heart rate. Stimulating electrodes are implanted in the perivascular space around each carotid sinus and the lead bodies are connected to a pulse generator (20, 23). With the Rheos system developed by CVRx, the internally implantable pulse generator is completely programmable externally by radiofrequency control. This allows for controlled current delivery throughout the day. In our experimental studies summarized below, the pulse generator has been programmed to deliver continuous impulses throughout the cardiac cycle, and the degree of blood pressure reduction has been controlled during the initial days of carotid sinus stimulation by adjusting the intensity of activation (1–7 V). Frequency of activation has ranged from 30 to 100 Hz, but variations within these extremes have not appeared to have a major impact on the magnitude of pressure reduction. Pulse duration has been 0.5 ms. It should be noted that because electrical stimulation of the carotid sinus does bypass mechanotransduction at the level of the baroreceptor itself, extrapolation of these findings to the role of the baroreflex in long-term control of arterial pressure should be made with caution, because most of the resetting of the baroreflex occurs at the level of the baroreceptor itself.
CENTRAL NERVOUS SYSTEM RESPONSES TO STIMULATION OF THE CAROTID SINUS
Acute findings in anesthetized animals and chronic observations in animals subjected to baroreceptor deafferentation (sinoaortic denervation) suggested the concept of central nervous system resetting to the prevailing baroreceptor input or the notion that central mechanisms develop to offset baroreceptor-induced changes in sympathetic activity and arterial pressure (4, 5, 16). Therefore, it was important to determine whether the initial suppression of sympathetic activity and attendant lowering of blood during baroreflex activation is sustained over long time periods. In studies conducted during 3 wk of carotid sinus stimulation, the intensity of activation was programmed to produce an initial reduction in arterial pressure of ∼20 mmHg during the first day of activation (21). In response to this degree of baroreflex activation, there was an ∼45% decrease in whole body NE spillover to plasma (an index of central sympathetic outflow) and a reduction in heart rate of ∼ 15 beats/min. Most significantly, these responses were sustained over the 3-wk period of carotid sinus stimulation (Fig. 1). Thus central compensations do not diminish the chronic suppression of sympathetic activity and attendant lowering of arterial pressure, or the bradycardia, in response to continuous stimulation of baroreceptor afferents.
Fig. 1.
Changes in mean arterial pressure and heart rate during chronic baroreflex activation (n = 6). Values are means ± SE. [Borrowed with permission from (21).].
In addition to reducing arterial pressure, baroreflex activation decreased spontaneous blood pressure variability. The potential significance of this response has recently been discussed by Schillaci et al. (28). These investigators summarized investigations indicating that increased blood pressure variability might predict organ damage and cardiovascular events over and above the contribution provided by average blood pressure in patients with hypertension. This indicates that continuous carotid sinus stimulation leads to reduced blood pressure fluctuations with potential beneficial cardiovascular consequences.
In contrast to the normal pulse-synchronous discharge of baroreceptors, the continuous delivery of impulses into the brain throughout the cardiac cycle during carotid sinus stimulation also raised the issue of whether this might disturb the normal dynamic control of heart rate by the baroreflex. Therefore, heart rate variability and spontaneous baroreflex sensitivity were determined from 18-h recordings of beat-to-beat systolic arterial pressure and pulse intervals (21). Surprisingly, there were significant increases in both measures, not decreases, indicating that baroreflex activation enhanced heart rate variability, a negative predictor of adverse cardiac events, while enhancing the sensitivity of the natural baroreflex control of heart rate. This suggests that electrical activation of the carotid baroreflex does not disrupt the negative feedback loop linking the baroreceptors with blood pressure and heart rate control via the autonomic nervous system. As a depressed cardiac parasympathetic reflex is an important predictor of cardiac morbidity and mortality independent of blood pressure changes, the central processing of natural afferent impulses during carotid sinus stimulation during normal blood pressure fluctuations and the resultant increase in cardiac baroreflex sensitivity may provide clinical benefit even beyond suppression of sympathetic activity and lowering of blood pressure.
Within the overarching issue related to the characteristics of baroreceptor signaling, these findings beg the question of whether a discharge pattern of carotid sinus stimulation reproducing the natural entrainment to the cardiac cycle would be more physiologically relevant and of added clinical benefit by having more efficient sustained reciprocal effects on sympathetic and parasympathetic activity. This determination has not yet been made.
RENAL-BODY FLUID CONTROL OF ARTERIAL PRESSURE
A fundamental law of the circulation is that arterial pressure is the product of cardiac output and total peripheral resistance. Because short-term regulation of arterial pressure is critically dependent on the rapid changes in peripheral resistance and cardiac output evoked by the baroreflex, often times little consideration is given to the potential importance of baroreflexes in long-term control of arterial pressure by altering renal excretory function and consequently total body fluid volume. In this regard, long-term regulation of arterial pressure is closely linked to volume homeostasis through the effect of pressure on sodium excretion, that is, by pressure natriuresis (5, 9). According to this concept, alterations in renal excretory function would be required to sustain long-term sympathetically mediated changes in arterial pressure. More specific to sympathoinhibition during carotid sinus stimulation, sympathetically mediated decreases in peripheral resistance would be expected to cause only transient reductions in arterial pressure unless they were also associated with concurrent renal actions to increase the sensitivity of pressure natriuresis. This is because the kidneys would retain sodium and water and continue to do so until arterial pressure returned to control levels. On the other hand, if inhibition of sympathetic activity were also to include actions on the kidneys to increase renal excretory function, fluid retention would be minimized and reductions in arterial pressure would be sustained because pressure natriuresis would be shifted to a lower pressure. Thus an increase in renal excretory function would be expected to be a prerequisite for achieving fluid balance at a reduced arterial pressure during baroreflex activation. An opposing view is that alterations in peripheral resistance and cardiac function can lead to chronic changes in arterial pressure while kidney function somehow spontaneously adapts to pressure changes allowing for achievement of sodium balance (1, 26 30). Contrary to experimental and theoretical evidence, the implication of this viewpoint is that arterial pressure does not have a long-term effect on sodium excretion and that the pressure natriuresis mechanism is not a long-term controller of body fluid volume and arterial pressure.
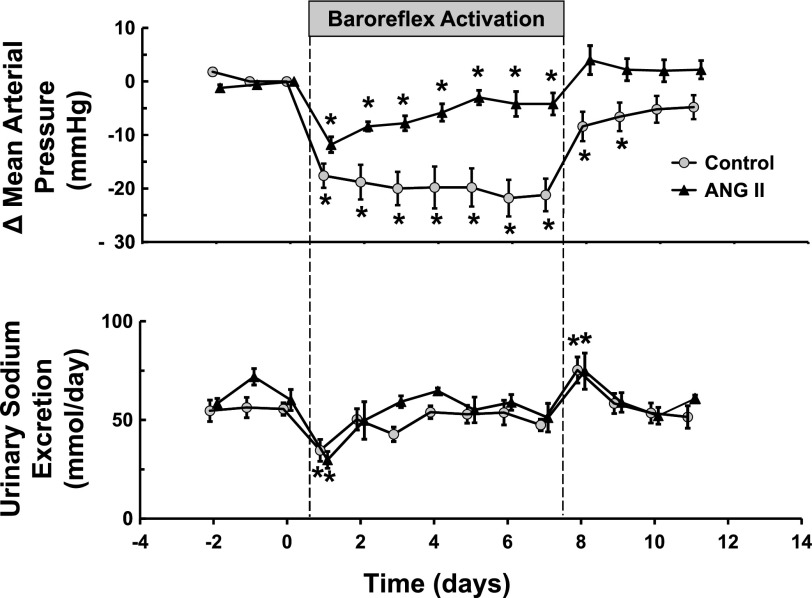
If baroreflex activation chronically increases renal excretory function, a question frequently asked is why is there sodium retention rather than increased sodium excretion during the onset of carotid sinus stimulation (Fig. 2)? This can be explained by the relative quantitative effects of sympathoinhibition on the peripheral vasculature and renal excretory function, with the former leading to a fall in arterial pressure exceeding the shift of the renal set point of pressure natriuresis to a lower pressure. Indeed, the opposite response occurs during chronic infusion of NE, with increased natriuresis acutely, as circulating NE has strong peripheral vasoconstrictor effects but weak protracted antinatriuretic actions. Consequently, infusion of NE leads to modest hypertension that is associated with loss of sodium and reduced body fluid volume (15). But what accounts for the increased renal excretory function that permits fluid balance at a reduced arterial pressure during baroreflex activation?
Fig. 2.
Changes in mean arterial pressure and sodium excretion before and after induction of ANG II hypertension. Values are means ± SE (n = 6). P < 0.05 vs. control [Borrowed with permission from (17).].
RENAL NERVES LINK SUPPRESSION OF CENTRAL SYMPATHETIC OUTFLOW TO INCREASED RENAL EXCRETORY FUNCTION DURING BAROREFLEX ACTIVATION
Increases in RSNA have direct renal actions that decrease sodium excretion by promoting sodium reabsorption and by decreasing glomerular filtration rate and renal blood flow. In addition, because increases in RSNA stimulate renin release, the generation of ANG II contributes indirectly to the antinatriuretic effects of renal adrenergic stimulation (6). Although renal nerve activity has not been measured during chronic electrical stimulation of the carotid baroreflex, there is considerable evidence suggesting that suppression of RSNA normally contributes to sustained reductions in arterial pressure during chronic baroreflex activation. First, as indicated above, electrical activation of the carotid baroreflex has sustained effects to suppress central sympathetic outflow (21). Furthermore, experimental studies in chronically instrumented animals have clearly demonstrated that the natural activation of the baroreflex in hypertension has sustained effects to suppress RSNA and promote sodium excretion (16, 24–25). Thus sustained baroreflex-mediated increases in renal excretory function attributable to suppression of RSNA would be expected to increase the sensitivity of the pressure natriuresis and allow sodium balance at a reduced arterial pressure. As discussed below, baroreflex-mediated suppression of RSNA is also consistent with the observed inhibitory effects of baroreflex activation on renin secretion.
RENAL NERVES ARE NOT THE ONLY MECHANISM INVOLVED IN ACHIEVING LONG-TERM REDUCTIONS IN ARTERIAL PRESSURE DURING PROLONGED BAROREFLEX ACTIVATION
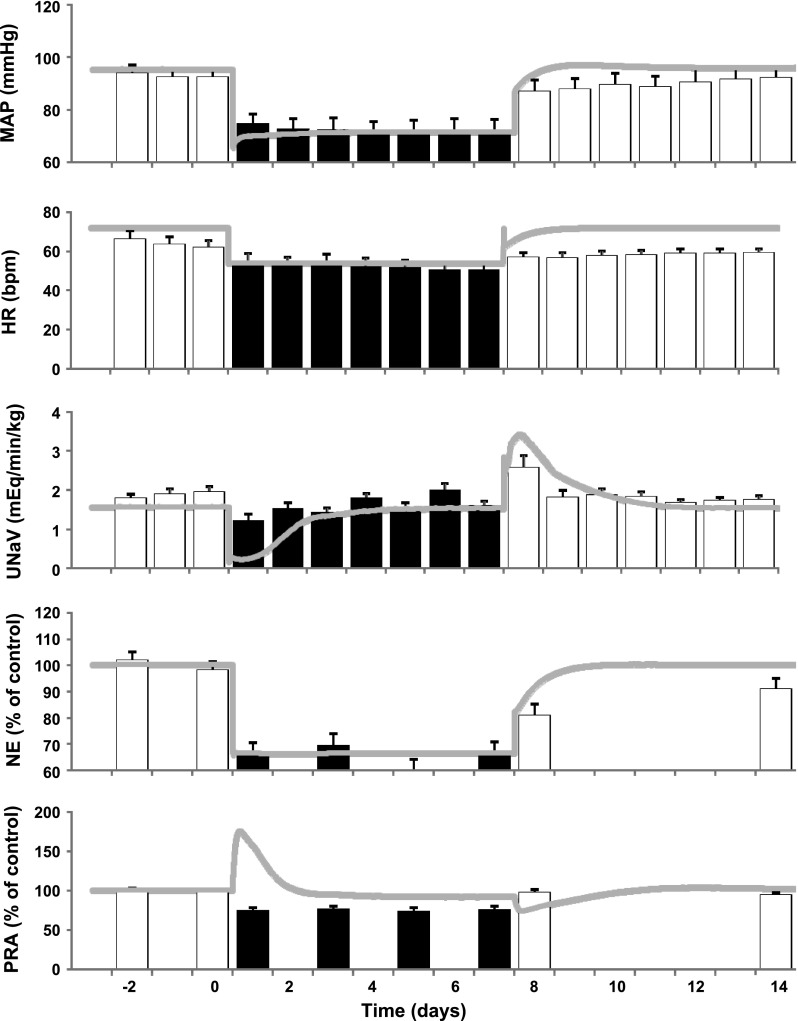
If suppression of RSNA increases the sensitivity of pressure natriuresis and in so doing chronically lowers arterial pressure, one would expect greater sodium retention and reduced blood pressure lowering during baroreflex activation in the absence of the renal nerves. Indeed, this was the case when determinations were made before and after renal denervation in the same dogs (18). However, because differences in sodium excretion and arterial pressure were not statistically significant and chronic reductions in arterial pressure still occurred after renal denervation, suppression of RSNA cannot be the sole mechanism that accounts for blood pressure lowering during baroreflex activation. To some, this observation may seem incongruous with the concept that the kidneys play a critical role in long-term control of arterial pressure during chronic changes in sympathetic activity. However, the above study also identified increased plasma atrial natriuretic peptide and decreased plasma protein concentration as two additional factors that may contribute to increased renal excretory function during chronic baroreflex activation. To provide further quantitative insight into the complex interactions of the integrated neurohormonal mechanisms that may account for blood pressure lowering during baroreflex activation, simulations were conducted using an established mathematical model of human physiology (12), now developed as HumMod (11). The model in its entirety is freely available for download and peruse, together with a detailed tutorial for specific simulations, in the supplemental data to the published paper (http://onlinelibrary.wiley.com/doi/10.1111/j.1440–1681.2009.05291.x/suppinfo). As a first step, we simulated 7 days of baroreflex activation and compared the results to actual experimental data (Fig. 3). In this simulation, arterial baroreceptor input into the central nervous system was increased to 70% of normal and fixed at this level for the 7 days to mimic the constant electrical stimulation of the carotid sinus. This level of activation was chosen to produce the same day 1 decrease in arterial pressure as observed experimentally (23). In response to the increased baroreceptor afferent input, the simulation indicated an ∼50% decrease in RSNA, which was sustained for the entire week of baroreflex activation. Most importantly, the hemodynamic, neurohormonal, and sodium excretory responses in the simulation closely matched the actual experimental data during baroreflex activation (Fig. 3), providing important validation of the accuracy of the model. Subsequent simulations indicated that by clamping RSNA at control levels and, therefore, preventing suppression of RSNA, the initial sodium retention with baroreflex activation was more pronounced, leading to more intense activation of redundant natriuretic mechanisms, which increased their effects on renal excretory function and their contribution to the chronic lowering of arterial pressure. More specifically, the simulations identified increased secretion of atrial natriuretic peptide and increased renal interstitial fluid pressure as two natriuretic responses of increased importance in shifting pressure natriuresis to a lower arterial pressure after renal denervation. In summary, mathematical models and simulations are important because they provide insight into complex integrative relationships that are not intuitively obvious, and they lead to testable hypotheses. However, it is important to emphasize that the above predictions from these simulations have not been critically tested in experimental studies and, therefore, additional cycles of experimentation and model refinement will be required to better understand the integrative influences on sodium excretion and long-term changes in arterial pressure during global suppression of sympathetic activity.
Fig. 3.
Hemodynamic, renal excretory, and neurohormonal responses to 1 wk of carotid baroreflex activation: comparison between experimental data [bars redrawn with permission from (23)] and QHP2008 simulation (line). MAP, mean arterial pressure; HR, heart rate: UNaV, urinary sodium excretion; NE, plasma norepinephrine concentration; PRA, plasma renin activity. [Borrowed with permission from (12).].
INTERACTION OF THE CAROTID BAROREFLEX WITH THE RENIN-ANGIOTENSIN SYSTEM IN LONG-TERM CONTROL OF ARTERIAL PRESSURE
A notable response in both normotensive and hypertensive dogs is that reductions in arterial pressure as great as 20–25 mmHg during baroreflex activation do not lead to an increase in plasma renin activity (PRA; 17–18, 20–23). In contrast, other studies in chronically instrumented dogs show that reductions in renal perfusion pressure ≥15 mmHg by means other than baroreflex activation lead to marked increases in PRA (14, 32). Therefore, baroreflex activation must have an inhibitory influence on renin secretion. On the basis of parallel changes in renin secretion and renal adrenergic stimulation (6), it is likely that pressure-dependent renin release is counteracted by suppression of RSNA during baroreflex activation. Thus we hypothesized that were it not for this inhibitory effect on renin secretion, the chronic blood pressure lowering effects of baroreflex activation would be greatly diminished.
This hypothesis was tested during equivalent degrees of carotid sinus stimulation before and during continuous infusion of ANG II (17). Studies were performed in the same dogs at an infusion rate that produced ∼3- to 5-fold increases in plasma ANG II and aldosterone concentration and a concomitant increase in MAP of ∼35 mmHg. Acute reductions in MAP during the first hour of baroreflex activation were pronounced and comparable with those achieved before induction of ANG II hypertension. However, as illustrated in Fig. 2, despite continuous baroreflex activation, this acute robust fall in arterial pressure was not sustained chronically in the presence of moderately higher and constant levels of plasma ANG II. This study demonstrates a clear dichotomy between the acute and long-term effects of sympathoinhibition on arterial pressure, with the acute effects being dominated by changes in peripheral resistance and cardiac function and the latter being dependent on the more sluggish renal mechanisms for control of body fluid volume. Thus, on the basis of the weak long-term antihypertensive response to baroreflex activation during ANG II hypertension, it would appear that increases in renal excretory function during inhibition of sympathetic activity are substantially diminished in the presence of even small increases in circulating ANG II. This study supports the hypothesis that baroreflex-mediated suppression of renin secretion counteracts increases in renin release that otherwise would greatly diminish the magnitude of pressure fall during baroreflex activation.
ELECTRICAL ACTIVATION OF THE BAROREFLEX ABOLISHES OBESITY HYPERTENSION
Many patients with resistant hypertension are obese and obesity-related hypertension has an important neurogenic component (3, 7, 10). Furthermore, obesity-hypertension, unlike hypertension produced by ANG II infusion, is associated with increased RSNA. Therefore, because baroreflex activation has sustained effects to inhibit RSNA, consideration was given to the possibility that electrical stimulation of the baroreflex would abolish the hypertension associated with weight gain in dogs fed a high-fat diet. This model of hypertension is particularly relevant to obesity in human subjects because it exhibits many of the same hemodynamic, neurohormonal, renal, and metabolic changes (10, 22).
As illustrated in Fig. 4, after 32 days on a diet supplemented with cooked beef fat, MAP was increased 17 mmHg along with a targeted weight gain of ∼50% (22). Heart rate also increased substantially. Similar to the response in dogs with ANG II hypertension, there was a substantial acute decrease in arterial pressure with baroreflex activation. However, in marked contrast to the weak chronic antihypertensive response in dogs with ANG II levels fixed modestly above control by infusion (Fig. 2), baroreflex activation abolished the hypertension and greatly diminished the tachycardia associated with weight gain (Fig. 4). Furthermore, the sustained lowering of blood pressure occurred concurrently with significant reductions in both plasma NE concentration and PRA, suggesting that sympathetic activation and neurally mediated renin secretion contributed significantly to the maintenance of the hypertension. After terminating baroreflex activation, measures of hemodynamics and neurohormones returned to prestimulation levels. Subsequently, to determine the importance of renal-specific sympathoinhibition to the antihypertensive effects of baroreflex activation, these same canines were subjected to bilateral renal denervation. Remarkably, within 2 days after renal denervation the hypertension was abolished. Furthermore, as with baroreflex activation, abolition of hypertension was associated with suppression of PRA, indicating once again the link between RSNA and renin secretion in long-term control of arterial pressure. In contrast to baroreflex activation, however, renal denervation did not lower circulating levels of NE, indicating sustained sympathetic drive to the peripheral circulation and highlighting the relative importance of renal specific sympathetic activation in sustaining the hypertension.
Fig. 4.
Changes in mean arterial pressure and heart rate during chronic baroreflex activation in dogs with obesity-induced hypertension (n = 6). Values are means ± SE. *P < 0.05 vs. day 32. Lean control values (open bars) are also presented as reference. [Borrowed with permission from (22).].
The importance of the renal nerves as a mediator of neurogenic hypertension is also supported by recent clinical studies demonstrating impressive antihypertensive effects of catheter-based endovascular radiofrequency ablation of the renal nerves in patients with resistant hypertension (29, 31). However, in contrast to the above findings in dogs with obesity hypertension indicating that renal denervation does not decrease global sympathetic activity, a report in a patient with resistant hypertension indicated that ablation of the renal nerves decreased systemic sympathetic activity and arterial pressure (29). This latter observation suggests that putative sensory afferent signals from the kidneys to the brain may contribute to central sympathetic outflow. It will be of interest to see if this observation is confirmed in a patient population of sufficient size.
Taken together, these studies in dogs with ANG II and obesity hypertension emphasize the importance of renal sympathoinhibition and concomitant suppression of renin secretion in mediating the long-term blood pressure lowering effects of baroreflex activation. However, beyond lowering blood pressure in patients with hypertension, one should not discount the potential therapeutic benefit of reducing heightened sympathetic outflow to organs other than the kidneys and improving autonomic balance to the heart.
POTENTIATION OF THE BLOOD PRESSURE LOWERING EFFECTS OF ANTIHYPERTENSIVE DRUGS BY BAROREFLEX ACTIVATION
The lowering of arterial pressure with some drugs commonly used for hypertension therapy, including diuretics and calcium channel blockers, activates the sympathetic nervous system, probably via a baroreflex-mediated mechanism. This sympathetic activation may attenuate the antihypertensive efficacy of these agents. Indeed, studies in dogs clearly demonstrate that the long-term lowering of blood pressure during administration of adrenergic blocking agents and calcium channel blockers leads to substantial sympathetic activation and concomitant stimulation of the renin-angiotensin system (13, 19). However, suppression of central sympathetic outflow by baroreflex activation decreases arterial pressure further and, in the absence of β-blockade, PRA as well (13, 19). Thus, as in obesity hypertension, baroreflex activation has impressive blood pressure lowering effects by suppressing heightened sympathetic activity and concomitant neural stimulation of renin release.
CONCLUSIONS AND PERSPECTIVES
The technology to chronically activate the baroreflex by electrical stimulation of the carotid sinus has provided further insight into the controversy regarding the role of baroreflexes in long-term control of arterial pressure. However, of greater significance, this technology has provided a unique tool to elucidate the neural mechanisms that account for chronic reductions in arterial pressure in response to suppression of central sympathetic outflow. The experimental studies conducted over time courses of several weeks and summarized above indicate that the suppression of central sympathetic outflow and the concomitant lowering of blood pressure are sustained chronically during electrical stimulation of the carotid sinus, discounting the importance of central resetting in diminishing the responses to baroreflex afferent input. Furthermore, current clinical trials indicate that the sympathoinhibitory effects of carotid sinus stimulation persist over much longer time periods (2, 20, 27). In these trials, reductions in systolic pressure of ∼ 30 mmHg have been sustained for more than 2 years in patients with resistant hypertension subjected to baroreflex activation therapy. Experimental studies also emphasize the importance of regional suppression of sympathetic activity to the kidneys in mediating the chronic lowering of blood pressure during baroreflex activation. This increase in renal excretory function during baroreflex activation permits fluid balance at a reduced arterial pressure and is achieved, in part, by neural suppression of renin secretion. However, there are clearly additional mechanisms beyond renal sympathoinhibition that promote sodium excretion during baroreflex activation.
Subjects with resistant hypertension are commonly obese, and antihypertensive agents reported to increase sympathetic activation are usually included in the regimen of drugs used for hypertensive therapy (2–3, 27, 29, 31). Thus increased sympathetic activity may be more prevalent than widely appreciated and contribute to the intractable hypertension in this patient population. If so, this may account for recent findings in clinical trials indicating impressive long-term antihypertensive responses to baroreflex activation therapy in patients with resistant hypertension (2, 20, 27).
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-51971.
DISCLOSURES
T. E. Lohmeier Consultant fees, Scientific Advisory Board, CVRx.
AUTHOR CONTRIBUTIONS
Author contributions: T.E.L. and R.I. conception and design of research; T.E.L. and R.I. performed experiments; T.E.L. and R.I. analyzed data; T.E.L. and R.I. interpreted results of experiments; T.E.L. and R.I. prepared figures; T.E.L. drafted manuscript; T.E.L. and R.I. edited and revised manuscript; T.E.L. and R.I. approved final version of manuscript.
REFERENCES
- 1. Bie P. Blood volume, blood pressure and total body sodium: internal signalling and output control. Acta Physiol (Oxf) 195: 187–196, 2009. [DOI] [PubMed] [Google Scholar]
- 2. Bisognano JD, Bakris G, Nadim MK, Sanchez L, Kroon AA, Schafer J, de Leeuw PW, Sica DA. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: results from the double-blind, randomized, placebo-controlled Rheos Pivotal trial. J Am Coll Cardiol 58: 765–773, 2011. [DOI] [PubMed] [Google Scholar]
- 3. Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica A, Ferdinand K, Giles TD, Falkner B, Carey RM. Resistant hypertension diagnosis evaluation, treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 51: 1403–1419, 2008. [DOI] [PubMed] [Google Scholar]
- 4. Chapleau MW, Hajduczok G, Abboud FM. Resetting of the arterial baroreflex: peripheral and central mechanisms. In: Reflex Control of the Circulation, edited by Zucker IH, Gilmore JP. Boca Raton, FL: CRC, 1991, p. 165–194. [Google Scholar]
- 5. Cowley AW., Jr Long-term control of arterial pressure. Physiol Rev 72: 231–300, 1992. [DOI] [PubMed] [Google Scholar]
- 6. DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev 77: 75–197, 1997. [DOI] [PubMed] [Google Scholar]
- 7. Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension 48: 787–796, 2006. [DOI] [PubMed] [Google Scholar]
- 8. Guyton AC, Coleman TC, Granger HJ. Circulation: overall regulation. Annu Rev Physiol 34: 13–46, 1972. [DOI] [PubMed] [Google Scholar]
- 9. Guyton AC. Arterial pressure and hypertension. Philadelphia: Saunders, 1980. [Google Scholar]
- 10. Hall JE, da Silva AA, Brandon E, Stec DE, Ying Z, Jones DW. Pathophysiology of obesity-induced hypertension and target organ damage. In: Comprehensive Hypertension, edited by Lip GYH, Hall JE. Philadelphia: Elsevier, 2007, p. 447–468. [Google Scholar]
- 11. Hester RL, Brown AJ, Husband L, Iliescu R, Pruett D, Summers R, Coleman TG. HumMod: a modeling environment for the simulation of integrative human physiology. Frontiers Physiol 2: 1–12, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iliescu R, Lohmeier TE. Lowering of blood pressure during chronic suppression of central sympathetic outflow: insight from computer simulations. Clin Exptl Pharmacol Physiol 37: e24–33, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iliescu R, Irwin ED, Georgakopoulos D, Lohmeier TE. Renal responses to chronic suppression of central sympathetic outflow. Hypertension 60: 749–756, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kirchheim HR, Finke R, Hackenthal E, Lowe W, Persson P. Baroreflex sympathetic activation increases threshold pressure for pressure-dependent renin release in conscious dogs. Pflügers Arch 405: 127–135, 1985. [DOI] [PubMed] [Google Scholar]
- 15. Lohmeier TE, Carroll RG. Chronic potentiation of vasoconstrictor hypertension by adrenocorticotropic hormone. Hypertension 4, Suppl II: II-138–II-148, 1982. [PubMed] [Google Scholar]
- 16. Lohmeier TE, Drummond HA. The baroreflex in the pathogenesis of hypertension. In: Comprehensive Hypertension, edited by Lip GYH, Hall JE. Philadelphia: Elsevier, 2007, p. 265–279. [Google Scholar]
- 17. Lohmeier TE, Dwyer TM, Hildebrandt DA, Irwin ED, Rossing MA, Sedar DJ, Kieval RS. Influence of prolonged baroreflex activation on arterial pressure in angiotensin hypertension. Hypertension 46: 1194–1200, 2005. [DOI] [PubMed] [Google Scholar]
- 18. Lohmeier TE, Hildebrandt DA, Dwyer TM, Barrett AM, Irwin ED, Rossing MA, Kieval RS. Renal denervation does not abolish sustained baroreflex-mediated reductions in arterial pressure. Hypertension 49: 373–379, 2007. [DOI] [PubMed] [Google Scholar]
- 19. Lohmeier TE, Hildebrandt DA, Dwyer TM, Iliescu R, Irwin ED, Cates AW, Rossing MA. Prolonged activation of the baroreflex decrease arterial pressure even during chronic adrenergic blockade. Hypertension 53: 833–838, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lohmeier TE, Iliescu R. Chronic lowering of blood pressure by carotid baroreflex activation. Mechanisms and potential for hypertension therapy. Hypertension 57: 880–886, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lohmeier TE, Iliescu R, Dwyer TM, Irwin ED, Cates AW, Rossing MA. Sustained suppression of sympathetic activity and arterial pressure during chronic activation of the carotid baroreflex. Am J Physiol Heart Circ Physiol 299: H402–H409, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lohmeier TE, Iliescu R, Liu B, Henegar JR, Maric-Bilkan C, Irwin ED. Systemic and renal-specific sympathoinhibition in obesity hypertension. Hypertension 59: 331–338, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lohmeier TE, Irwin ED, Rossing MA, Sedar DJ, Kieval RS. Prolonged activation of the baroreflex produces sustained hypotension. Hypertension 43: 306–311, 2004. [DOI] [PubMed] [Google Scholar]
- 24. Lohmeier TE, Lohmeier JR, Haque A, Hildebrandt DA. Baroreflexes prevent neurally-induced sodium retention in angiotensin hypertension. Am J Physiol Regul Integr Comp Physiol 279: R1437–R1448, 2000. [DOI] [PubMed] [Google Scholar]
- 25. Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular diseases. Physiol Rev 90: 513–557, 2010. [DOI] [PubMed] [Google Scholar]
- 26. Osborn JW, Averina VA, Fink GD. Current computational models do not reveal the importance of the nervous system in long-term control of arterial pressure. Exp Physiol 94: 389–395, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scheffers IJM, Kroon AA, Schmidli J, Jordan J, Tordoir JJM, Mohaupt MG, Luft FC, Haller H, Menne J, Engeli S, Ceral J, Eckert S, Erglis A, Narkiewicz K, Philipp T, de Leeuw PW. Novel baroreflex activation therapy in resistant hypertension. J Am Coll Cardiol 56: 1254–1258, 2010. [DOI] [PubMed] [Google Scholar]
- 28. Schillaci G, Pucci G, Parati G. Blood pressure variability. An additional target for antihypertensive treatment? Hypertension 58: 155–160, 2011. [DOI] [PubMed] [Google Scholar]
- 29. Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med 361: 932–934, 2009. [DOI] [PubMed] [Google Scholar]
- 30. Seelinger E, Andersen JL, Bie P, Reinhardt HW. Elevated renal perfusion pressure does not contribute to natriuresis induced by isotonic saline infusion in freely moving dogs. J Physiol 559: 939–951, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Symplicity HTN2 Investigators. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial). Lancet 376: 1903–1909, 2011. [DOI] [PubMed] [Google Scholar]
- 32. Yang HM, Lohmeier TE, Kivlign SD, Carroll RG, Smith MJ., Jr Sustained increases in plasma epinephrine concentration do not modulate renin release. Am J Physiol Endocrinol Metab 257: E57–E64, 1989. [DOI] [PubMed] [Google Scholar]