Abstract
Inactivity-related diseases are becoming a huge burden on Western society. While there is a major environmental contribution to metabolic health, the intrinsic properties that predispose or protect against particular health traits are harder to define. We used rat models of inborn high running capacity (HCR) and low running capacity (LCR) to determine inherent differences in mitochondrial volume and function, hypothesizing that HCR rats would have greater skeletal muscle respiratory capacity due to an increase in mitochondrial number. Additionally, we sought to determine if there was a link between the expression of the orphan nuclear receptor neuron-derived orphan receptor (Nor)1, a regulator of oxidative metabolism, and inherent skeletal muscle respiratory capacity. LCR rats were 28% heavier (P < 0.0001), and fasting serum insulin concentrations were 62% greater than in HCR rats (P = 0.02). In contrast, HCR rats had better glucose tolerance and reduced adiposity. In the primarily oxidative soleus muscle, maximal respiratory capacity was 21% greater in HCR rats (P = 0.001), for which the relative contribution of fat oxidation was 20% higher than in LCR rats (P = 0.02). This was associated with increased citrate synthase (CS; 33%, P = 0.009) and β-hydroxyacyl-CoA (β-HAD; 33%, P = 0.0003) activities. In the primarily glycolytic extensor digitum longus muscle, CS activity was 29% greater (P = 0.01) and β-HAD activity was 41% (P = 0.0004) greater in HCR rats compared with LCR rats. Mitochondrial DNA copy numbers were also elevated in the extensor digitum longus muscles of HCR rats (35%, P = 0.049) and in soleus muscles (44%, P = 0.16). Additionally, HCR rats had increased protein expression of individual mitochondrial respiratory complexes, CS, and uncoupling protein 3 in both muscle types (all P < 0.05). In both muscles, Nor1 protein was greater in HCR rats compared with LCR rats (P < 0.05). We propose that the differential expression of Nor1 may contribute to the differences in metabolic regulation between LCR and HCR phenotypes.
Keywords: intrinsic running capacity, mitochondria, skeletal muscle, metabolism, neuron-derived orphan receptor 1
the ability to deliver and utilize O2 is an essential component of metabolic regulation, with an individual's maximal aerobic power [maximal O2 consumption (V̇o2 max)] a good correlate of whole body health status (13). Aerobic power is determined by the interaction of intrinsic (i.e., genetic) and environmental (i.e., lifestyle) factors, with the heritability of endurance capacity estimated to be 40% or higher (10). However, V̇o2 max and its phenotypic expression (i.e., endurance running ability) can be rapidly modified by embarking on a vigorous exercise training program or, conversely, by adopting an inactive lifestyle. While such phenotypic plasticity can be harnessed to study the time course of progression (or reversal) of a number of metabolic disease traits, the contribution of potential intrinsic mechanisms to such diseases becomes difficult to define because of the confounding impact of numerous environmental influences (13).
Through two-way artificial selection, we have generated an animal model of low or high aerobic exercise capacity [low (LCR) or high running capacity (HCR), respectively] from a population of genetically heterogeneous rats. Divergent artificial selection for a complex trait (e.g., superior aerobic phenotype) produces a useful genetic model to study gene-exercise interactions because contrasting allelic variation is concentrated at the extremes from one generation to the next. In this model, 11 generations of two-way selection produced rats that differ substantially in aerobic exercise capacity (374% difference between LCR and HCR rats, P < 0.0001) while simultaneously presenting with markedly different metabolic and cardiovascular disease risk factors (48).
Because skeletal muscle mitochondrial respiration is a limiting factor for V̇o2 max, and because skeletal muscle oxidative capacity correlates with whole body insulin sensitivity (4), it has been proposed that differences in mitochondrial function in LCR-HCR animals are the major determinants of their divergent running capacities and metabolic phenotypes. In support of this contention, HCR rats are better at using O2, an adaptive variation likely to be present at the level of the skeletal muscle (10). Results from a series of studies from our laboratory (16, 17, 35) and others (24, 25, 41, 45, 48) have provided direct evidence that skeletal muscle from HCR rats has superior substrate handling ability (i.e., “metabolic flexibility”) and mitochondrial enzyme activities compared with LCR rats. However, there is some contention as to whether this is due to increases in the mitochondrial reticulum (35) or an enhanced capacity of some or all of the oxidative enzymes (24, 41, 45). Additionally, we have shown that the differences in metabolic flexibility are linked to β-adrenergic signaling through the nuclear receptor (NR)4A orphan NR neuron-derived clone (Nur)77 (16, 17). The NR4A family of orphan NRs [Nur77, Nurr1, and neuron-derived orphan receptor (Nor)1] has recently emerged as a key player in the regulation of a number of important metabolic processes (4, 16, 31, 33). All three have been linked to the regulation of skeletal muscle substrate handling and whole body glucose homeostasis (4, 16, 31, 33), and Nor1 has been shown to be essential for oxidative metabolism (32) and may also be important in promoting several oxidative adaptations in the muscle (21).
Since previous studies have been inconclusive as to whether skeletal muscle respiratory capacity is due to an increased number of mitochondria (35) or increased mitochondrial activity (24, 41), we aimed to determine the ex vivo activity and protein expression of the key electron transfer system (ETS) respiratory complexes in muscle from LCR and HCR rats. We hypothesized that skeletal muscle respiratory capacity would be greater in HCR rats compared with LCR rats and that this difference would be due to a greater mitochondrial density in the muscle. Furthermore, we hypothesized that the expression of Nor1 would be greater in the muscle of HCR rats compared with LCR rats.
METHODS
Experimental animals.
Rat models for LCR and HCR were derived from genetically heterogeneous N:NIH stock rats by artificial selection for treadmill running capacity, as previously described (12). Female offspring of either LCR rats (N = 12, generation 27) or HCR rats (N = 12, generation 27) were housed under a controlled 12:12-h light-dark cycle at a constant temperature of 22°C. Animals were provided ad libitum access to water and a standard chow diet. Breeding and phenotyping of parent rats was conducted at the University of Michigan (Ann Arbor, MI). Rats arrived at RMIT University at ∼8 wk of age and were allowed to acclimate to the RMIT Animal Facility for 2 wk before the commencement of any experimental procedures. This study was undertaken with approval from both the University of Michigan and RMIT University Animal Ethics Committees.
Fasting blood and insulin measurements.
After a 5-h fast, ∼15-μl aliquot of blood was collected via a tail cut, and the glucose concentration was measured on a hand-held glucometer (Roche Diagnostics, Castle Hill, NSW, Australia). A separate aliquot of blood (∼15–20 μl) was allowed to clot over ice and centrifuged to obtain serum. Serum was kept frozen at −20°C for later analysis of fasting serum insulin concentrations using a commercially available ELISA (no. 80-INSRT-E01, ALPCO Immunoassays,).
Intraperitoneal glucose tolerance testing.
After the fasting blood collection, rats received a single glucose bolus (2 g/kg body wt) via intraperitoneal injection, and blood glucose concentrations were monitored at 30-min intervals throughout the subsequent 120 min. Additional aliquots of blood were collected at 30, 60, and 120 min postinjection for the later determination of serum insulin concentrations in response to the glucose challenge.
Physical activity monitoring.
Before being monitored, rats were familiarized with activity monitoring chambers comprising infrared beams in the x-, y-, and z-axes (MED Associates, St. Albans, VT) during three 10-min sessions in the week before testing. Spontaneous activity was monitored during both diurnal (1200 hours) and nocturnal (0200 hours) sessions. Horizontal, vertical, and ambulatory locomotor activities were determined in 20-s intervals over 2 × 10-min sessions.
Tissue collection and analyses.
Tissue collection procedures were conducted after a 5-h fast in 11-wk-old rats, which were weighed and anesthetized with pentobarbital sodium (60 mg/kg body wt), and hindlimb muscles were immediately excised. To limit any possible differences that might occur as a result of differing cage activity, the soleus and extensor digitorum longus (EDL) muscles were chosen for study. The soleus (∼87% type I fibers and ∼12% type IIA fibers) is predominantly a postural muscle and therefore has a high daily activity level, whereas many of the fibers in the EDL muscle (∼2% type I fibers, ∼42% type IIA fibers, and ∼56% type IIB fibers) are recruited only during rapid, high-intensity tasks (1). Similar experiments were performed on soleus and EDL portions from the same leg. The left soleus muscle was placed in ice-cold muscle preservation media [BIOPS; 50 mM K+-MES, 20 mM taurine, 0.5 mM DTT, 6.56 mM MgCl2, 5.77 mM ATP, 15 mM phosphocreatine, and 20 mM imidazole (pH 7.1 adjusted with 5 N KOH at 0°C); Ca2+-EGTA buffer (10 mM; 2.77 mM CaK2EGTA + 7.23 mM K2EGTA, 0.1 μM free calcium)] for respirometry experiments (34, 36, 42), whereas the contralateral soleus muscle was carefully dissected into longitudinal strips from tendon to tendon (15–20 mg/strip) and placed in pregassed Krebs-Henseleit buffer (KHB) for measurements of basal and insulin-stimulated glucose uptake. The remaining hindlimb muscles were freeze clamped in liquid N2 and stored at −80°C for later analyses. Periovarian fat pads were removed and weighed as indicators of adiposity.
Glucose uptake.
All reagents used were obtained from Sigma-Aldrich (Castle Hill, NSW, Australia) unless otherwise specified. Soleus muscle strips from the right leg were allowed to recover for 30 min in preoxygenated KHB [118.5 mM NaCl, 25 mM NaHCO3, 4.74 mM KCl, 1.19 mM MgSO4·7H2O, 1.18 mM KH2PO4, and 2.5 mM CaCl2 (pH 7.4)] containing 32 mM mannitol, 2 mM pyruvate, 8 mM glucose, and 0.1% fatty-acid free BSA. Vials were maintained at 30°C in a shaking water bath throughout the experiment. Basal and insulin-stimulated glucose uptake were determined according to the method of Young et al. (49) with modifications (8, 17, 44). After the recovery incubation, muscle strips were incubated for an additional 30 min in continuously gassed modified KHB with or without insulin (60 nM, Humulin, Eli Lilly, Indianapolis, IN). Muscle strips were next washed for 10 min in KHB modified with 40 mM mannitol, 2 mM pyruvate, 0.1% fatty-acid free BSA, and 60 nM insulin, if it was present in the previous incubation. After glucose washout, strips were transferred to vials containing oxygenated KHB containing 0.1% fatty-acid free BSA, 2 mM pyruvate, 1 mM 2-[1,2-3H]deoxyglucose (1 mCi/ml, Perkin-Elmer), 39 mM d-[1-14C]-mannitol (0.1 μCi/ml, Perkin-Elmer), and 60 nM insulin, if it was present in the previous incubations. After 20 min, the reaction was stopped by washing the muscle strips in KHB twice, blotting the tissue on filter paper, and then freeze clamping it with tongs precooled in liquid N2. Muscle strips were weighed, digested in 10% tricarboxylic acid (TCA), and transferred to vials containing 5 ml scintillation fluid (Ultima Gold XR, Perkin-Elmer). Duplicate aliquots were counted in a liquid scintillation counter (Packard Tri-Carb, Perkin-Elmer) set for simultaneous 3H and 14C counting. Rates of basal and insulin-stimulated skeletal muscle 2-[1,2-3H]deoxyglucose transport were calculated as previously described (15).
High-resolution respirometry.
Approximately 10 mg of the soleus (n = 10 soleus muscles/group) were mechanically separated over ice using fine forceps (34) and then chemically permeablized with saponin (50 μg/ml in BIOPS) for 30 min. This was followed by a 10-min wash period in mitochondrial respiratory media [MIR05; 110 mM sucrose, 60 mM K-lactobionate (ACROS Organics), 0.5 mM EGTA, 3 mM MgCl2, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES (adjusted to pH 7.1 with KOH at 37°C), and 1 g/l fatty acid-free BSA] (36). Duplicate tissue samples (2–3.5 mg) were transferred to the chambers of an O2K-Oxygraph high-resolution respirometer (Oroboros, Innsbruck, Austria) containing 2 ml MIR06 (MIR05 plus 280 IU/ml catalase) and calibrated to air saturation. Individual chambers were oxygenated to ∼485 nmol/ml with pure O2 (BOC Australia). Chambers were maintained at a temperature of 37°C, and O2 saturation was kept between 450 and 300 nmol/ml with regular titrations of H2O2. The remaining, nonpermeabilized muscle was frozen for later analyses.
Substrate-uncoupler-inhibitor titration protocol.
Malate (2 mM) was added, and the mass-specific O2 flux (in pmol·s−1·mg wet wt−1) was stabilized for 5–10 min. Subsequently, tissue samples were subjected to a substrate-uncoupler-inhibition titration (SUIT) protocol, which sequentially evaluates an electron-transferring flavoprotein (ETF; fat oxidation) in leak state through the addition of octanoylcarnitine (0.2 mM, TOCRIS Bioscience; state 2 respiration), oxidative phosphorylation (OXPHOS) with electron flux through the ETF by titration of ADP (1 and 2.5 mM; state 3 respiration), OXPHOS with electron flux through the ETF and complex I (CI) with the addition of glutamate (10 mM), OXPHOS with electron flux through the ETF, CI, and complex II (CII) with the addition of succinate (10 mM), maximal coupled OXPHOS with electron flux through the ETF, CI, and CII with the addition of ADP (5 mM), the integrity of the outer mitochondrial membrane with the addition of cytochrome c (10 μM), the maximal capacity of the ETS by uncoupling with a stepped titration of FCCP (0.5 and 1 μM), uncoupled respiration with electron flux through the ETF and CII with the addition of the CI inhibitor rotenone (0.5 μM), and residual O2 consumption with the addition of antimycin A (2.5 μM). Additionally, complex IV (CIV) activity was determined after the addition of the artificial electron donor N,N,N,N,N′,N′-tetramethyl-p-phenylenediamine dihydrochloride (0.5 mM) in the presence of ascorbate (2 mM). The reaction was terminated by the addition of NaN3 (50 mM), after which the chambers were reoxygenated for the determination of auto-oxidation. Mass-specific O2 flux was determined using DATLAB (Oroboros) from steady-state O2 flux normalized to tissue wet weight and adjusted for instrumental background, residual O2 consumption, and auto-oxidation. In addition, flux control ratios (results normalized to the maximal capacity of the ETS) were calculated to determine mitochondrial function independent of mitochondrial density.
Mitochondrial enzyme activities.
Muscle homogenates (n = 10 homogenates/group) were prepared over ice from freeze-clamped soleus and EDL muscles (10–20 mg) in buffer [175 mM KCl and 2 mM EDTA (pH 7.4), 1:50 or 1:100 dilution]. Homogenates were then subjected to three freeze-thaw cycles. Citrate synthase (CS) and β-hydroxyacyl-CoA dehydrogenase (β-HAD) activities were determined according to the methods of Srere (CS) (37) and Bergmeyer (β-HAD) (2) with modifications as previously described (38).
Nucleic acid extraction and quantification.
DNA and RNA were each extracted from ∼10–30 mg of frozen soleus and EDL. RNA was extracted using TRIzol reagent (Invitrogen, Mulgrave, Australia), and DNA was extracted using a commercially available kit (Qiagen, Doncaster, Australia). A NanoDrop 1000 spectrophotometer (Nanodrop Technologies) was used to determine the quality and quantity of both extracts. For each nucleic acid, an aliquot was taken from each sample (n = 7 samples/group) and pooled. The pooled RNA or DNA sample was then quantified and serially diluted to produce standard curves for inclusion in each PCR run.
Reverse transcription and real-time PCR.
First-strand cDNA synthesis was performed for RNA extracts using Superscript VILO (Invitrogen). Relative mRNA expression was determined using commercially available Taqman primer/probe sets (Applied Biosystems, Mulgrave, Australia) for peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α (cat. no. Rn00580241_m1) and fibronectin type III domain-containing 5 (FNDC5; cat. no. Rn01519161_m1). GAPDH (cat. no. Rn01775763_g1) was included as a housekeeping gene to normalize threshold cycle (CT) values. Quantification was performed in duplicate using a 72-well Rotor-Gene 3000 Centrifugal Real-Time Cycler (Corbett Research, Mortlake, Australia). PCR conditions were as follows: 2 min at 50°C and 10 min at 95°C followed by 40 cycles of 95°C for 15 s and 65°C for 60 s. Calculations were made using the ΔΔCT method (20).
Mitochondrial DNA copy number determination.
Mitochondrial (mt)DNA copy numbers were determined in DNA extracts as previously described (38) using Taqman primer/probe sets for mitochondrial-encoded NADH dehydrogenase-1 (cat. no. Rn03296764_s1) and nuclear-encoded GAPDH. DNA samples were subjected to the same PCR conditions as described above. mtDNA copy numbers were calculated as described by Venegas and Halberg (43).
Immunoblot analysis.
Approximately 50 mg of frozen soleus or EDL was homogenized in buffer [50 mM Tris·HCl (pH 7.5), 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 50 mM NaF, 5 mM Na pyrophosphate, 10% glycerol, 1% Triton X-100, 10 μg/ml trypsin inhibitor, 2 μg/ml aprotinin, 1 mM benzamidine, and 1 mM PMSF; 1:8 dilution] and centrifuged at 20,000 g for 30 min at 4°C. The protein concentration of the supernatant was determined using the bicinchoninic acid method (Pierce). Muscle lysates containing 10 μg protein were prepared in 4× Laemmli buffer, subjected to SDS-PAGE, and then transferred to polyvinylidene difluoride membranes. Membranes were blocked (5% nonfat dry milk) for 1 h at room temperature and then incubated overnight at 4°C with primary antibodies specific for the following proteins: PGC-1 (∼100 kDa, no. ab3242, Chemicon), FNDC5 (∼28 kDa, no. ab93373, Abcam,), Nur77 (∼48 kDa, no. sc5569, Santa Cruz Biotechnology), Nor1 (∼68 kDa, no. 92777, Abcam), glucose transporter 4 (∼47 kDa, no. ab654, Abcam), fatty acid translocase/CD36 (∼80 kDa, no. 17044, Abcam), CI (∼18 kDa), CII (∼25 kDa), complex III (CIII; ∼45 kDa), CIV subunit I (∼37 kDa), and complex V (CV; ∼52 kDa) of the ETS (no. MA604, MitoSciences), CIV subunit II (∼25 kDa, no. MS405, MitoSciences), CIV subunit IV (∼15 kDa, no. MS407, MitoSciences), CS (∼52 kDa, no. ab96600, Abcam), and uncoupling protein 3 (UCP3; ∼35 kDa, no. PA1-055, Affinity BioReagents). α-Tubulin (∼50 kDa, Sigma) was used as a protein loading control. Protein expression was detected using enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ) and quantified by densitometry.
Statistical analyses.
All values are expressed as means ± SEM. Two-way ANOVA was used to analyze data from the intraperitoneal glucose tolerance test (IP-GTT). Physical activity and glucose uptake data were analyzed using one-way ANOVA with a Student-Newman-Keuls post hoc test. An unpaired t-test was used to compare groups for all other analyses unless otherwise specified. Statistical analyses were performed using Graph Pad Prism software. Significance is reported where P < 0.05.
RESULTS
HCR and LCR rats have divergent metabolic health parameters.
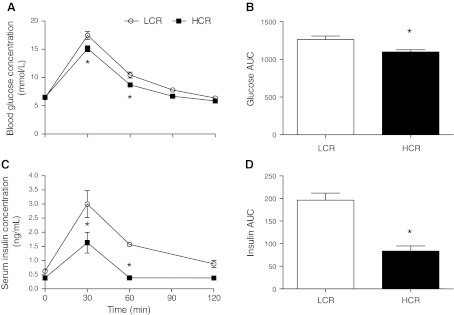
The metabolic health characteristics of each rat phenotype are shown in Table 1. Although there were no differences in the ages of the animals, LCR rats were 28% heavier than HCR rats (P < 0.0001) and had 48% more periovarian fat (P = 0.02). There were no differences in interscapular brown adipose masses. Fasting blood glucose concentrations were not different, but fasting serum insulin concentrations were 62% higher in LCR rats compared with HCR rats (P = 0.02). HCR rats were more glucose tolerant than LCR rats, as determined by the area under the glucose curve during the IP-GTT (LCR rats: 1,267 ± 47.4 and HCR rats: 1,100 ± 27.8, P = 0.01; Fig. 1, A and B). Similarly, the insulin response to the IP-GTT was 135% greater in LCR rats compared with HCR rats, as determined by the area under the insulin curve during the IP-GTT (LCR rats: 196.2 ± 15.8 and HCR rats: 83.6 ± 11.3, P = 0.001; Fig. 1, C and D).
Table 1.
Body composition and metabolic parameters
| LCR Rats | HCR Rats | |
|---|---|---|
| Age, days | 80.9 ± 0.6 | 81.0 ± 0.6 |
| Body weight, g | 214.1 ± 3.7 | 167.7 ± 4.2* |
| Periovarian fat pad mass, g | 1.2 ± 0.1 | 0.8 ± 0.1* |
| Interscapular brown adipose mass, mg | 183.0 ± 13.7 | 185.1 ± 10.2 |
| Fasting blood glucose concentration, mM | 6.5 ± 0.1 | 6.5 ± 0.2 |
| Fasting serum insulin concentration, ng/ml | 0.62 ± 0.07 | 0.39 ± 0.01* |
Values are means ± SE; N = 8–12 rats/group. LCR rats, rats with low running capacity; HCR rats, rats with high running capacity.
P < 0.05.
Fig. 1.
Glucose tolerance test. A: blood glucose responses over 120 min. B: areas under the blood glucose curve (Glucose AUC). C: serum insulin responses over 120 min. D: areas under the serum insulin curve (Insulin AUC). HCR rats, rats with high running capacity; LCR rats, rats with low running capacity. Values are means ± SE; N = 8–10. *P < 0.05.
The glucose uptake response to insulin is enhanced in HCR rats.
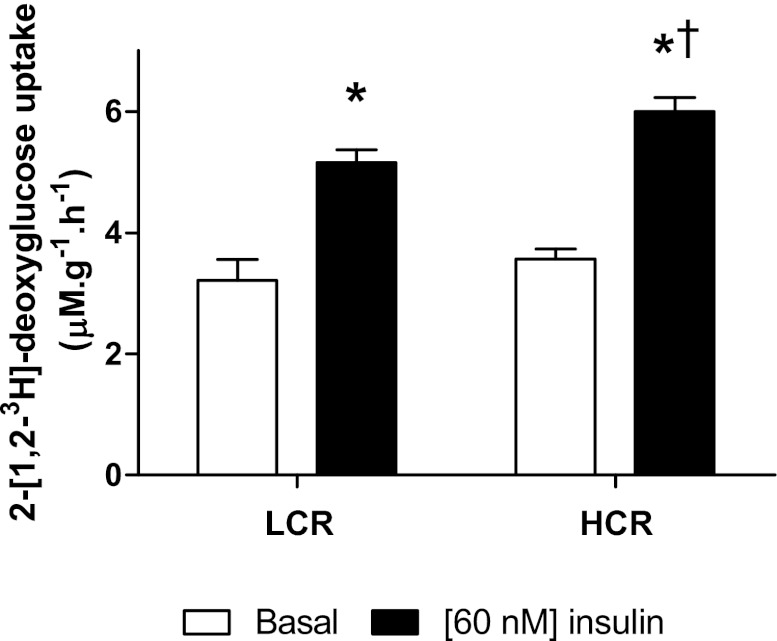
Rates of glucose uptake in the soleus are shown in Fig. 2. There were no differences in the rate of basal glucose uptake between LCR and HCR rats (3.21 ± 0.35 and 3.57 ± 0.16 μM·g−1·h−1, respectively). Insulin stimulation increased glucose uptake in both groups (LCR rats: 5.16 ± 0.21 μM·g−1·min−1, P < 0.0001, and HCR rats: 6.00 ± 0.23 μM·g−1·min−1, P < 0.0001), and this increase was of a greater magnitude in HCR rats (14%, P = 0.02).
Fig. 2.
Basal and insulin-stimulated glucose uptake in the soleus muscles of LCR and HCR rats. Values are means ± SE; N = 11. *P < 0.05, different from the basal value; †P < 0.05 different from the LCR value under the same conditions.
HCR rats are more active than LCR rats.
Physical activity data for each group are shown in Table 2. During the diurnal period, HCR rats completed a greater number of horizontal beam breaks (P = 0.0004), spent more time ambulatory (P = 0.02) and less time stationary (P = 0.02), and traveled a greater total distance (P = 0.04) compared with LCR rats. During the nocturnal phase, HCR rats completed fewer horizontal beam breaks, had fewer stereotypic movements, and spent more time stationary than they did during the diurnal phase. For LCR rats, movement patterns were not different during diurnal or nocturnal testing periods. Compared with LCR rats during the nocturnal period, HCR rats spent more time ambulatory (P = 0.007) and completed a greater number of horizontal beam breaks (P = 0.07). Distance traveled or time spent stationary was not different between phenotypes during the nocturnal monitoring period.
Table 2.
Physical activity levels of animals
| Day |
Night |
|||
|---|---|---|---|---|
| LCR rats | HCR rats | LCR rats | HCR rats | |
| No movement, % | 67.1 ± 0.7‡ | 61.9 ± 1.9*† | 70.9 ± 1.6 | 71.4 ± 2.7‡ |
| Stereotypic movement, % | 22.8 ± 0.6 | 22.3 ± 0.5† | 20.4 ± 1.1‡ | 17.8 ± 1.2*†‡ |
| Ambulatory movement, % | 7.5 ± 0.3‡ | 10.0 ± 0.9* | 7.4 ± 0.4 | 10.6 ± 0.6*† |
| Horizontal beam breaks, counts | 54.8 ± 3.4‡ | 133.6 ± 16.7*† | 49.8 ± 6.5‡ | 76.0 ± 12.4‡ |
| Total distance traveled, cm | 1280.0 ± 79.3‡ | 1721.0 ± 187.9*† | 1185.0 ± 79.4‡ | 1336.0 ± 132.1‡ |
Values are means ± SE; N = 8–12 rats/group.
P < 0.05, different from LCR rats during the day;
P < 0.05, different from LCR rats during the night;
P < 0.05, different from HCR rats during the day.
Mitochondrial respiratory capacity is greater in HCR rats compared with LCR rats.
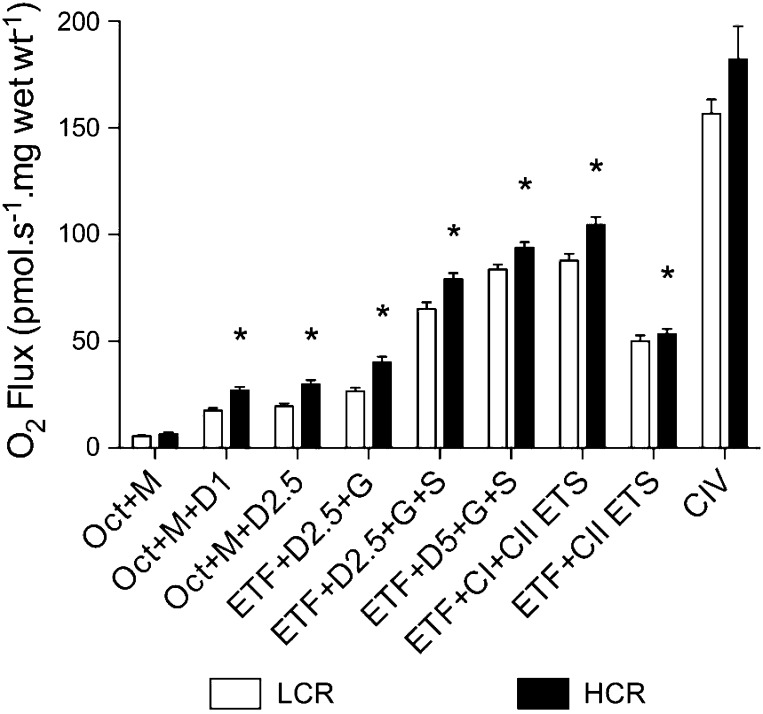
To evaluate the capacity of the ETS and thus determine maximal O2 flux through the mitochondria, we measured O2 flux during a SUIT protocol with substrates for the ETF, CI, CII, and CIV (Fig. 3). State 2 respiration with electron leak through the ETF was not different between groups (LCR rats: 5.55 ± 0.42 pmol·s−1·mg wet wt−1 and HCR rats: 6.61 ± 0.83 pmol·s−1·mg wet wt−1); however, state 3 respiration (OXPHOS) with electron flux through the ETF was greater in HCR rats than in LCR rats at 1 mM ADP (17.6 ± 1.2 and 27.9 ± 1.7 pmol·s−1·mg wet wt−1, respectively) and 2.5 mM ADP (30.68 ± 1.97 and 19.54 ± 1.35 pmol·s−1·mg wet wt−1, respectively). State 3 respiration with electron flux through both the ETF and CI was greater in HCR rats compared with LCR rats (41.27 ± 2.44 and 26.77 ± 1.59 pmol·s−1·mg wet wt−1, respectively), and when flux through the ETF was taken into account, CI values for HCR rats remained elevated (HCR rats: 11.73 ± 2.14 pmol·s−1·mg wet wt−1 and LCR rats: 7.62 ± 0.22 pmol·s−1·mg wet wt−1, P = 0.09). State 3 respiration with electron flux through the ETF, CI, and CII was greater in HCR rats compared with LCR rats at submaximal (80.56 ± 2.79 and 65.29 ± 3.10 pmol·s−1·mg wet wt−1, respectively) and maximal (95.52 ± 2.34 and 83.74 ± 2.34 pmol·s−1·mg wet wt−1, respectively) ADP saturation. Uncoupled OXPHOS with electron flux through the ETF and CII was greater in HCR rats compared with LCR rats (54.70 ± 2.19 and 46.72 ± 1.00 pmol·s−1·mg wet wt−1, respectively, P = 0.007). Maximal uncoupled flux through the ETS (ETF, CI, and CII) was greater in HCR rats compared with LCR rats (106.50 ± 3.54 and 87.86 ± 3.20 pmol·s−1·mg wet wt−1, respectively). CIV activity, as determined through the use of artificial electron donors, was not different between groups (LCR rats: 156.70 ± 6.7 pmol·s−1·mg wet wt−1 and HCR rats: 169.7 ± 10.35 pmol·s−1·mg wet wt−1). To assess O2 flux independent of mitochondrial density, we calculated the flux control ratio for each substrate combination. These are shown in Table 3.
Fig. 3.
Mass-specific O2 consumption in the soleus muscles of LCR and HCR rats. Oct, octanoylcarnitine; M, malate; D1, 1 mM ADP; D22.5, 2.5 mM ADP; ETF, electron-transferring flavoprotein; G, glutamate; S, succinate, D5, 5 mM ADP; ETS, electron transfer system (uncoupled respiration); CIV, respiratory complex IV (cytochrome c oxidase). Values are means ± SE; N = 11. *P < 0.05.
Table 3.
Mitochondrial flux control ratios
| LCR Rats | HCR Rats | |
|---|---|---|
| ETF and leak (state 2) | 0.07 ± 0.01 | 0.06 ± 0.01 |
| ETF and OXPHOS (state 3) | 0.23 ± 0.01 | 0.29 ± 0.02* |
| ETF, CI, and OXPHOS (state 3) | 0.32 ± 0.01 | 0.39 ± 0.2* |
| ETF, CI, CII, and OXPHOS (state 3, submaximal) | 0.77 ± 0.02 | 0.76 ± 0.03 |
| ETF, CI, CII, and OXPHOS (state 3, maximal) | 0.96 ± 0.02 | 0.91 ± 0.03 |
| ETF and CII (uncoupled, state 4) | 0.57 ± 0.01 | 0.51 ± 0.01* |
Values are means ± SE; N = 8–12 rats/group. ETF, electron-transferring flavoprotein; OXPHOS, oxidative phosphorylation; CI, complex I; CII, complex II.
P < 0.05.
Increased O2 flux in muscle from HCR rats is associated with greater mitochondrial enzyme activity and increased mtDNA copy numbers.
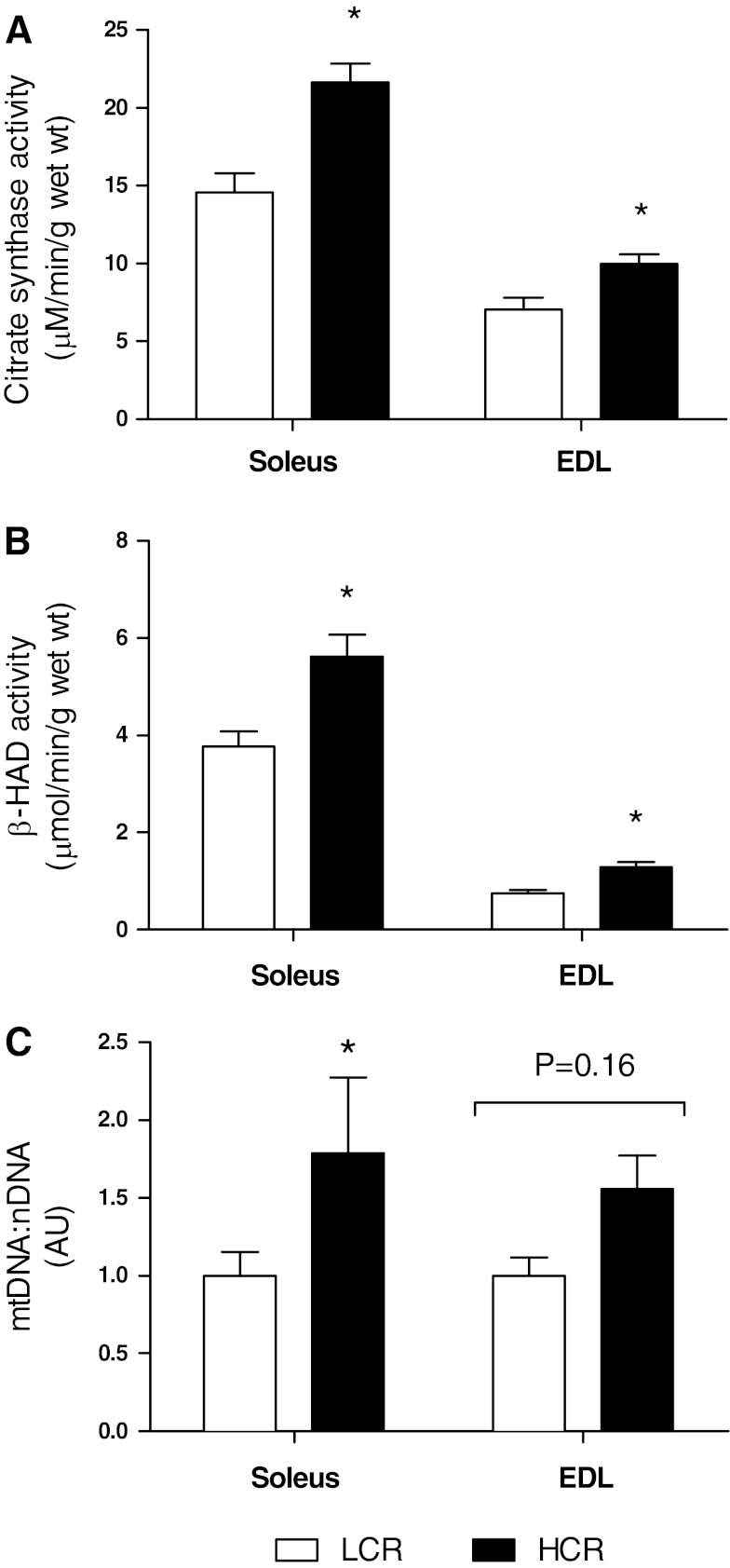
CS activity was greater in HCR rats compared with LCR rats in both the soleus (HCR rats: 21.64 ± 1.21 μmol·min−1·g wet wt−1 and LCR rats: 14.58 ± 1.23 μmol·min−1·g wet wt−1, P = 0.009; Fig. 4A) and EDL (HCR rats: 9.98 ± 0.62 μmol·min−1·g wet wt−1 and LCR rats: 7.06 ± 0.77 μmol·min−1·g wet wt−1, P = 0.01; Fig. 4A) muscles. β-HAD activity was also greater in HCR rats compared with LCR rats in the soleus (HCR rats: 5.62 ± 0.45 μmol·min−1·g wet wt−1 and LCR rats: 3.78 ± 0.31 μmol·min−1·g wet wt−1, P = 0.003; Fig. 4B) and EDL (HCR rats: 1.29 ± 0.11 μmol·min−1·g wet wt−1 and LCR rats: 0.75 ± 0.066 μmol·min−1·g wet wt−1, P = 0.0004; Fig. 4B) muscles. In the EDL, mtDNA copy numbers were elevated in HCR rats compared with LCR rats (11,810 ± 1,640 and 7,584 ± 891, respectively, P = 0.049; Fig. 4C). The mtDNA copy number was 44% greater in the soleus of HCR rats (HCR rats: 11,930 ± 3,235 and LCR rats: 6,665 ± 1,015, P = 0.16; Fig. 4C).
Fig. 4.
Enzyme activities and relative mitochondrial (mt)DNA copy numbers in both the soleus and EDL muscles of LCR and HCR rats. A: citrate synthase (CS) activity (N = 10). B: β-hydroxyacyl-CoA (β-HAD) activity (N = 10). C: mtDNA-to-nuclear (n)DNA ratio [mtDNA:nDNA; in arbitrary units (AU), N = 7]. Values are means ± SE. *P < 0.05.
Muscle-specific protein expression of PGC-1α and FNDC5.
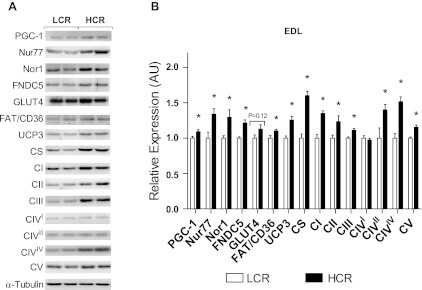
The relative expression of PGC-1α mRNA was not different between groups for either soleus or EDL muscles (data not shown). PGC-1α protein expression was not different in the soleus (Fig. 5), although a small but significant increase was observed in the EDL of HCR rats (9%, P = 0.03; Fig. 6). Expression of the PGC-1α-dependent FNDC5 gene was not different between groups in either muscle type (data not shown). Similarly, FNDC5 protein expression was not different in the soleus (Fig. 5). FNDC5 protein expression was greater in the EDL of HCR rats (17%, P = 0.0002; Fig. 6).
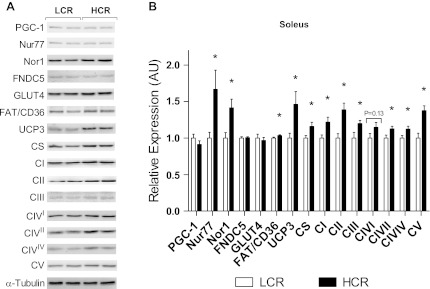
Fig. 5.
Protein content of the soleus muscle from LCR and HCR rats as determined by Western blot analysis. PGC-1, peroxisome proliferator-activated receptor-γ coactivator-1; Nur77, neuron-derived clone 77; Nor1, neuron-derived orphan receptor 1; FNDC5, fibronectin type III domain-containing 5; GLUT4, glucose transporter 4; FAT/CD36, fatty acid translocase/CD36; UCP3, uncoupling protein 3; CI, complex I; CII, complex II; CIII, complex III; CIVI, CIV subunit I; CIVII, CIV subunit II; CIVIV, CIV subunit IV; CV, complex V. Values are means ± SE; N = 8–12. *P < 0.05.
Fig. 6.
Protein content of the EDL muscle from LCR and HCR rats as determined by Western blot analysis. Values are means ± SE; N = 8–12. *P < 0.05.
Nor1 expression is greater in HCR rats.
In both the soleus and EDL muscles, protein expression of the orphan NR Nor1 was greater in HCR rats (soleus: 21%, P = 0.009, and EDL: 23%, P = 0.016; Figs. 5 and 6). Expression of Nur77 (soleus: 40%, P = 0.02, and EDL: 25%, P = 0.01) and NR4A target proteins fatty acid translocase/CD36 (soleus: 4%, P = 0.04, and EDL: 9%, P = 0.02) and UCP3 (soleus: 32%, P = 0.01, and EDL: 20%, P = 0.0003) was also increased in both muscle types from HCR rats (P < 0.05; Figs. 5 and 6).
Mitochondrial protein expression is greater in HCR rats.
In the soleus muscle, expression of all individual ETS complex proteins was greater in HCR rats compared with LCR rats (P < 0.05; Fig. 5) except for CIV subunit I. Additionally, expression of CS was 14% greater in HCR rats compared with LCR rats (P = 0.03; Fig. 5). In the EDL muscle, expression of all individual ETS complexes was greater in HCR rats compared with LCR rats (P < 0.05; Fig. 6) except for CIV subunit I, which was not different. CS protein expression was 38% greater in HCR rats (P < 0.0001; Fig. 6).
DISCUSSION
The motivation for developing the LCR/HCR model system originated from noting the strong statistical association between low endurance exercise capacity and increased morbidity and mortality in humans (4). From this, we formulated the general hypothesis that aerobic energy metabolism is a central mechanistic determinant of the divide between disease and health, which we termed the “aerobic hypothesis.” As an unbiased test of this hypothesis, we applied divergent artificial selection for intrinsic LCR and HCR. As predicted by this hypothesis, disease risks and reduced longevity segregated strongly with low aerobic capacity (14). Here, we provide specification for features that can explain the intrinsic aerobic exercise capacity differences between the LCR and HCR and, ostensibly, disease risks.
Alterations to mitochondrial function typically occur in parallel with disease progression, and although environmental influences undoubtedly play an important role in the development of metabolic diseases (6), there is a growing body of evidence to suggest that a substantial genetic component underlies many complex metabolic disorders (22, 29). Here, we present data showing that skeletal muscle respiratory capacity is greater in oxidative muscle from HCR rats compared with LCR rats (Fig. 3) and that this enhanced respiratory capacity is likely due to greater relative mitochondrial enzyme activities [particularly those with a role in fat oxidation (Figs. 3 and 4)] coupled with increased mitochondrial number (Figs. 4–6). This increase in muscle oxidative capacity is associated with an increase in the protein expression of the NR Nor1 (Figs. 5 and 6), which has previously been linked to the adaptive response of skeletal muscle in response to exercise training, (21, 50) and the expression of several proteins involved in glucose metabolism (4, 16, 31, 33). These novel findings complement our previous reports in which we have shown that there are a greater number of mitochondria present in the glycolytic muscle from HCR rats (35) and that muscle substrate handling is superior in these animals (15–17).
Low aerobic capacity, independent of physical activity levels, is a key predictor of early mortality (23, 47). Along with a reduced lifespan (14), LCR rats express a number of characteristics common to metabolic disease phenotypes, such as increased body weight and adiposity (Table 1) (10, 24, 25, 39), hyperinsulinemia (Table 1) (25, 40, 48), and impaired glucose tolerance (Fig. 1) (24, 25, 35). In contrast, HCR rats live 6–8 mo longer (14) and present with superior metabolic health, as characterized by resistance to weight gain (24–26) and an increased capacity for the uptake and oxidation of both glucose (Fig. 2) (17, 35) and long-chain fatty acids (17, 24, 25, 35). The differential expression of these characteristics appears to be linked to muscle oxidative capacity (25), although previous studies have been inconclusive with regard to the precise mechanisms involved. As noted, HCR rats have superior O2 handling capacity, an adaptive variation that resides at the level of the muscle (10). We (35, 48) have previously reported a greater number of mitochondria in the muscle of HCR rats, although results from previous studies have not supported this observation (24, 45) and have concluded that the difference in oxidative capacity is not due to mitochondrial size or number (24, 41, 45) but to a difference in mitochondrial enzyme activity.
To address this issue, we performed respirometry experiments, monitoring O2 consumption through the mitochondria of permeabilized soleus muscles from LCR and HCR rats in response to specific substrate combinations. In contrast to individual enzyme activity assays, this is a dynamic measurement that reflects the integrated activities of β-oxidation, the TCA, cycle and the ETS. During ADP-stimulated (state 3) respiration, we observed greater O2 consumption with electron flux through the ETF, CI, and CII. Additionally, maximal activity of the ETS was greater in muscles from HCR rats. Previously, we have shown that the oxidation of the long-chain fatty acid palmitate is greater in in vitro soleus muscle incubations from HCR rats (17) and during hindlimb perfusion experiments (35). Here, we confirmed that the capacity for total lipid oxidation is enhanced in the muscle of HCR rats and that this is due to the increased activity of mitochondrial enzymes involved in the oxidation of fatty acids. Specifically, respiratory flux control ratios indicated that independent of mitochondrial volume, the relative contribution of the ETF to maximal flux was greater in HCR rats when the muscle was exposed to the medium-chain fatty acid octanoylcarnitine (Table 3). Furthermore, β-HAD activity (the third enzyme of the β-oxidation pathway) was greater in muscles from HCR rats (Fig. 4B). In contrast, when data for the remaining substrate combinations were normalized to maximal uncoupled respiratory capacity, combined O2 flux was not different, which suggests that the coupling of OXPHOS and CI and CII activity are similar for LCR and HCR rats. This indicates that along with an increased capacity for fat oxidation per mitochondrion, an increase in the mitochondrial reticulum is also likely to contribute to the increased respiratory capacity of muscles from HCR rats. Since respirometry and enzyme activity measurements are indirect determinants of mitochondrial content that must be supported by additional measurements, we also measured mtDNA copy numbers (Fig. 4C) and the expression of a number of mitochondrial proteins (Figs. 5 and 6) in both soleus and EDL muscles. We found that the mtDNA copy number was greater in both muscles under investigation and, moreover, that the expression of a number of mitochondrial proteins was increased in both muscle types, supporting our hypothesis of a greater mitochondrial volume in the muscle of HCR rats. Furthermore, the TCA cycle protein CS, which has long been regarded as a surrogate marker of mitochondrial volume, was expressed to a greater degree (Figs. 5 and 6) along with elevated maximal activities (Fig. 4A) in both the soleus and EDL muscles of HCR rats. This difference in skeletal muscle respiratory capacity is no doubt a major contributing factor to the overall phenotype of these rats. The greater capacity for lipid oxidation observed in HCR rats is likely to result in the sparing of glycogen during exercise. Indeed, even in the untrained state, HCR rats exhibit muscle substrate storage profiles similar to that of endurance-trained athletes. Increased fuel availability as a result of greater intramuscular storage of glycogen and triglycerides (16, 17, 35), along with an increased capacity for lipid oxidation and overall enhanced rates of OXPHOS, would allow HCR rats to run longer distances than LCR rats.
Another finding of the present study is the elevated protein levels of FNDC5, which was confined to the glycolytic muscle of HCR rats. This protein has recently been identified as a PGC-1α-dependent membrane protein that is cleaved to become the putative myokine irisin (3). Although we were unable to measure circulating irisin concentrations, the reduced adiposity and superior glucose tolerance exhibited by HCR rats may, at least in part, be related to the increased expression of FNDC5 in the glycolytic muscle. Indeed, exercise training has been shown to increase the concentration of irisin in both mice and humans (3), so it is quite plausible that HCR rats have higher concentrations of circulating irisin than LCR rats. Furthermore, the “browning” effect seen in white adipose tissue after irisin administration (3) may not be restricted to this tissue, and we cannot discount the possibility that irisin elicits a similar effect on glycolytic skeletal muscle. Characterization of the adipose tissue from LCR and HCR rats will determine whether differences in mitochondrial function are restricted to the muscle or if they are also present in other highly plastic tissues.
Previously, we (16) have shown that the orphan NR Nur77 and its downstream targets are expressed to a lesser extent in LCR rats and that this is linked to impaired β-adrenergic signaling in the skeletal muscle. Another NR4A NR, Nor1, shares a high sequence homology with Nur77 and has been shown to be induced under many of the same conditions (31). Pearen et al. (32) demonstrated that Nor1 is essential for oxidative metabolism in cultured myotubes and that muscle-specific Nor1 transgenic mice have a more oxidative skeletal muscle phenotype, a greater running capacity, and better glucose tolerance compared with their wild-type littermates (30). Here, we provide evidence showing that HCR rats have greater Nor1 protein levels in both the soleus and EDL muscles (Figs. 5 and 6) and suggest that Nor1 may serve as a possible mechanism for increasing the cell's oxidative machinery. In support of a possible Nor1-mediated response, we found no differences in PGC-1α mRNA expression between phenotypes. Furthermore, little (in the EDL; Fig. 6) or no difference (in the soleus; Fig. 5) in PGC-1 protein levels was observed, suggesting that an alternative program is responsible for the greater respiratory capacity seen in the muscle of HCR rats. At present, little is known about the molecular mechanisms through which Nor1 is likely to drive changes in oxidative metabolism. Thus, more indepth analyses of the function of Nor1 and clarification of its primary targets will be essential in determining its precise role in the regulation of skeletal muscle metabolism.
Physical training increases skeletal muscle insulin sensitivity and oxidative capacity and induces a rapid increase in Nor1 transcription (21, 46, 50). Although both LCR and HCR animals were kept under identical housing and feeding regimens that excluded formal exercise training, we cannot discount the possibility that habitual activity levels may play a role in the development of their distinct phenotypes. Therefore, we monitored the activity of the rats during both diurnal and nocturnal periods and found that HCR rats were consistently more active than LCR rats (Table 2). This observation is in agreement with others (26, 27, 40) who report differences in the habitual activity levels of LCR and HCR rats from earlier generations. Furthermore, Novak et al. (28) identified different activity levels in another strain of obesity-resistant rats, whereas others (7, 19) identified a type of “inactivity physiology” in humans. Since there is a link between intrinsic exercise capacity and daily habitual activity in rodents, and a link between inactivity and poor metabolic health in humans (7, 11, 18, 27), the extent to which spontaneous cage activity contributes to the distinct phenotypes of these rodent models is an important question that needs to be addressed. Although controlling for habitual activity is difficult, we propose that it is partially responsible for the divergent metabolic phenotype displayed by these two rat strains, as we (9, 17) have previously shown that exercise training of LCR rats reverses many of negative metabolic health traits observed in these animals.
In summary, we used an animal model in which artificial selection for HCR and LCR (in the total absence of exercise training) simultaneously controls for unknown environmental influences and allows the two phenotypes to act as controls for one another. In addition to their divergent running capacities, these rats displayed differences in a number of metabolic health traits and, importantly, markedly different muscle oxidative capacities that underlie the major differences between phenotypes. Given that the NR Nor1 has been shown to play a key role in metabolic regulation and is necessary for oxidative metabolism, we propose that the differential expression of Nor1 observed in HCR and LCR rats may contribute to their distinct phenotypes and overall metabolic regulation. “Gain/loss of function” experiments will be required to confirm this hypothesis.
GRANTS
This work was funded, in part, by Australian Heart Foundation Grant-In-Aid G09M4348 (to J. A. Hawley). The LCR-HCR rat model system was funded by National Institutes of Health (NIH) Grant R24-RR-017718 (to L.G. Koch and S. L. Britton) and is currently supported by National Institutes of Health Grant ROD012098A (to L. G. Koch and S. L. Britton). Additional support was provided by NIH Grant RO1-DK-077200 (to S. L. Britton).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.J.S., N.K.S., L.G.K., S.L.B., and J.A.H. conception and design of research; E.J.S. performed experiments; E.J.S. analyzed data; E.J.S. interpreted results of experiments; E.J.S. prepared figures; E.J.S. drafted manuscript; E.J.S., N.K.S., L.G.K., S.L.B., and J.A.H. edited and revised manuscript; E.J.S., N.K.S., L.G.K., S.L.B., and J.A.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge Molly Kalahar and Lori Gilligan for expert care of the rat colony and Cesare Granata, Christopher Hedges, and Fabio Serpiello for providing technical support for the respirometry experiments.
The LCR and HCR model can be made available for collaborative study (contact brittons@umich.edu or lgkoch@umich.edu).
REFERENCES
- 1. Armstrong RB, Laughlin H. Blood flows within and among rat muscles as a function of time during high speed treadmill exercise. J Physiol 344: 189–208, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bergmeyer HU. Methods of Enzymatic Analysis. Weinheim: Verlag Chemie, 1974 [Google Scholar]
- 3. Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481: 463–468, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Briand O, Helleboid-Chapman A, Ploton M, Hennuyer N, Carpentier R, Pattou F, Vandewalle B, Moerman E, Gmyr V, Kerr-Conte J, Eeckhoute J, Staels B, Lefebvre P. The nuclear orphan receptor Nur77 is a lipotoxicity sensor regulating glucose-induced insulin secretion in pancreatic beta-cells. Mol Endocrinol 26: 399–413, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bruce CR, Anderson MJ, Carey AL, Newman DG, Bonen A, Kriketos AD, Cooney GJ, Hawley JA. Muscle oxidative capacity is a better predictor of insulin sensitivity than lipid status. J Clin Endocrinol Metab 88: 5444–5451, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Hahn SH, Kerfoot S, Vasta V. Assay to measure oxidized and reduced forms of CoQ by LC-MS/MS. Methods Mol Biol 837: 169–179, 2012 [DOI] [PubMed] [Google Scholar]
- 7. Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 56: 2655–2667, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Hansen PA, Gulve EA, Holloszy JO. Suitability of 2-deoxyglucose for in vitro measurement of glucose transport activity in skeletal muscle. J Appl Physiol 76: 979–985, 1994 [DOI] [PubMed] [Google Scholar]
- 9. Haram PM, Kemi OJ, Lee SJ, Bendheim MO, Al-Share QY, Waldum HL, Gilligan LJ, Koch LG, Britton SL, Najjar SM, Wisloff U. Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity. Cardiovasc Res 81: 723–732, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Howlett RA, Gonzalez NC, Wagner HE, Fu Z, Britton SL, Koch LG, Wagner PD. Selected contribution: skeletal muscle capillarity and enzyme activity in rats selectively bred for running endurance. J Appl Physiol 94: 1682–1688, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Johannsen DL, Welk GJ, Sharp RL, Flakoll PJ. Differences in daily energy expenditure in lean and obese women: the role of posture allocation. Obesity (Silver Spring) 16: 34–39, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics 5: 45–52, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Koch LG, Britton SL. Divergent selection for aerobic capacity in rats as a model for complex disease. Integr Comp Biol 45: 405–415, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Koch LG, Kemi OJ, Qi N, Leng SX, Bijma P, Gilligan LJ, Wilkinson JE, Wisloff H, Hoydal MA, Rolim N, Abadir PM, van Grevenhof EM, Smith GL, Burant CF, Ellingsen O, Britton SL, Wisloff U. Intrinsic aerobic capacity sets a divide for aging and longevity. Circ Res 109: 1162–1172, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lessard SJ, Rivas DA, Chen ZP, Bonen A, Febbraio MA, Reeder DW, Kemp BE, Yaspelkis BB, 3rd, Hawley JA. Tissue-specific effects of rosiglitazone and exercise in the treatment of lipid-induced insulin resistance. Diabetes 56: 1856–1864, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Lessard SJ, Rivas DA, Chen ZP, van Denderen BJ, Watt MJ, Koch LG, Britton SL, Kemp BE, Hawley JA. Impaired skeletal muscle beta-adrenergic activation and lipolysis are associated with whole-body insulin resistance in rats bred for low intrinsic exercise capacity. Endocrinology 150: 4883–4891, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lessard SJ, Rivas DA, Stephenson EJ, Yaspelkis BB, 3rd, Koch LG, Britton SL, Hawley JA. Exercise training reverses impaired skeletal muscle metabolism induced by artificial selection for low aerobic capacity. Am J Physiol Regul Integr Comp Physiol 300: R175–R182, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, Jensen MD, Clark MM. Interindividual variation in posture allocation: possible role in human obesity. Science 307: 584–586, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Levine JA, Miller JM. The energy expenditure of using a “walk-and-work” desk for office workers with obesity. Br J Sports Med 41: 558–561, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Mahoney DJ, Parise G, Melov S, Safdar A, Tarnopolsky MA. Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J 19: 1498–1500, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34: 267–273, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 346: 793–801, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Naples SP, Borengasser SJ, Rector RS, Uptergrove GM, Morris EM, Mikus CR, Koch LG, Britton SL, Ibdah JA, Thyfault JP. Skeletal muscle mitochondrial and metabolic responses to a high-fat diet in female rats bred for high and low aerobic capacity. Appl Physiol Nutr Metab 35: 151–162, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Noland RC, Thyfault JP, Henes ST, Whitfield BR, Woodlief TL, Evans JR, Lust JA, Britton SL, Koch LG, Dudek RW, Dohm GL, Cortright RN, Lust RM. Artificial selection for high-capacity endurance running is protective against high-fat diet-induced insulin resistance. Am J Physiol Endocrinol Metab 293: E31–E41, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Novak CM, Escande C, Burghardt PR, Zhang M, Barbosa MT, Chini EN, Britton SL, Koch LG, Akil H, Levine JA. Spontaneous activity, economy of activity, and resistance to diet-induced obesity in rats bred for high intrinsic aerobic capacity. Horm Behav 58: 355–367, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Novak CM, Escande C, Gerber SM, Chini EN, Zhang M, Britton SL, Koch LG, Levine JA. Endurance capacity, not body size, determines physical activity levels: role of skeletal muscle PEPCK. PLoS One 4: e5869, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Novak CM, Kotz CM, Levine JA. Central orexin sensitivity, physical activity, and obesity in diet-induced obese and diet-resistant rats. Am J Physiol Endocrinol Metab 290: E396–E403, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100: 8466–8471, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pearen MA, Eriksson NA, Fitzsimmons RL, Goode JM, Martel N, Andrikopoulos S, Muscat GE. The nuclear receptor, Nor-1, markedly increases type II oxidative muscle fibers and resistance to fatigue. Mol Endocrinol 26: 372–384, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pearen MA, Muscat GE. Minireview: Nuclear hormone receptor 4A signaling: implications for metabolic disease. Mol Endocrinol 24: 1891–1903, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pearen MA, Myers SA, Raichur S, Ryall JG, Lynch GS, Muscat GE. The orphan nuclear receptor, NOR-1, a target of β-adrenergic signaling, regulates gene expression that controls oxidative metabolism in skeletal muscle. Endocrinology 149: 2853–2865, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Pei L, Waki H, Vaitheesvaran B, Wilpitz DC, Kurland IJ, Tontonoz P. NR4A orphan nuclear receptors are transcriptional regulators of hepatic glucose metabolism. Nat Med 12: 1048–1055, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Pesta D, Gnaiger E. High-resolution respirometry. OXPHOS protocols for human cells and permeabilized fibres from small biopisies of human muscle. In: Mitochondrial Bioenergetics: Methods and Protocols, edited by Palmeira C, Moreno A. New York: Humana, 2011, p. 25–58 [DOI] [PubMed] [Google Scholar]
- 35. Rivas DA, Lessard SJ, Saito M, Friedhuber AM, Koch LG, Britton SL, Yaspelkis BB, 3rd, Hawley JA. Low intrinsic running capacity is associated with reduced skeletal muscle substrate oxidation and lower mitochondrial content in white skeletal muscle. Am J Physiol Regul Integr Comp Physiol 300: R835–R843, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Skladal D, Sperl W, Schranzhofer R, Krismer M, Gnaiger E, Margreiter R, Gellerich FN. Preservation of mitochondrial functions in human skeletal muscle during storage in high energy preservation solution (HEPS). In: What Is Controlling Life? Modern Trends in BioThermoKinetics 3, edited by Gnaiger E, Gellerich FN, Wyss M. Innsbruck: Innsbruck Univ. Press, 1994, p. 268–271 [Google Scholar]
- 37. Srere PA. Citrate synthase. In: Methods in Enzymology. -13: Citric Acid Cycle, edited by Lowenstein JM. Waltham, MA: Academic, 1969, p. 3–11 [Google Scholar]
- 38. Stephenson EJ, Camera DM, Jenkins TA, Lee JS, Kosari S, Hawley JA, Stepto NK. Skeletal muscle respiratory capacity is enhanced in rats consuming an obesogenic Western diet. Am J Physiol Endocrinol Metab 302: E1541–E1549, 2012 [DOI] [PubMed] [Google Scholar]
- 39. Swallow JG, Wroblewska AK, Waters RP, Renner KJ, Britton SL, Koch LG. Phenotypic and evolutionary plasticity of body composition in rats selectively bred for high endurance capacity. J Appl Physiol 109: 778–785, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thyfault JP, Rector RS, Uptergrove GM, Borengasser SJ, Morris EM, Wei Y, Laye MJ, Burant CF, Qi NR, Ridenhour SE, Koch LG, Britton SL, Ibdah JA. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol 587: 1805–1816, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tweedie C, Romestaing C, Burelle Y, Safdar A, Tarnopolsky MA, Seadon S, Britton SL, Koch LG, Hepple RT. Lower oxidative DNA damage despite greater ROS production in muscles from rats selectively bred for high running capacity. Am J Physiol Regul Integr Comp Physiol 300: R544–R553, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Veksler VI, Kuznetsov AV, Sharov VG, Kapelko VI, Saks VA. Mitochondrial respiratory parameters in cardiac tissue: a novel method of assessment by using saponin-skinned fibers. Biochim Biophys Acta 892: 191–196, 1987 [DOI] [PubMed] [Google Scholar]
- 43. Venegas V, Halberg MC. Measurement of mitochondrial DNA copy number. Methods Mol Biol 837: 327–335, 2012 [DOI] [PubMed] [Google Scholar]
- 44. Viollet B, Andreelli F, Jorgensen SB, Perrin C, Geloen A, Flamez D, Mu J, Lenzner C, Baud O, Bennoun M, Gomas E, Nicolas G, Wojtaszewski JF, Kahn A, Carling D, Schuit FC, Birnbaum MJ, Richter EA, Burcelin R, Vaulont S. The AMP-activated protein kinase α2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest 111: 91–98, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Walsh B, Hooks RB, Hornyak JE, Koch LG, Britton SL, Hogan MC. Enhanced mitochondrial sensitivity to creatine in rats bred for high aerobic capacity. J Appl Physiol 100: 1765–1769, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Wang J, Rakhade M. Utility of array CGH in molecular diagnosis of mitochondrial disorders. Methods Mol Biol 837: 301–312, 2012 [DOI] [PubMed] [Google Scholar]
- 47. Wei M, Kampert JB, Barlow CE, Nichaman MZ, Gibbons LW, Paffenbarger JRS, Blair SN. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA 282: 1547–1553, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernstrom M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science 307: 418–420, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Young DA, Uhl JJ, Cartee GD, Holloszy JO. Activation of glucose transport in muscle by prolonged exposure to insulin. Effects of glucose and insulin concentrations. J Biol Chem 261: 16049–16053, 1986 [PubMed] [Google Scholar]
- 50. Zambon AC, McDearmon EL, Salomonis N, Vranizan KM, Johansen KL, Adey D, Takahashi JS, Schambelan M, Conklin BR. Time- and exercise-dependent gene regulation in human skeletal muscle. Genome Biol 4: R61, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]