Abstract
To examine potential age-specific adaptations in skeletal muscle size and myofiber contractile physiology in response to aerobic exercise, seven young (YM; 20 ± 1 yr) and six older men (OM; 74 ± 3 yr) performed 12 wk of cycle ergometer training. Muscle biopsies were obtained from the vastus lateralis to determine size and contractile properties of isolated slow [myosin heavy chain (MHC) I] and fast (MHC IIa) myofibers, MHC composition, and muscle protein concentration. Aerobic capacity was higher (P < 0.05) after training in both YM (16 ± 2%) and OM (13 ± 3%). Quadriceps muscle volume, determined via MRI, was 5 ± 1 and 6 ± 1% greater (P < 0.05) after training for YM and OM, respectively, which was associated with an increase in MHC I myofiber cross-sectional area (CSA), independent of age. MHC I peak power was higher (P < 0.05) after training for both YM and OM, while MHC IIa peak power was increased (P < 0.05) with training in OM only. MHC I and MHC IIa myofiber peak and normalized (peak force/CSA) force were preserved with training in OM, while MHC I peak force/CSA and MHC IIa peak force were lower (P < 0.05) after training in YM. The age-dependent adaptations in myofiber function were not due to changes in protein content, as total muscle protein and myofibrillar protein concentration were unchanged (P > 0.05) with training. Training reduced (P < 0.05) the proportion of MHC IIx isoform, independent of age, whereas no other changes in MHC composition were observed. These data suggest relative improvements in muscle size and aerobic capacity are similar between YM and OM, while adaptations in myofiber contractile function showed a general improvement in OM. Training-related increases in MHC I and MHC IIa peak power reveal that skeletal muscle of OM is responsive to aerobic exercise training and further support the use of aerobic exercise for improving cardiovascular and skeletal muscle health in older individuals.
Keywords: sarcopenia, physical activity, elderly, endurance, V̇o2 max
primary aging is associated with reduced functional capacity, which is likely attributed to deterioration of aerobic capacity and loss of muscle mass. Physical activity is routinely recommended to attenuate the loss in physical function with aging; therefore, the development of efficient and effective exercise interventions is of high importance. While the benefits of aerobic exercise on cardiovascular and metabolic health for older adults have been well described (6), the influence of this exercise mode on skeletal muscle mass and function is less understood. Reports on the influence of aerobic exercise training on muscle mass in older adults have been equivocal, with some finding no influence (24, 45, 46, 52), but several others observing significant whole muscle hypertrophy (19, 27, 29, 42). The discrepancy in the literature is likely due to variations in exercise program characteristics and subject populations.
Our laboratory recently reported that 12 wk of aerobic exercise training improved aerobic capacity and drastically increased whole muscle mass in older women (19, 27). These robust adaptations were associated with improved whole muscle power production, myosin heavy chain (MHC) I myofiber contractile function, and an increase in MHC I protein composition (19, 27, 28). The alterations in myofiber contractile physiology were consistent with previous reports in young subjects after aerobic training (50), aerobically trained athletes (17), and run-trained rodents (41); however, the observed change in myofiber contractile properties in concert with whole muscle hypertrophy was unique. These findings revealed that a single-mode exercise program (i.e., cycle ergometry) significantly improved aerobic capacity and muscle mass, marking it as an efficient exercise intervention for cardiovascular and muscle health. However, because these studies were conducted only in older women, it cannot be determined if these responses are age and/or sex specific. Therefore, the purpose of the present investigation was to examine the influence of aerobic exercise training on whole muscle and single myofiber size and contractile function in healthy, untrained young (YM) and older men (OM).
MATERIALS AND METHODS
Subjects
Thirteen men, seven YM and six OM, volunteered to participate in this investigation (Table 1). Each young subject provided a detailed medical history, and each older subject underwent a thorough physical examination, which included a blood chemistry profile, pulmonary function test, and resting and exercise electrocardiograms. Subjects were excluded based on the following criteria: 1) body mass index ≥28 kg/m2; 2) type 1 or type 2 diabetes; 3) uncontrolled hypertension; 4) active cancer, cancer in remission, or having received treatment for any form of cancer in the previous 5 yr; 5) coronary artery disease; 6), cardiovascular disease (e.g., peripheral arterial disease, peripheral vascular disease); 7) abnormal thyroid function; 8) engaged in regular aerobic or resistance exercise more than one time per week for 20 min or longer during the previous year; 9) chronic and/or regular nonsteroidal anti-inflammatory drug consumption; and 10) any condition that presents a limitation to exercise training (e.g., severe arthritis, chronic obstructive pulmonary disease, neuromuscular disorder, moderate or severe cognitive impairment, Alzheimer's disease, vertigo, dizziness). Four OM were on cholesterol-lowering medications (i.e., statins), three were on blood pressure medications (non-β-blocker), and five were on medications for prostate health. Written, informed consent was obtained from each subject for all procedures following approval by the Institutional Review Board of Ball State University.
Table 1.
Subject characteristics, physical performance, and whole muscle size and function before and after 12 wk of aerobic training in young and older men
| Young Men (n = 7) |
Older Men (n = 6) |
|||||
|---|---|---|---|---|---|---|
| Pre | Post | %Δ | Pre | Post | %Δ | |
| Subject characteristics | ||||||
| Age‡, yr | 20 ± 1 | 74 ± 3 | ||||
| Body weight, kg | 85 ± 4 | 84 ± 4 | N/A | 82 ± 4 | 81 ± 1 | N/A |
| Graded exercise test | ||||||
| Aerobic capacity†‡#, l/min | 3.4 ± 0.2 | 3.9 ± 0.2 | 16 ± 2 | 1.8 ± 0.1 | 2.0 ± 0.1 | 13 ± 3 |
| Maximum heart rate‡, beats/min | 199 ± 1 | 194 ± 2 | N/A | 146 ± 5 | 149 ± 7 | N/A |
| Peak workload†‡, W | 261 ± 12 | 313 ± 13 | 20 ± 2 | 143 ± 6 | 172 ± 9 | 21 ± 6 |
| Whole muscle | ||||||
| Quadriceps volume†, cm3 | 1,052 ± 62 | 1,101 ± 64 | 5 ± 1 | 930 ± 28 | 984 ± 24 | 6 ± 1 |
| Knee extensor power‡, W | 925 ± 74 | 955 ± 81 | N/A | 509 ± 45 | 522 ± 57 | N/A |
Values are means ± SE; n, no. of subjects. Pre, before training; Post, after training; Δ, change; N/A, not applicable. P < 0.05, main effect for
time,
group, and
interaction.
General Study Design
Eligible volunteers underwent a series of baseline measurements for the determination of aerobic capacity using a graded exercise test on a cycle ergometer, whole muscle volume assessed with magnetic resonance imaging, whole muscle function (i.e., power) of the knee extensor muscle group, and a muscle biopsy procedure for the assessment of single-muscle fiber size, contractile mechanics, and protein morphology. Upon completion of the baseline measurements, subjects performed 12 wk of progressive aerobic exercise training. Following the training intervention, all subjects repeated the testing procedures that occurred at baseline.
Experimental Procedures
Aerobic capacity/graded exercise test.
Subjects performed a graded exercise test for the assessment of aerobic power (V̇o2max) before and after the 12-wk aerobic training intervention. Older subjects performed the physician supervised test on an electronically braked cycle ergometer (SensorMedics Ergometrics 800) beginning at a very low workload (∼10 W), and the workload was progressively increased 10 W in 1-min stages until exhaustion with a total test time of 10–12 min. During the test, subject's heart rate, blood pressure, rating of perceived exertion, and electrocardiogram were monitored, and ventilation and expired air samples were measured by a metabolic cart (TrueOne 2400 Metabolic System, ParvoMedics) for the determination of O2 uptake.
Young subjects performed the test on an electronically braked cycle ergometer (Velotron; RacerMate, Seattle, WA). During the test, subjects performed a warm-up consisting of 2 min at 50–100 W and 2 min at 100–150 W. Thereafter, the workload was increased in 25- to 50-W increments every 2 min until respiratory exchange ratio reached ∼1.0. Subsequent workloads were increased 25 W every minute until exhaustion. During the test, subjects′ perceived exertion and heart rate were recorded and respiratory gases were measured with gas analyzers (Applied Electrochemistry, Ameteck S3A and CD3A).
Whole muscle volume/magnetic resonance imaging.
Proton magnetic resonance images of the thigh were measured before and after the 12-wk aerobic training intervention using a Siemen's Symphony 1.5-T imaging system at standard settings (repetition time/echo time = 2,000/9 ms) as our laboratory has previously described (19, 27). Bilateral scans were obtained after 1 h of supine rest to avoid the influence of potential fluid shifts (1). Subjects were positioned with an adjustable foot restraint for fixation of joint angles and thus muscle lengths. Contiguous, 1-cm interleaved serial scans were obtained from the head of femur to the articular surface of the femur. Magnetic resonance images were electronically transferred to a personal computer (Macintosh Power PC) and analyzed with National Institutes of Health Image software (version 1.60) using manual planimetry. Average muscle cross-sectional area (CSA) was taken as the average of each slice from the first distal image containing m. rectus femoris and the last proximal image not containing the gluteal muscle. The average CSA (cm2) was taken as the average of all analyzed slices for the vastii (vastus lateralis, vastus medialis, vastus intermedius) and rectus femoris and summed for total quadriceps femoris. Muscle volume (cm3) was calculated by multiplying the CSA by the slice thickness (8 mm) for all analyzed images. The same investigator made all measurements in a blinded fashion.
Skeletal muscle function.
Peak power of the knee extensor muscle group was assessed before and after the 12-wk aerobic training intervention using an inertial ergometer (Inertial Technology) connected to a strain gauge load cell and potentiometer interfaced with a personal computer (Gateway E-4200) (19, 27). Following multiple orientation sessions to become familiar with the knee extensor device, subjects performed three identical sessions separated by at least 2 days. Before any testing, subjects performed a 5-min warm-up on a stationary bicycle at a self-selected intensity, as well as stretching exercises of the lower limbs followed by small loads on the resistance apparatus. Subjects completed three maximal attempts with 3-min rest between attempts. The highest concentric power output recorded throughout the full range of motion was used for data analysis.
Muscle biopsy procedure.
Percutaneous needle biopsies were obtained from the vastus lateralis muscle according to the methods of Bergstrom (2) before and after the 12-wk aerobic training intervention. The muscle specimen was immediately divided into longitudinal sections, dissected free of adipose and connective tissue, frozen in liquid nitrogen, or processed for single muscle fiber physiology, as described below. Posttraining muscle biopsies were obtained 48 h after the last exercise session.
Aerobic exercise training protocol.
Subjects performed 12 wk of aerobic exercise training on cycle ergometer (Monark Ergomedic 828E). Exercise intensity was standardized among subjects, relative to their maximal aerobic capacity, by utilizing the heart rate reserve method, as our laboratory has previously described (19). The ergometer workload was adjusted in order for subjects to maintain target heart rate, and subjects exercised at an average pedal frequency between 65 and 85 revolutions/min (RPMs). A total of 42 exercise sessions were performed. Duration (20–45 min), intensity (60–80% heart rate reserve), and frequency (3–4 sessions/wk) of exercise were progressively increased throughout the 12 wk to optimize the training response. The last 5 wk of the exercise program consisted of four 45-min sessions at 80% intensity per week. The training program was identical to the protocol our laboratory has previously used in older subjects (19, 27, 28), and a detailed outline of the training program has been previously published (28). Each exercise session was monitored in its entirety by a member of the investigative team to ensure that the prescribed exercise intensity and duration were obtained. Additionally, subject's body weight was measured and recorded before each exercise session (3–4 times/wk), and subjects were counseled, if necessary, to make modifications in dietary intake to maintain body weight.
Single-fiber physiology studies.
A bundle of muscle fibers was placed in cold skinning solution for the determination of contractile properties. The skinning solution contained the following (in mM): 125 potassium propionate, 2.0 EGTA, 4.0 ATP, 1.0 MgCl2, 20.0 imidazole (pH 7.0), and 50% (vol/vol) glycerol. Fibers were kept in this solution for a minimum of 1 day, but not longer than 4 wk (25). Individual muscle fibers were analyzed for diameter, peak force (Po), shortening velocity (Vo), and power characteristics. Detailed descriptions and illustrations of these procedures have been presented in our laboratory's previous work (48, 49, 51).
SINGLE FIBER SIZE.
A video camera (Sony CCD-IRIS, DXC-107A) connected to the microscope and interfaced to a computer allowed viewing on a computer monitor and storage of the digitized images of the muscle fibers during the experiment. Fiber diameter was determined from a captured computer image taken with the fiber briefly suspended in air (<3 s). Fiber width (diameter) was determined at three points along the length of the captured computer image using public domain software (National Institutes of Health Image version 1.61) and averaged to provide a mean diameter measurement. Fiber CSA was determined from the fiber diameter with the assumption that the fiber forms a circular shape while suspended in air, as we have previously done (35, 47–49, 51).
FORCE DETERMINATION (Po).
Resting force was monitored with the fiber in pCa 9.0 solution, and then the fiber was maximally activated in pCa 4.5 solution. Po in milliNewtons was determined in each fiber by computer subtraction of the force baseline from the peak in the pCa 4.5 solution. Normalized force (Po/CSA) was determined from the relationship of Po and fiber CSA.
UNLOADED CONTRACTION VELOCITY DETERMINATION (Vo).
Fiber Vo was measured by the slack test technique, as described by Edman (11). Briefly, the fiber was brought to peak tension and then rapidly shortened so that tension returned to baseline. The time between the onset of slack and redevelopment of tension (i.e., period of unloaded shortening) was measured by computer analyses. Four different slack distances (each <15% of FL) were used for each fiber and the slack length was plotted as a function of the duration of unloaded shortening. Velocity [fiber length (FL)/s] was calculated by dividing the slope of the fitted line by the fiber segment length, and the data were normalized to a sarcomere length of 2.50 μm.
POWER DETERMINATION.
Submaximal isotonic load clamps were performed on each fiber for determination of force-power parameters. Each fiber segment was fully activated and subjected to a series of three isotonic load steps, resulting in a total of 15–18 isotonic contractions. Po and Vo data points derived from the isotonic contractions were fit using the Hill equation (21). Only individual experiments with r2 ≥ 0.98 were included for analysis. Fiber power was calculated from the fitted force-velocity parameters [Po, maximum velocity (Vmax), and a/Po, where a is a force constant]. Absolute peak power (μN·FL·s−1) was defined as the product of force (in μN) and Vo (Vmax; in FL/s), while normalized power (W/l) was defined as the product of normalized force, (i.e., force/fiber CSA) and Vo (Vmax; in FL/s).
MHC composition.
Following the single muscle fiber physiological measurements, each fiber was solubilized in 80 μl of 10% SDS sample buffer and stored at −20°C until assayed. Mixed-muscle homogenate MHC composition was determined from the isolated myofibrillar fraction (see below). To determine MHC composition, samples were run on a Hoefer SE 600 gel electrophoresis system that consisted of a 3.5% (wt/vol) acrylamide stacking gel with 5% separating gel at 4°C. Following gel electrophoresis, gels were silver-stained, as described by Giulian et al. (14). MHC isoforms were identified according to final relative migration position from the SDS-PAGE/silver staining. The myofibers were categorized as MHC I, IIa, IIx, I/IIa, I/IIa/IIx, I/IIx, and IIa/IIx. The relative proportion of the MHC isoforms (I, IIa, IIx) in the homogenates was determined by densitometry (FluorChem SP, Alpha Innotech).
Water content and protein concentration.
The wet weight of muscle samples (∼10 mg) was determined on a precision microbalance at −35°C, and samples were then freeze-dried for 72 h. The dry weight of each muscle sample was then determined at −35°C. Muscle water content was determined from the difference in dry and weight wet for each muscle sample and expressed as percentage of initial wet weight. Each muscle sample was then homogenized in 40 volumes of cold buffer (250 mM sucrose, 100 mM potassium chloride, 20 mM imidazole, and 5 mM EDTA; pH 6.8) in a ground glass homogenizer. Samples were then centrifuged at 1,600 g for 30 min at 4°C. The pellet contained myofibrillar proteins and was washed once with 100 μl of homogenizing buffer and once with 100 μl of purified and deionized water, with each wash followed by centrifugation at 1,600 g for 20 min at 4°C. Myofibrillar proteins were dissolved by adding 150 μl of 0.3 N NaOH to the remaining pellet, and insoluble proteins (i.e., collagen) were removed by centrifugation at 4,000 g for 20 min at 4°C (9). The resulting supernatant was used to determine myofibrillar concentration using the bicinchoninic acid assay (Thermo Scientific, Rockford, IL), with bovine serum albumin used as the protein standard, as our laboratory has previously performed (48).
Statistical analysis.
Data are expressed as means and SE. Statistical significance for group means for all main outcome variables was assessed with a two-way (group × time) ANOVA with repeated measures on time (pre and post). Training responses, expressed as absolute or relative (%) change, between groups were assessed using an unpaired t-test. Single muscle fiber physiology variables were averaged in a fiber-type-specific manner and pooled by subject for each group at each time point. The average of the subject means was used for statistical analysis. In the presence of a main effect, a Bonferroni post hoc analysis was performed to make pairwise comparisons. A P value of <0.05 (P < 0.05) was considered statistically significant.
RESULTS
Subject Characteristics and Exercise Compliance
Each subject completed all 42 exercise sessions for an exercise compliance of 100%.
Total mechanical work performed during the training protocol was approximately twofold higher (P < 0.05) in young (16,437 ± 1,080 kJ) compared with older (8,831 ± 463 kJ) subjects. Average cycling cadence (expressed as RPM) during the training period was higher (P < 0.05) in YM (81 ± 1 RPM) compared with OM (70 ± 1 RPM).
Aerobic Capacity
Significant (P < 0.05) main effects for time, group, and interaction were present for aerobic capacity (Table 1). Aerobic capacity was higher (P < 0.05) in YM, and the training protocol increased (P < 0.05) aerobic capacity by 16 ± 2 and 13 ± 3% for the YM and OM, respectively. The absolute increase in cycling V̇o2max was greater (P < 0.05) in the YM (0.5 ± 0.1 l/min) compared with the OM (0.2 ± 0.1 l/min).
Whole Muscle Volume and Function
A significant (P < 0.05) main effect for time was present for whole muscle volume, indicating that the training protocol increased whole muscle size, independent of age (Table 1). There were no differences (P > 0.05) in the absolute amount of muscle mass gain between groups. Peak power was greater (P < 0.05) in YM, and the training protocol did not influence (P > 0.05) knee extensor power in either group. Cycling peak power output during the graded exercise test was ∼20% higher (P < 0.05) after training in both groups.
Single Muscle Fiber Physiology
A total of 666 individual myofibers, 353 from YM and 313 from OM, were isolated and successfully examined for size and contractile properties. Pretraining analysis consisted of 145 MHC I myofibers, 127 MHC IIa, 13 MHC I/IIa, 39 MHC IIa/IIx, and 15 pure MHC IIx. Posttraining analysis consisted of 142 MHC I myofibers, 126 MHC IIa, 11 MHC I/IIa, 42 MHC IIa/IIx, and 6 pure MHC IIx. MHC distribution for single fibers examined for contractile function was similar between groups. Due to the low amount of hybrid fibers studied, presentation and discussion of results are restricted to MHC I (slow) and MHC IIa (fast) myofibers.
Myofiber size.
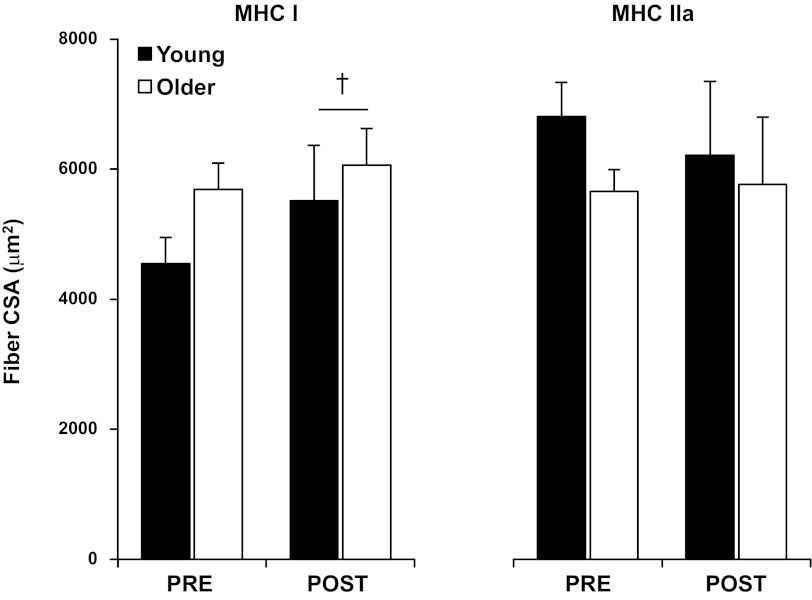
Aerobic training increased (P < 0.05) CSA of MHC I fibers, independent of age (Fig. 1). Training did not significantly (P > 0.05) influence MHC IIa fiber CSA. No differences were present between groups for either fiber type before or after training.
Fig. 1.
Cross-sectional area (CSA) of slow [myosin heavy chain (MHC) I] and fast (MHC IIa) myofibers before (PRE) and after (POST) 12 wk of progressive aerobic exercise training in young (YM) and older men (OM). Values are means ± SE. †P < 0.05, main effect for time.
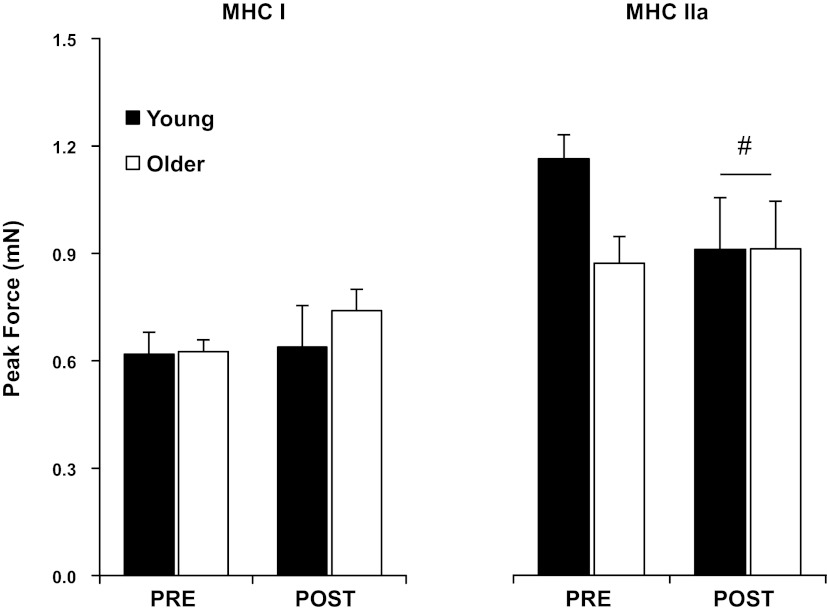
Myofiber force production.
Po for the MHC I fibers was not different (P > 0.05) between groups and was unaltered with aerobic training (Fig. 2 and Table 2). Normalized force production (Po/CSA) of MHC I fibers was higher (P < 0.05) pretraining in YM compared with OM and was reduced (P < 0.05) with training in YM only, such that no age-specific differences were present after training.
Fig. 2.
Peak force (Po) of individual slow (MHC I) and fast (MHC IIa) myofibers PRE and POST 12 wk of progressive aerobic exercise training in YM and OM. Values are means ± SE. #P < 0.05, interaction between groups.
Table 2.
Contractile properties of slow (MHC I) and fast (MHC IIa) single muscle fibers before and after 12 wk of aerobic training in young and older men
| Young Men (n = 7) |
Older Men (n = 6) |
|||||||
|---|---|---|---|---|---|---|---|---|
| MHC I |
MHC IIa |
MHC I |
MHC IIa |
|||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Po/CSA#, kN/m2 | 137 ± 3 | 118 ± 6* | 176 ± 5 | 154 ± 9 | 111 ± 9 | 124 ± 3 | 162 ± 13 | 163 ± 7 |
| Vo, FL/s | 0.73 ± 0.05 | 0.83 ± 0.07 | 2.79 ± 0.21 | 2.86 ± 0.23 | 0.82 ± 0.05 | 0.94 ± 0.07 | 2.69 ± 0.15 | 2.84 ± 0.19 |
| Normalized power#, W/l | 1.8 ± 0.1 | 1.6 ± 0.1 | 11.7 ± 0.9 | 10.3 ± 1.2 | 1.5 ± 0.1 | 1.9 ± 0.1* | 10.2 ± 1.0 | 12.0 ± 1.1 |
Values are means ± SE; n, no. of subjects. MHC, myosin heavy chain; Po, peak force; Vo, shortening velocity; FL, fiber length.
P < 0.05, interaction for MHC I.
P < 0.05, compared with corresponding Pre.
Po of MHC IIa fibers trended (P = 0.07) to be higher pretraining in YM compared with OM. Significant (P < 0.05) main effects for time and interaction were present for MHC IIa fibers, indicating that aerobic training reduced MHC IIa Po, which was due primarily to a reduction in YM. No age or training differences were noted for MHC IIa Po/CSA.
Myofiber contraction velocity.
Maximum unloaded Vo was not different (P < 0.05) between groups and was unaltered with aerobic training for both MHC I and MHC IIa fibers, although a trend (P = 0.08; main effect for time) to increase Vo for MHC I fibers was observed (Table 2). Vmax was not changed in either fiber type with aerobic training.
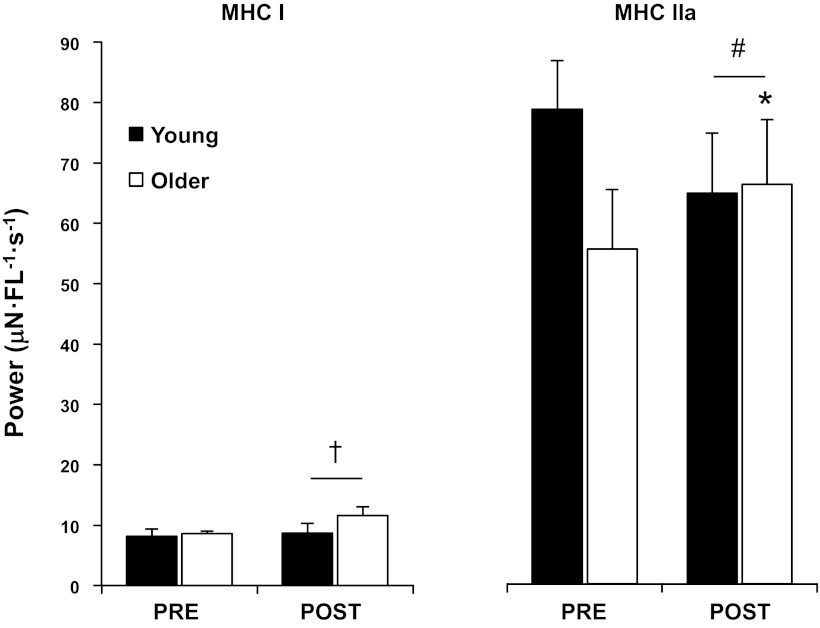
Myofiber power.
Aerobic training increased (P < 0.05) peak absolute power of MHC I fibers, independent of age (Fig. 3 and Table 2). Peak power of MHC IIa fibers trended (P = 0.08) to be higher in YM compared with OM pretraining. A significant interaction (P < 0.05) between groups revealed that aerobic training increased peak absolute power of MHC IIa fibers in OM only. Similarly, MHC I peak power normalized to cell volume trended (P = 0.08) to be higher in YM compared with OM pretraining. A significant interaction (P < 0.05) between groups showed that aerobic training increased normalized power of MHC I fibers in OM only. Normalized power of MHC IIa fibers was not different (P > 0.05) between groups and was unaltered by the training program.
Fig. 3.
Peak absolute power of individual slow (MHC I) and fast (MHC IIa) myofibers PRE and POST 12 wk of progressive aerobic exercise training in YM and OM. Aerobic training resulted in a general increase in MHC I peak power, while increases in MHC IIa peak power were specific to older men. Values are means ± SE. †P < 0.05, main effect for time. #P < 0.05, interaction between groups. *P < 0.05, compared with corresponding PRE.
Force velocity parameters.
a/Po, which describes the curvature of the force-velocity curve, and M, defined as the percentage of Po at which peak power occurs, were lower (P < 0.05; main effect for time) after training for the MHC I fibers. a/Po and M for MHC IIa fibers were not influenced by the training program.
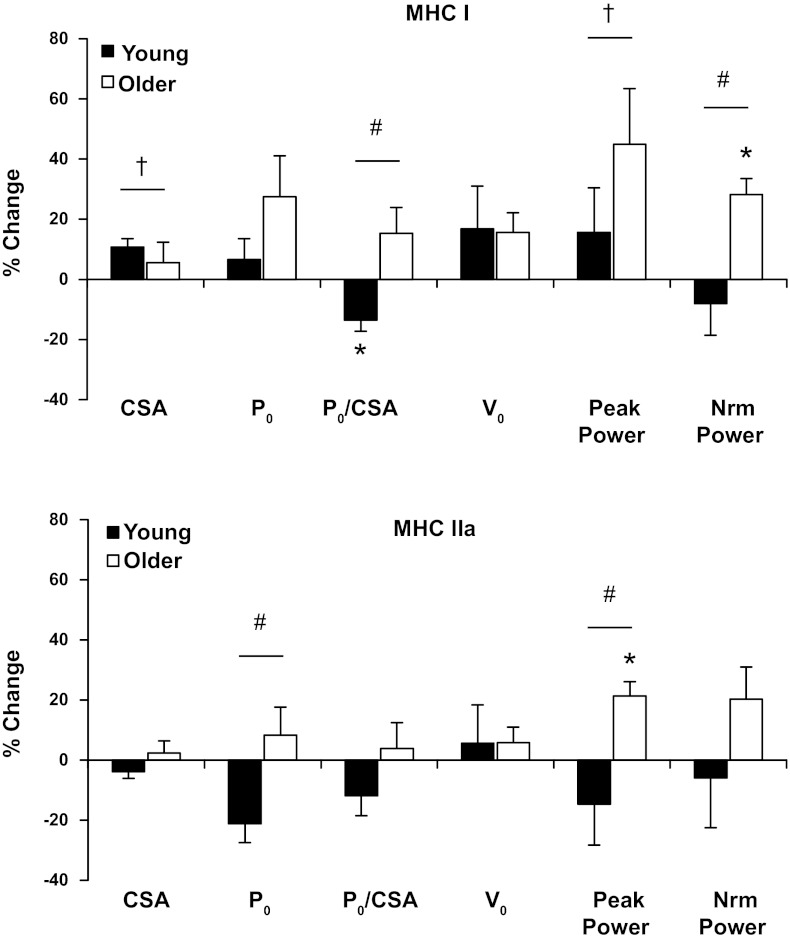
Percent change with training for both fiber types for myofiber CSA, force, contraction velocity, and power production is presented in Fig. 4, and comparison between groups (expressed as %difference from YM) is presented in Fig. 5.
Fig. 4.
Percent change in contractile variables in response to 12 wk of progressive aerobic exercise training for slow (MHC I) and fast (MHC IIa) myofibers for YM and OM. Values are means ± SE. Vo, shortening velocity; Nrm power, normalized power. †P < 0.05, main effect for time. #P < 0.05, interaction between groups. *P < 0.05, compared with corresponding PRE.
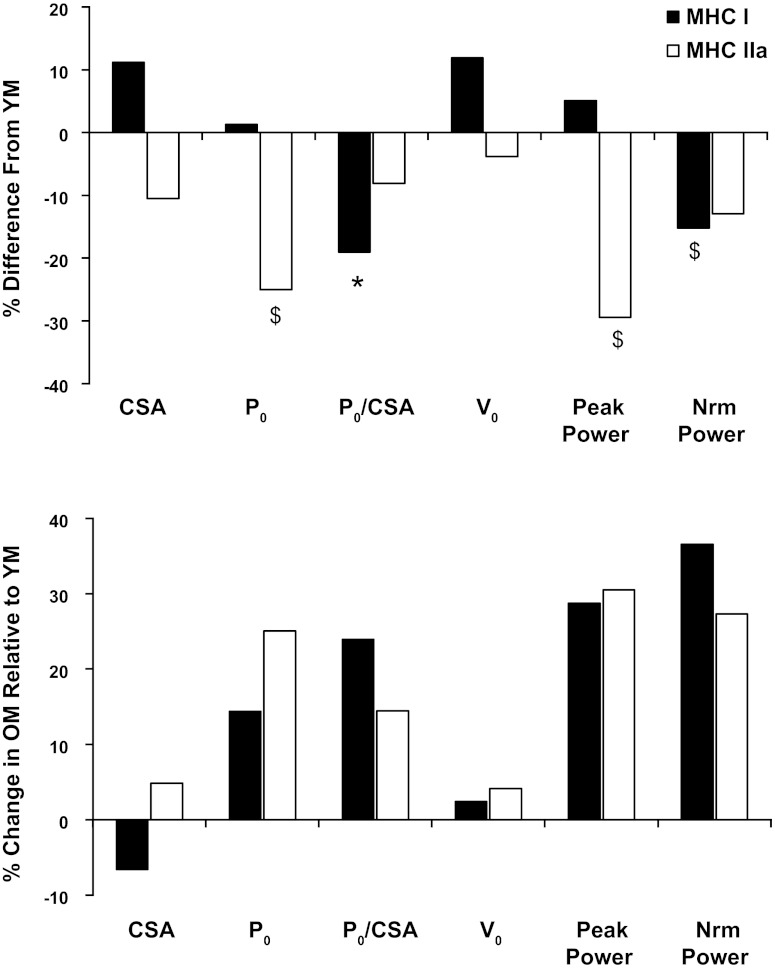
Fig. 5.
Age differences in myofiber contractile function at baseline and with training. Top: baseline differences in myofiber contractile function expressed as percent difference from YM. Bottom: percent change in myofiber contractile function with training for OM in relation to YM, determined by the percent difference from YM posttraining minus percent difference from YM pretraining. A positive value represents an improvement in contractile function for OM relative to the YM. *P < 0.05 between groups. $P < 0.10 between groups.
Muscle Myofibrillar Protein Concentration and Water Content
No differences were observed between groups or with training for total muscle protein or myofibrillar protein concentration. Muscle water content decreased (P < 0.05) with aerobic training, independent of age. Muscle water content was not different between YM (76.8 ± 0.4% pretraining; 75.1 ± 0.4% posttraining) and OM (77.8 ± 0.9% pretraining; 76.1 ± 0.9% posttraining).
MHC Composition
The aerobic training program reduced (P < 0.05) the proportion of MHC IIx isoform, independent of age (Table 3). The proportion of MHC IIa isoform displayed a trend (P = 0.1) to be higher posttraining.
Table 3.
MHC composition determined from mixed muscle homogenates before and after 12 wk of aerobic training in young and older men
| Young Men (n = 7) |
Older Men (n = 6) |
|||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| MHC I | 32 ± 4 | 34 ± 3 | 42 ± 4 | 44 ± 6 |
| MHC IIa | 55 ± 4 | 63 ± 3 | 43 ± 7 | 47 ± 6 |
| MHC IIx† | 13 ± 6 | 4 ± 2 | 15 ± 4 | 9 ± 4 |
Values are means ± SE in percent; n, no. of subjects.
P < 0.05, main effect for time.
DISCUSSION
The primary goal of this investigation was to examine the influence of a progressive aerobic exercise training program on aerobic capacity, whole muscle and single muscle fiber size, and contractile function in YM (20 ± 1 yr) and OM (74 ± 3 yr). Our laboratory has previously reported that a similar training program elicited substantial muscle hypertrophy and robust improvements in muscle function in a cohort of older women (19, 27, 28). The primary findings from the present study are that aerobic training 1) improved aerobic capacity in both YM and OM; 2) induced significant whole muscle hypertrophy independent of age; and 3) improved peak power production of isolated slow (MHC I) and fast (MHC IIa) myofibers in OM. These data are the first evidence that aerobic exercise training can improve MHC IIa myofiber contractile function in older individuals. These findings support our previous research and further solidify the concept that aerobic exercise training should be considered an effective and efficient exercise modality for combating the decline of aerobic capacity and loss of muscle mass that is characteristic of the normal aging process.
Peak aerobic power (i.e., V̇o2max) is commonly used as a measure of functional capacity in the elderly and is of clinical significance as a powerful predictor of mortality in men (33). The age-related decline in aerobic capacity has been well described (3, 37), but can be improved with exercise training, as shown in the present study and previously (19, 31, 43). Loss of muscle mass may be responsible for up to 50% of the age-related reduction in aerobic capacity (13, 34, 38); therefore, the development of aerobic exercise prescriptions that increase both muscle mass and aerobic capacity, as in the present study, have important implications for older adults. The ∼1 metabolic equivalent improvement observed in the OM with training may translate into a 12% improvement in survival and expanded functional reserve above the threshold for independence (∼5 metabolic equivalent) (26, 33). Given the apparent relationship between skeletal muscle mass and aerobic capacity, surprisingly little attention has been paid to developing aerobic exercise interventions that target skeletal muscle mass.
To our knowledge, this is the first investigation to report similar amounts of muscle hypertrophy in young and older subjects in response to aerobic training. While the anabolic potential of aerobic exercise is not always appreciated, the present data are in agreement with previous reports that aerobic training induces significant whole muscle hypertrophy in sedentary healthy individuals of various ages (19, 22, 23, 27, 29, 30, 42). It should be noted that aerobic exercise-induced muscle hypertrophy is not specific to cycling exercise, as Schwartz and colleagues (42) originally reported thigh muscle hypertrophy in OM following a walk/jog program. The observed muscle hypertrophy appears to be driven primarily by an increase in slow (MHC I) muscle fiber size, which is consistent with previous research examining young and older subjects after aerobic training programs <6 mo in duration (15, 19). Long-duration (>6 mo) training studies and cross-sectional analysis of aerobically trained athletes support the potential for aerobic exercise to induce fast (MHC IIa) muscle fiber hypertrophy (7, 17). The gains in muscle mass following chronically performed aerobic exercise are supported by a growing body of literature showing that aerobic exercise acutely (10, 18, 20, 32, 44) and chronically (36, 45) elevates muscle protein synthesis in young and older individuals. These findings are particularly important for older individuals, as a single-mode (i.e., cycle ergometry) exercise program utilized in the present study increased muscle mass and improved aerobic capacity, which are two primary risk factors associated with the negative health consequences of aging.
The exercise program in the present investigation set exercise intensity as a function of aerobic capacity, such that both groups exercised at the same relative intensity. Maximal aerobic capacity and peak workload were ∼50% lower in the OM; therefore, the accumulated mechanical work performed during the 12-wk training program was twofold higher in the YM. Despite the drastic differences in total work performed, both groups exhibited similar amounts of absolute quadriceps muscle hypertrophy (∼50 cm3). These data imply that the OM were more efficient at translating work performed into muscle mass accretion. An explanation for this is not readily apparent, but may be related to differences in tension placed on the muscle during training, although exercise intensity was relatively equivalent. Cycle exercise performed at 75% aerobic capacity, similar to the exercise intensity performed in the present study, corresponds to 38% of maximal dynamic muscle force (40). On average, the OM exercised at a lower cycling cadence (i.e., RPM) compared with the young (81 vs. 70 RPM), suggesting that the relative tension on the muscle for each contraction was greater in the older subjects at the same relative exercise intensity (39). Over the 12-wk duration of the training program, the lower pedal frequency resulted in ∼19,000 less muscle contractions performed by the OM. Additionally, structural differences in tendon and connective tissue in older individuals (5) may influence the efficiency of force transmission from the muscle, resulting in a greater muscle force production to achieve the same relative external force output. Collectively, these factors may contribute to the observation that the OM appeared to be more efficient at translating mechanical work output into muscle hypertrophy.
Adaptations at the myofiber level in response to the aerobic exercise program were more prevalent in the OM as highlighted by increases in both MHC I and IIa power accompanied with the preservation of myofiber force. The improvement in MHC IIa myofiber power is particularly relevant due to their superior functional performance and is unique to the OM, as our laboratory has previously shown that increases in myofiber power with aerobic training are limited to MHC I fibers in older women (19), identifying a potential sex-based fiber-type-specific adaptation in older individuals. For comparison, resistance training improves slow and fast myofiber power in both YM (53) and OM (51), suggesting that the improvements in MHC IIa function with aerobic training in the present study are age dependent. The preservation of myofiber force production with aerobic training in the OM is consistent with a cross-sectional investigation, suggesting that aerobic training maintains myofiber force production with aging (8), while the reduction in myofiber force production in YM after training is consistent with other reports of aerobically trained skeletal muscle (17). The age-dependent adaptations in myofiber contractile function cannot be explained by differences in protein content, as the training did not measurably alter total or myofibrillar protein concentration in either cohort of men. Interestingly, muscle water content was reduced with aerobic training in both YM and OM in the present study, which is consistent with what we have observed in highly trained aerobic male athletes (unpublished observations) but in contrast to our laboratory's observations in older women (19). The disparity between the present study and our laboratory's previous work in older women may be due to training-induced changes in muscle fiber type or MHC composition. Older women had a large increase in MHC I protein after aerobic training (28), while the men in the present study underwent reductions in MHC IIx composition with no significant change in MHC I isoform. Because MHC I fibers contain less concentration of myosin relative to MHC IIa fibers (4), a robust fiber-type shift may result in measurable changes in myofibrillar protein at the mixed-muscle level. Collectively, these data suggest that cellular adaptations to aerobic training are age and sex dependent and highlight the need for additional work to examine the underlying basis for these adaptations.
A potential explanation for the age-dependent adaptations to aerobic training at the myofiber level is the initial, pretraining contractile profile. Four variables, MHC I normalized force, MHC I normalized power, MHC IIa Po, and MHC IIa peak power, displayed a significant interaction between the YM and OM with training. Interestingly, all four variables at least trended (P < 0.10) to be lower in the OM at the pretraining time point, and these differences were no longer evident after training (Fig. 5). Examining the alterations in contractile function with training (Fig. 4), in combination with the posttraining contractile profiles of the YM and OM, reveals a pattern that suggests the muscle is converging to a common level of myofiber contractile function (i.e., force and power), independent of age. This suggests that there is a defined amount of force and power production at the myofiber level that is necessary to optimally perform cycle exercise at a given relative exercise intensity. Due to the energetic demands of chronically performing aerobic exercise, strategic adaptations of the myofiber contractile parameters would likely result in a power output necessary to meet the functional demands in the most energetically efficient manner, which appears to be the case in the YM. Within a given fiber type, force production and ATP utilization are proportional to the number of cross-bridges in the strong-bound state (12, 16). Therefore, any adaptation that permits a myofiber to meet its functional demands with a lower force production, as observed in the MHC IIa fibers of the YM, would likely be associated with less energy utilization per contraction, which may be advantageous for aerobically trained skeletal muscle. Interestingly, this pattern was only evident in the YM, revealing an age-specific adaptation. Although several aspects of myofiber contractile function were lower pretraining in the OM, these data show that skeletal myofibers from OM, particularly MHC IIa fibers, retain the plasticity to adapt to the functional demands placed on them.
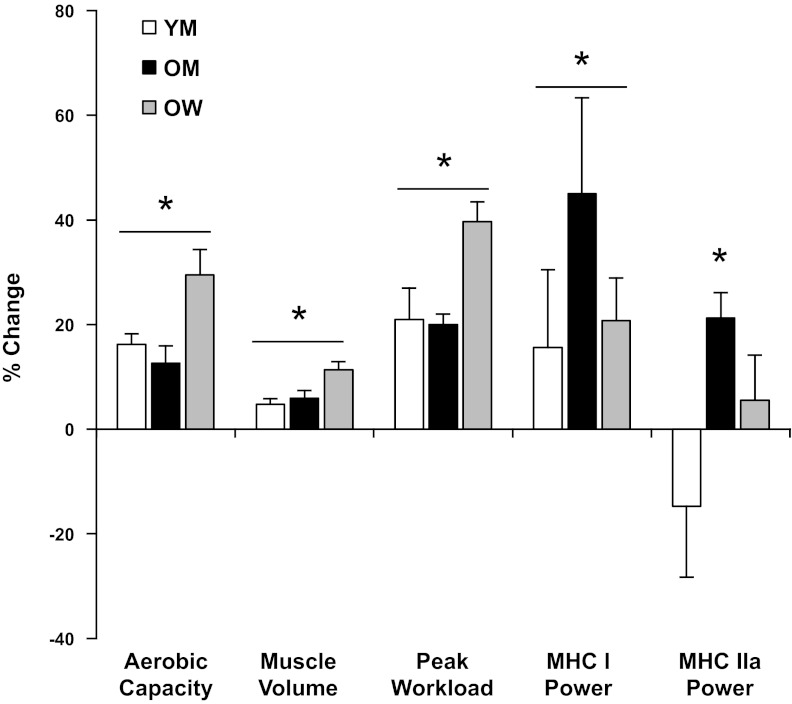
In summary, our findings demonstrate that a single-mode exercise program improved aerobic capacity and induced significant muscle hypertrophy in both YM and OM. Although whole body and whole muscle adaptations were relatively similar between groups, alterations in myofiber contractile function were age dependent, being more prominent and overall beneficial in the OM (Figs. 4 and 5), highlighted by improved power and preservation of force production of both slow and fast myofibers from the OM. Combining data from the present investigation with results from our laboratory's previous studies in older women (19, 27, 28) reveals that this exercise program can elicit beneficial adaptations at the whole body, whole muscle, and muscle cell levels, particularly in older individuals, that carry important clinical implications for overall health and well-being (Fig. 6). Based on these findings, we propose that aerobic exercise training performed at sufficient intensity (60–80% V̇o2max) should be considered an important aspect of the exercise recommendations for combating the decline of aerobic capacity and loss of muscle mass that occurs with the normal aging process.
Fig. 6.
Aerobic exercise training responses for key whole body and individual myofiber functional variables in YM, OM, and older women (OW). Values are means ± SE, expressed as percent change with training. Data from OW have been presented previously (17). *P < 0.05 for a difference between PRE and POST.
GRANTS
This research was funded by National Institute on Aging Grant AG032127 and National Aeronautics and Space Administration Grant NNJ06HF59G.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.P.H., A.R.K., L.A.K., T.A.T., and S.W.T. conception and design of research; M.P.H., A.R.K., M.K.U., J.M.H., K.M., and L.A.K. performed experiments; M.P.H., A.R.K., and M.K.U. analyzed data; M.P.H., A.R.K., M.K.U., T.A.T., and S.W.T. interpreted results of experiments; M.P.H. prepared figures; M.P.H. drafted manuscript; M.P.H., A.R.K., M.K.U., J.M.H., L.A.K., T.A.T., and S.W.T. edited and revised manuscript; M.P.H., A.R.K., M.K.U., J.M.H., K.M., L.A.K., T.A.T., and S.W.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Paul Reidy and Tina Vannatta for assistance with subject training.
REFERENCES
- 1. Berg HE, Tedner B, Tesch PA. Changes in lower limb muscle cross-sectional area and tissue fluid volume after transition from standing to supine. Acta Physiol Scand 148: 379–385, 1993 [DOI] [PubMed] [Google Scholar]
- 2. Bergstrom J. Muscle electrolytes in man. Scand J Clin Lab Invest Suppl 68: 1–110, 1962 [Google Scholar]
- 3. Buskirk ER, Hodgson JL. Age and aerobic power: the rate of change in men and women. Fed Proc 46: 1824–1829, 1987 [PubMed] [Google Scholar]
- 4. Carroll CC, Carrithers JA, Trappe TA. Contractile protein concentrations in human single muscle fibers. J Muscle Res Cell Motil 25: 55–59, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Carroll CC, Dickinson JM, Haus JM, Lee GA, Hollon CJ, Aagaard P, Magnusson SP, Trappe TA. Influence of aging on the in vivo properties of human patellar tendon. J Appl Physiol 105: 1907–1915, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, Skinner JS. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc 41: 1510–1530, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol 72: 1780–1786, 1992 [DOI] [PubMed] [Google Scholar]
- 8. D'Antona G, Pellegrino MA, Carlizzi CN, Bottinelli R. Deterioration of contractile properties of muscle fibres in elderly subjects is modulated by the level of physical activity. Eur J Appl Physiol 100: 603–611, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Dickinson JM, Lee JD, Sullivan BE, Harber MP, Trappe SW, Trappe TA. A new method to study in vivo protein synthesis in slow- and fast-twitch muscle fibers and initial measurements in humans. J Appl Physiol 108: 1410–1416, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Durham WJ, Casperson SL, Dillon EL, Keske MA, Paddon-Jones D, Sanford AP, Hickner RC, Grady JJ, Sheffield-Moore M. Age-related anabolic resistance after endurance-type exercise in healthy humans. FASEB J 24: 4117–4127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edman KA. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol 291: 143–159, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fitts RH, McDonald KS, Schluter JM. The determinants of skeletal muscle force and power: their adaptability with changes in activity pattern. J Biomech 24, Suppl 1: 111–122, 1991 [DOI] [PubMed] [Google Scholar]
- 13. Fleg JL, Lakatta EG. Role of muscle loss in the age-associated reduction in V̇o2max. J Appl Physiol 65: 1147–1151, 1988 [DOI] [PubMed] [Google Scholar]
- 14. Giulian GG, Moss RL, Greaser M. Improved methodology for analysis and quantitation of proteins on one- dimensional silver-stained slab gels. Anal Biochem 129: 277–287, 1983 [DOI] [PubMed] [Google Scholar]
- 15. Gollnick PD, Armstrong RB, Saltin B, Saubert CWt Sembrowich WL, Shepherd RE. Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol 34: 107–111, 1973 [DOI] [PubMed] [Google Scholar]
- 16. Han YS, Geiger PC, Cody MJ, Macken RL, Sieck GC. ATP consumption rate per cross bridge depends on myosin heavy chain isoform. J Appl Physiol 94: 2188–2196, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Harber M, Trappe S. Single muscle fiber contractile properties of young competitive distance runners. J Appl Physiol 105: 629–636, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Harber MP, Crane JD, Dickinson JM, Jemiolo B, Raue U, Trappe TA, Trappe SW. Protein synthesis and the expression of growth-related genes are altered by running in human vastus lateralis and soleus muscles. Am J Physiol Regul Integr Comp Physiol 296: R708–R714, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Harber MP, Konopka AR, Douglass MD, Minchev K, Kaminsky LA, Trappe TA, Trappe S. Aerobic exercise training improves whole muscle and single myofiber size and function in older women. Am J Physiol Regul Integr Comp Physiol 297: R1452–R1459, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harber MP, Konopka AR, Jemiolo B, Trappe SW, Trappe TA, Reidy PT. Muscle protein synthesis and gene expression during recovery from aerobic exercise in the fasted and fed states. Am J Physiol Regul Integr Comp Physiol 299: R1254–R1262, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Hill AV. The heat of shortening and the dynamic constants of muscle. Proc R Soc London Ser B 126: 136–195, 1938 [Google Scholar]
- 22. Hudelmaier M, Wirth W, Himmer M, Ring-Dimitriou S, Sanger A, Eckstein F. Effect of exercise intervention on thigh muscle volume and anatomical cross-sectional areas–quantitative assessment using MRI. Magn Reson Med 64: 1713–1720, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Izquierdo M, Hakkinen K, Ibanez J, Kraemer WJ, Gorostiaga EM. Effects of combined resistance and cardiovascular training on strength, power, muscle cross-sectional area, and endurance markers in middle-aged men. Eur J Appl Physiol 94: 70–75, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Jubrias SA, Esselman PC, Price LB, Cress ME, Conley KE. Large energetic adaptations of elderly muscle to resistance and endurance training. J Appl Physiol 90: 1663–1670, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Julian FJ, Moss RL, Waller GS. Mechanical properties and myosin light chain composition of skinned muscle fibres from adult and new-born rabbits. J Physiol 311: 201–218, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kokkinos P, Myers J, Faselis C, Panagiotakos DB, Doumas M, Pittaras A, Manolis A, Kokkinos JP, Karasik P, Greenberg M, Papademetriou V, Fletcher R. Exercise capacity and mortality in older men: a 20-year follow-up study. Circulation 122: 790–797, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Konopka AR, Douglass MD, Kaminsky LA, Jemiolo B, Trappe TA, Trappe S, Harber MP. Molecular adaptations to aerobic exercise training in skeletal muscle of older women. J Gerontol A Biol Sci Med Sci 65: 1201–1207, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Konopka AR, Trappe TA, Jemiolo B, Trappe SW, Harber MP. Myosin heavy chain plasticity in aging skeletal muscle with aerobic exercise training. J Gerontol A Biol Sci Med Sci 66: 835–841, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lovell DI, Cuneo R, Gass GC. Can aerobic training improve muscle strength and power in older men? J Aging Phys Act 18: 14–26, 2010 [DOI] [PubMed] [Google Scholar]
- 30. McPhee JS, Williams AG, Degens H, Jones DA. Inter-individual variability in adaptation of the leg muscles following a standardised endurance training programme in young women. Eur J Appl Physiol 109: 1111–1118, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Meredith CN, Frontera WR, Fisher EC, Hughes VA, Herland JC, Edwards J, Evans WJ. Peripheral effects of endurance training in young and old subjects. J Appl Physiol 66: 2844–2849, 1989 [DOI] [PubMed] [Google Scholar]
- 32. Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol 567: 1021–1033, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 346: 793–801, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Proctor DN, Joyner MJ. Skeletal muscle mass and the reduction of V̇o2max in trained older subjects. J Appl Physiol 82: 1411–1415, 1997 [DOI] [PubMed] [Google Scholar]
- 35. Raue U, Slivka D, Minchev K, Trappe S. Improvements in whole muscle and myocellular function are limited with high-intensity resistance training in octogenarian women. J Appl Physiol 106: 1611–1617, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Robinson MM, Turner SM, Hellerstein MK, Hamilton KL, Miller BF. Long-term synthesis rates of skeletal muscle DNA and protein are higher during aerobic training in older humans than in sedentary young subjects but are not altered by protein supplementation. FASEB J 25: 3240–3249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Robinson S. Experimental studies of physical fitness in relation to age. Arbeitsphysiologie 10: 251–323, 1938 [Google Scholar]
- 38. Rosen MJ, Sorkin JD, Goldberg AP, Hagberg JM, Katzel LI. Predictors of age-associated decline in maximal aerobic capacity: a comparison of four statistical models. J Appl Physiol 84: 2163–2170, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Sargeant AJ, Hoinville E, Young A. Maximum leg force and power output during short-term dynamic exercise. J Appl Physiol 51: 1175–1182, 1981 [DOI] [PubMed] [Google Scholar]
- 40. Sargeant AJ, Jones DA. The significance of motor unit variability in sustaining mechanical output of muscle. Adv Exp Med Biol 384: 323–338, 1995 [DOI] [PubMed] [Google Scholar]
- 41. Schluter JM, Fitts RH. Shortening velocity and ATPase activity of rat skeletal muscle fibers: effects of endurance exercise training. Am J Physiol Cell Physiol 266: C1699–C1713, 1994 [DOI] [PubMed] [Google Scholar]
- 42. Schwartz RS, Shuman WP, Larson V, Cain KC, Fellingham GW, Beard JC, Kahn SE, Stratton JR, Cerqueira MD, Abrass IB. The effect of intensive endurance exercise training on body fat distribution in young and older men. Metabolism 40: 545–551, 1991 [DOI] [PubMed] [Google Scholar]
- 43. Seals DR, Hagberg JM, Hurley BF, Ehsani AA, Holloszy JO. Endurance training in older men and women. I. Cardiovascular responses to exercise. J Appl Physiol 57: 1024–1029, 1984 [DOI] [PubMed] [Google Scholar]
- 44. Sheffield-Moore M, Yeckel CW, Volpi E, Wolf SE, Morio B, Chinkes DL, Paddon-Jones D, Wolfe RR. Postexercise protein metabolism in older and younger men following moderate-intensity aerobic exercise. Am J Physiol Endocrinol Metab 287: E513–E522, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab 286: E92–E101, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Sipila S, Suominen H. Effects of strength and endurance training on thigh and leg muscle mass and composition in elderly women. J Appl Physiol 78: 334–340, 1995 [DOI] [PubMed] [Google Scholar]
- 47. Trappe S, Costill D, Thomas R. Effect of swim taper on whole muscle and single muscle fiber contractile properties. Med Sci Sports Exerc 33: 48–56, 2001 [PubMed] [Google Scholar]
- 48. Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol 552: 47–58, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Trappe S, Godard M, Gallagher P, Carroll C, Rowden G, Porter D. Resistance training improves single muscle fiber contractile function in older women. Am J Physiol Cell Physiol 281: C398–C406, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Trappe S, Harber M, Creer A, Gallagher P, Slivka D, Minchev K, Whitsett D. Single muscle fiber adaptations with marathon training. J Appl Physiol 101: 721–727, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Trappe S, Williamson D, Godard M, Porter D, Rowden G, Costill D. Effect of resistance training on single muscle fiber contractile function in older men. J Appl Physiol 89: 143–152, 2000 [DOI] [PubMed] [Google Scholar]
- 52. Verney J, Kadi F, Saafi MA, Piehl-Aulin K, Denis C. Combined lower body endurance and upper body resistance training improves performance and health parameters in healthy active elderly. Eur J Appl Physiol 97: 288–297, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Widrick JJ, Stelzer JE, Shoepe TC, Garner DP. Functional properties of human muscle fibers after short-term resistance exercise training. Am J Physiol Regul Integr Comp Physiol 283: R408–R416, 2002 [DOI] [PubMed] [Google Scholar]