Abstract
Mechanical ventilation inevitably exposes the delicate tissues of the airways and alveoli to abnormal mechanical stresses that can induce pulmonary edema and exacerbate conditions such as acute respiratory distress syndrome. The goal of our research is to characterize the cellular trauma caused by the transient abnormal fluid mechanical stresses that arise when air is forced into a liquid-occluded airway (i.e., atelectrauma). Using a fluid-filled, parallel-plate flow chamber to model the “airway reopening” process, our in vitro study examined consequent increases in pulmonary epithelial plasma membrane rupture, paracellular permeability, and disruption of the tight junction (TJ) proteins zonula occludens-1 and claudin-4. Computational analysis predicts the normal and tangential surface stresses that develop between the basolateral epithelial membrane and underlying substrate due to the interfacial stresses acting on the apical cell membrane. These simulations demonstrate that decreasing the velocity of reopening causes a significant increase in basolateral surface stresses, particularly in the region between neighboring cells where TJs concentrate. Likewise, pulmonary epithelial wounding, paracellular permeability, and TJ protein disruption were significantly greater following slower reopening. This study thus demonstrates that maintaining a higher velocity of reopening, which reduces the damaging fluid stresses acting on the airway wall, decreases the mechanical stresses on the basolateral cell surface while protecting cells from plasma membrane rupture and promoting barrier integrity.
Keywords: airway reopening, paracellular permeability, acute respiratory distress syndrome, ventilator-induced lung injury
acute lung injury (ali) and the more severe acute respiratory distress syndrome (ARDS) are conditions of the lung initiated by a traumatic insult (e.g., sepsis, smoke inhalation, aspiration, or primary pneumonia) that compromises the structural and functional integrity of the pulmonary epithelial and endothelial cell layers (46). Alterations of the diffuse network of tight junction (TJ) proteins that normally seal together neighboring pulmonary epithelial cells can significantly increase paracellular permeability, allowing proteinaceous edema fluid and inflammatory cells to flood the airways and alveoli (8). This alters respiratory mechanics and can play a pivotal role in determining the final prognosis of ALI and ARDS patients. In fact, ARDS patients unable to maintain a proper lung fluid balance are three times more likely to die than those with a sustained capacity to clear edema fluid (42, 45). Presently, ALI and ARDS are associated with a mortality rate of ∼40% (36, 39) and an annual number of deaths in the United States that is comparable to breast cancer, human immunodeficiency virus infection, and asthma (38).
Mechanical ventilation clearly is an indispensible life-sustaining therapy for ALI and ARDS; however, the wide range of irregular mechanical stresses and strains introduced during forced respiratory maneuvers contributes significantly to airway and alveolar damage, causing ventilator-induced lung injury (VILI). Ventilation strategies are now understood to play a critical role in determining the clinical outcome of mechanically ventilated individuals suffering from ALI or ARDS (1). It thus is essential that we establish more protective ventilator schemes that preclude excessive airway and alveolar stretch while avoiding repetitive collapse and reopening of atelectatic lung units (29).
The present study builds on a series of in vitro parallel plate flow chamber analyses that investigate alterations in the pulmonary epithelium resulting from mechanical stress when air is reintroduced into a liquid-occluded airway [i.e., “airway reopening” (16)]. The mechanical environment associated with two-phase (air-liquid) interfacial flows imparts a transient series of abnormal and potentially harmful surface stresses on the cellular epithelium, as first described by Bilek et al. (4) and depicted in Fig. 1. Although cell mechanotransduction and injury traditionally have been attributed to shear stress in vascular studies [e.g., reviewed extensively by Davies (15)], the mechanics governing single-phase steady flow do not provide a suitable description for this two-phase system. In fact, experimental results have repeatedly demonstrated that pulmonary epithelial membrane rupture (i.e., “cell wounding”) is enhanced at slower reopening velocities where shear stresses are negligible. Rather, the damaging mechanical stimulus in this system is the steep gradient in pressure generated near the traveling bubble tip, which can far exceed healthy physiological values during airway reopening (4, 21, 24, 43, 52, 53).
Fig. 1.
A: the reopening of fluid-filled, atelectatic airways generates abnormal fluid mechanical stresses and stress gradients that act along the airway walls (illustrated here by the shaded arrows) to alter both the structure and function of the lining cellular epithelium (as depicted within the circular windows below). B: this “airway reopening” process can be modeled using a parallel-plate flow chamber, as illustrated here in cross section. By culturing the base with pulmonary epithelial cells, this channel serves as a two-dimensional representation of a respiratory bronchiole with walls held in rigid apposition by a viscous liquid obstruction. “Reopening” occurs as a semi-infinite bubble of air is driven into the system at a velocity Ububble, clearing the “airway” of its liquid occlusion.
The experiments discussed herein were designed to enhance our understanding of the pulmonary epithelial injury response to the transient interfacial mechanical stresses imposed during airway reopening (i.e., “reopening stresses” or “fluid stresses”) that could exacerbate ALI or ARDS. Specifically, our goal was to characterize changes in the barrier integrity of wounded cells across a reopening-compromised pulmonary epithelial layer that could relate to patterns of lung injury and pulmonary edema during mechanical ventilation. To this end, we implemented an in vitro flow chamber system supplemented with an instructional computational investigation to elucidate mechanical stress-induced 1) plasma membrane rupture, 2) paracellular permeability increases, and 3) TJ protein alterations that result from airway reopening.
EXPERIMENTAL PROCEDURES
Cell Culture
Our study focuses on the effects of airway reopening on the expression of pulmonary epithelial TJs and the resulting changes in paracellular permeability. We worked with NCI H441 pulmonary epithelial cells (HTB-174; ATCC, Manassas, VA), which have been shown to develop a marked increase in transepithelial electrical resistance and solid, well-organized peripheral zonula occludens-1 (ZO-1) expression when cultured to confluence in the presence of the glucocorticoid dexamethasone (Fisher Scientific, Pittsburgh, PA) (20). Initially, H441 cells (passage numbers 57–62) were stored, propagated, and subcultured in tissue culture flasks using RPMI-1640 medium with l-glutamine (ATCC, Manassas, VA) that was supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) and 1% antibiotic-antimycotic (×100; Invitrogen, Carlsbad, CA) at 37°C and 5% CO2 (2). This complete growth medium was replaced daily. For reopening experiments, cells were then subcultured onto the glass coverslip base of a parallel-plate flow chamber at a seeding density of 2 × 104 cells/cm2 for 11–13 days in complete growth medium supplemented with 1 μM dexamethasone at 37°C and 5% CO2.
Reopening Experiments
H441 cells were subcultured onto the sterile coverslip base of a custom-built, parallel-plate flow chamber for 11–13 days until confluent and fully mature. At the start of each experiment, the cultured cells were carefully washed three times in warmed (37°C) phosphate-buffered saline (PBS; Invitrogen, Carlsbad, CA) for 5 min. The PBS used herein contained CaCl2 and MgCl2 to promote TJ stability (19, 35). Next, the base component of the flow chamber was slowly filled with warmed PBS, and the sterile acrylic top plate was positioned carefully to ensure the absence of air in the system. This fully assembled flow channel, with a gap width of 1.7 mm, served to model events on the level of the respiratory bronchioles.
To simulate airway reopening, an infusion syringe pump (KD Scientific, Holliston, MA) introduced air into the channel. This resulted in a steadily progressing semi-infinite bubble that pushed the occluding fluid from the outlet of the chamber, as depicted in Fig. 1B. To model the pathological state of airway reopening, airflow was set to one of two steady perfusion rates: fast, UF = 2.7 cm/s, or slow, US = 0.27 cm/s. These velocities are consistent with physiological flow rates using typical tidal volumes and the geometry of respiratory bronchioles based on the Weibel lung model (48). For the control condition, the sample was immersed in warmed PBS for the duration of a reopening experiment. The simplified geometry and controlled reopening rates of these experiments correspond well to computational models developed previously in this laboratory (4, 22) and as the instructional computational study described herein (see the model analysis).
Visualization
Following a UF- or US-reopening experiment, the H441 monolayers were prepared for visualization using the techniques described below, then placed directly onto the stage of a Nikon Eclipse TE2000-E inverted epifluorescence microscope (Nikon, Melville, NY). Cell layers were examined at ×20 magnification using a phase objective or a red [excitation (ex): 510–560 nm], green (ex: 450–490 nm), or blue (ex: 325–375 nm) emission filter with appropriate binning of pixels and equal exposure times for each image. All images were acquired along the flow centerline to eliminate edge effects. These were captured and stored using a microscope-mounted, cooled digital 12-bit charge-coupled device camera system (SensiCam QE, COOKE, Romulus, Michigan) and IPLab imaging software (Becton Dickinson, Franklin Lakes, NJ).
Paracellular Permeability Assays
The cell layers were examined for reopening-induced changes in paracellular permeability relative to control (no flow) conditions using a novel in vitro method developed and well-characterized by Cavanaugh and Margulies (8). Within the dismantled flow chamber base component, we immersed the cell layer in serum-free medium supplemented with 0.2 μM of a ouabain-BODIPY-FL conjugate [BODIPY-ouabain, ∼20 Å, ex/emission (em) ∼501/512 nm; Invitrogen, Carlsbad, CA] and 4 μM of the standard “dead cell” indicator ethidium homodimer-1 (EthD-1, ex/em ∼528/617 nm; Invitrogen, Carlsbad, CA). Ouabain has a specific, high affinity for the Na+-K+-ATPases that are present solely on the basolateral surface of the pulmonary epithelium, and the extremely low paracellular permeability of confluent H441 layers limits the apical-to-basolateral passage of this moderate-sized tracer molecule only to regions where the TJ network has been compromised (10, 14). In developing this paracellular permeability measurement technique, Cavanaugh and Margulies (8) also tested and ruled out various transcellular apical-to-basolateral routes for this molecule, including internalization by cells, receptor-mediated endocytosis, or binding due to plasma membrane rupture. Thus, by exposing the apical epithelial surface to fluorescently tagged ouabain and providing sufficient time to cross to the basolateral side in the present study, this molecule indicated which reopening scenarios resulted in the greatest increases in paracellular permeability. Each sample was bathed in the staining solution for 1 h at 37°C and 5% CO2 before the cells were washed in PBS and visualized. As such, the results herein report a cumulative measure of the paracellular flux of BODIPY-ouabain through the cell layer and the total quantity of membrane-compromised cells that developed within the first hour after a reopening event.
Images of basolateral pools of BODIPY-ouabain and of the nuclei of membrane-compromised cells, four fields per flow chamber and eight flow chambers per flow condition (control, UF and US), were captured using the green and red emission filters, respectively, of the inverted microscope, with equal exposure times and gain settings. To measure fluorescence intensity, these images were processed in the same way using the image analysis software ImageJ (National Institutes of Health, Bethesda, MD). The “Subtract Background” macro was implemented to remove nonuniform background fluorescence, and remaining noise was eliminated using a median filter by applying the “Despeckle” macro (6). Paracellular permeability from these final BODIPY-ouabain images then was quantified by adding all pixels of an intensity above threshold (i.e., the maximum pixel intensity within a randomly selected region of the control monolayers). In addition, the sum of all pixels comprising EthD-1 positive, wounded cells provided a measure of reopening-induced epithelial injury. In both cases, these data were then divided by the total image pixel count and are reported herein as “percentage of field area stained.” For a more detailed perspective of the localized changes in barrier function relative to the distributed wounded epithelial cells, we also provide mean BODIPY-ouabain (green) pixel intensities, ranging from 0 to 1, binned according to the minimum radial distance between each green pixel and the nearest red-fluorescing pixel of the EthD-1-stained nuclei in an image (see Figs. 5 and 10).
Fig. 5.
Distribution of the paracellular permeability indicator from wounded epithelial cells. Mean pixel intensities of the green paracellular permeability indicator BODIPY-ouabain (with a total intensity range of 0 to 1) after a single reopening event at either US (A) or UF (B) is shown. The horizontal axis represents the radial distance from each green pixel to the nearest red-fluorescing, EthD-1-stained nucleus in an image, scaled by the average minimum radial distance between red-fluorescing nuclei in that image, and binned into 0.036 unit columns. Each inset depicts the same data unscaled and binned into 1.305-μm columns.
Fig. 10.
Distribution of the paracellular permeability indicator from wounded epithelial cells. A: differences in the mean pixel intensities of the green paracellular permeability indicator BODIPY-ouabain (with a total intensity range of 0 to 1) after a single reopening event following US and UF reopening (i.e., slow-fast results). The horizontal axis represents the radial distance from each green pixel to the nearest red-fluorescing, EthD-1-stained nucleus in an image, scaled by the average minimum radial distance between red-fluorescing nuclei in that image and binned into 0.036 unit columns. B: the cumulative quantity of BODIPY-ouabain that has passed through the epithelium during US (solid line) or UF (dashed line) experiments.
TJ Studies
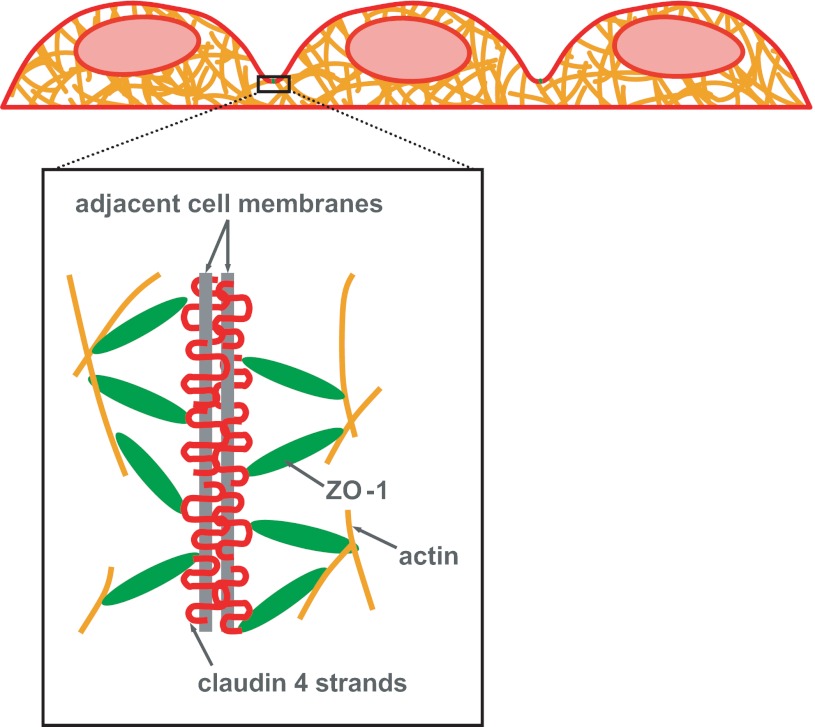
To gain a deeper understanding of the cell processes that influence the paracellular permeability of the pulmonary epithelium, we initiated a similar analysis of two key TJ proteins: ZO-1 and claudin-4. As shown in Fig. 2, claudin-4 is a strandlike transmembrane protein that attaches to TJ strands of adjacent cells and also internally to the cytoskeleton via cytosolic ZO-1. Together, these TJ proteins fuse adjacent cells of an epithelial monolayer and regulate paracellular permeability of an intact epithelium. Our TJ protein analysis occurs over 1 h, the same as the above permeability studies, to provide insight into the chronic, rather than acute, TJ damage initiated during our experimental model of airway reopening.
Fig. 2.
Tight junctions (TJs) seal together neighboring cells to prevent fluid and protein passage across the pulmonary epithelial cell layer. TJ protein strands (claudin-4) weave together adjacent cell membranes and are linked to the internal actin cytoskeleton via cytosolic TJ proteins [zonula occludens-1 (ZO-1)].
Antibody staining of claudin-4 and ZO-1 was based on the methods presented in Hermanns et al. (20), described in detail below. However, we modified their technique by fixing the monolayers with cold (−20°) methanol rather than 3.7% paraformaldehyde. In our hands, paraformaldehyde fixation with Triton X-100 permeabilization produced weak and inconsistent labeling of the TJ proteins particular to this investigation. Instead, we found that methanol (which simultaneously permeabilizes and fixes) yielded more uniform and consistent images with an overall higher protein content throughout the H441 cell layers (data not shown). A good review of the application-specific benefits and limitations of various fixation methods is provided by Wheatley and Wang (49).
Specifically, after the reopening bubble cleared the chamber, the monolayer was soaked in warmed serum-free RPMI-1640 medium for 1 h at 37°C and 5% CO2. The cell layer was rinsed in ice-cold PBS before its fixation by immersion in −20°C methanol for 12–18 h. The coverslip was air-dried and rehydrated by incubation for 30 min in 0.1% n-octyl-β-d-glucopyranoside in PBS (PBSO, Fisher Scientific, Pittsburgh, PA) containing 100 μM bis(sulfosuccinimidyl) suberate (BS3; Fisher Scientific, Pittsburgh, PA). Next, the epithelium was rinsed three times in PBSO for 5 min, and residual BS3 was quenched by soaking in 0.1 M ethylene diamine-HCl, pH 7.5 (Fisher Scientific, Pittsburgh, PA) for 15 min. After an additional set of three 5-min PBSO rinses, the coverslip was coated in blocking buffer (BB, 3% bovine serum albumin + 5% normal goat serum + PBS; Invitrogen, Carlsbad, CA) and incubated for 30 min. Next the BB was removed, and cells were incubated for 1 h with the 1° antibody, 2 μg/ml rabbit anti-ZO-1 and 1 μg/ml mouse anti-claudin-4 (Invitrogen, Carlsbad, CA), in BB. The monolayer was then washed three times using PBS and once in BB, 5 min apiece, before soaking for an additional 1 h in the 2° antibody, 3 μg/ml Alexa Fluor 488 goat anti-rabbit IgG (ex/em ∼500/520 nm; Invitrogen, Carlsbad, CA) and 0.4 μg/ml Alexa Fluor 568 goat anti-mouse IgG (ex/em ∼578/602 nm; Invitrogen, Carlsbad, CA) in BB, causing ZO-1 and claudin-4 to fluoresce green and red, respectively. After a final series of three PBS washes, 5 min apiece, excess liquid was removed, and ProLong Gold antifade reagent with 4,6-diamidino-2-phenylindole was applied, which prevented photobleaching and fluorescently labeled cell nuclei blue. The coverslip was then inverted onto a slide, and the following day its edges were sealed with clear nail polish (20).
Visualization and image analysis proceeded in a manner similar to the permeability studies detailed above. Again, we acquired four images per slide with eight slides per flow condition (control, UF and US). Using the green, red, and blue emission filters independently the distributions of ZO-1, claudin-4, and cell nuclei, respectively, were visualized within each randomly selected field using equal exposure times and gain settings. Image processing was carried out using the same algorithms as the permeability analysis: briefly, for each of the fluorescent colors, a background illumination map was generated from all images and then subtracted from the originals before a median filter removed residual noise. From these final images, we gauged the effects of our airway reopening model on ZO-1 and claudin-4 by summing the intensity values of all pixels that were above threshold and then dividing by the total cell count. These results were additionally normalized by the average ZO-1 or claudin-4 intensity per cell of the control group and are presented herein as means ± SE. Again, significant differences in our results were determined using a one-way ANOVA analysis and the Tukey post hoc test at P ≤ 0.05.
EXPERIMENTAL RESULTS
Paracellular Permeability
The photomicrographs of Fig. 3 depict the pattern of pulmonary epithelial wounding and increased paracellular permeability, labeled red with EthD-1 or green with BODIPY-ouabain, respectively, that have developed 1 h after a fast- (UF = 2.7 cm/s) or slow-velocity (US = 0.27 cm/s) experiment. The results of this study are summarized as means ± SE in Fig. 4, with paracellular permeability and cell wounding, respectively, quantified using the total area of green-fluorescing BODIPY-ouabain or of plasma membrane-compromised, EthD-1-positive cells relative to the total image area. This analysis shows for the first time that pulmonary epithelial paracellular permeability, in addition to cell wounding, is significantly increased (P < 0.05) as a result of interfacial flows. Specifically, for the range of velocities examined, we found that decreasing the reopening speed an order of magnitude significantly exacerbates pulmonary epithelial injury, with the area fraction of regions of enhanced paracellular permeability and of membrane-compromised cells increasing ∼1.8- and 3.5-fold, respectively. These observations coincide with the membrane-wounding studies of Bilek et al. (4), Kay et al. (24), and Yalcin et al. (53), further validating the conclusion that decreasing the velocity of reopening from UF to US causes significant pulmonary epithelial trauma. Also of note, the images of Fig. 3 suggest that the most significantly enhanced regions of barrier disruption tend to surround larger epithelial cells. We speculate that the topographic heterogeneities associated with such higher-profile pulmonary epithelial cells, which have been shown to locally magnify fluid stresses, particularly in junctional regions between adjacent cells (22), might further challenge the integrity of TJ proteins and cause this observed localized increase in paracellular permeability.
Fig. 3.
Fluorescent images of pulmonary epithelial layers stained for paracellular permeability using BODIPY-ouabain (green) and for plasma membrane rupture using ethidium homodimer-1 (EthD-1) (red). Images were captured 1 h after a fast (UF) or slow (US) reopening experiment or under control conditions. In the US image, representative areas of increased paracellular permeability that are relatively far removed from regions of cell wounding are indicated with white arrows. Scale bars are equivalent to 50 μm.
Fig. 4.
Percentage of the total field area stained with the green paracellular permeability indicator BODIPY-ouabain (shaded bars) or plasma membrane-compromised pulmonary epithelial cells with nuclei that stained positive (red) for EthD-1 (solid bars) 1 h post-airway reopening under UF, US, or control conditions. Results are means ± SE (n = 8 flow chambers for all groups). Significant differences (P < 0.05) between results: *permeability tests and #percent wounded assay.
Figure 5, A and B, demonstrate in detail the distribution of barrier dysfunction of the pulmonary epithelium with respect to wounded cells following slow or fast reopening, respectively. Here, the average pixel intensity of the green-fluorescing permeability indicator (Īouab) is presented as a function of the radial distance to the nearest red pixel of an EthD-1-stained cell nucleus. Since the maximum possible distance between green and red pixels within the US group is considerably shorter than that of the UF group due to the denser population of US-wounded cells, we scaled the independent (length) variable by the average distance between wounded (red) cells. This scaled representation serves to convey relative regional differences in paracellular permeability between the two groups. The primary plots of Fig. 5, A and B, show this scaled spatial distribution of paracellular transport [i.e., mean BODIPY-ouabain (green) pixel intensity], while Fig. 5 insets provide these data in the absence of scaling.
The intensity distributions of Fig. 5 show that Īouab is greatest near the origin of each plot (i.e., closest to EthD-1-stained nuclei). Assuming a monotonic relationship between pixel intensity and BODIPY-ouabain concentration (and thus also the paracellular permeability of the pulmonary epithelium), this indicates that regions of cell wounding are directly associated with enhanced barrier function disruption. Thus changes in cell polarity resulting from membrane disruption are likely to enhance paracellular permeability. However, these data also demonstrate that increased paracellular permeability exists in areas more distal to wounded cells (i.e., further from the plot origin), albeit to a much lesser degree. This indicates that diminished barrier function exists in the absence of pulmonary epithelial cell wounding and may be a direct stress response to the local mechanical stress field (see the Model Analysis below). Also of note, this analysis shows that the increases in pulmonary epithelial paracellular permeability following US reopening dominate those associated with UF reopening, both near to and far from a damaged epithelial cell.
TJ Analysis
The control images of Fig. 6 portray the distribution of claudin-4 and ZO-1 across a healthy, relatively impermeable pulmonary epithelium. Here, a prominent, continuous network of green-fluorescing ZO-1 and a fainter and more punctate system of claudin-4 colocalize at the plasma membrane to join individual cells across the entire cell layer. Claudin-4 and, to a lesser extent, ZO-1 are also found in the cellular cytosol. Following airway reopening, it is this intracellular TJ protein content, claudin-4 in particular, that experiences a discernable morphological change, increasing significantly under the more traumatic US conditions.
Fig. 6.
Pulmonary epithelial TJ protein immunofluorescence under control conditions (row 1) or 1 h after a fast (UF, row 2) or slow (US, row 3) reopening experiment. Isolated expression of claudin-4 is provided in column 1, while column 2 contains the corresponding ZO-1 expression. Claudin 4 (red) and ZO-1 (green) are merged in column 3, along with cell nuclei (blue). Scale bars represent 50 μm.
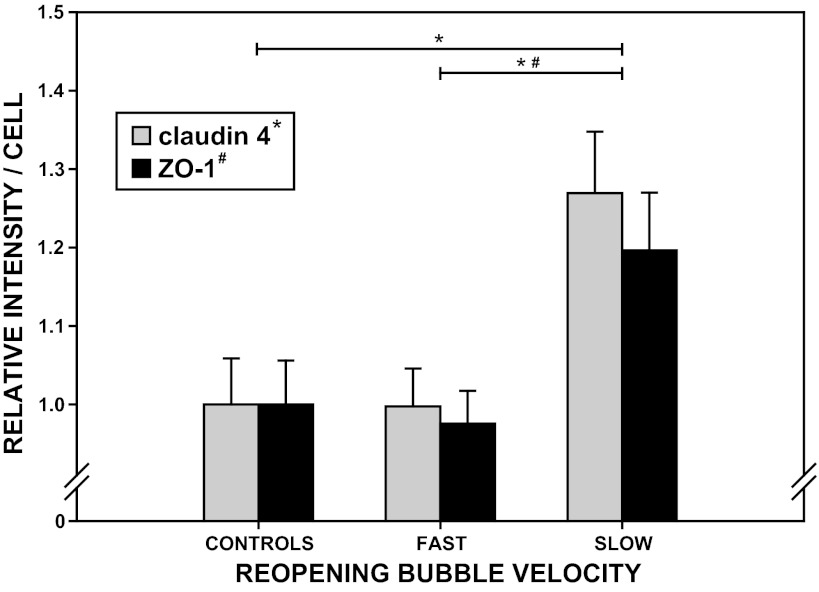
The total fluorescence intensity of ZO-1 and claudin-4 per cell, normalized to control values, are presented as means ± SE in Fig. 7. One-way ANOVA and Tukey post hoc comparisons indicated that the UF-to-US differences in ZO-1 and claudin-4 intensities were significant (P < 0.05). Consistent with our paracellular permeability and cell-wounding results, the greatest increase in both of these TJ proteins developed from US reopening. Conversely, the differences between UF and control results for both proteins were insignificant. In fact, with respect to the control group, only claudin-4 demonstrated a significant change in intensity, and this arose only in response to the more traumatic US-reopening condition.
Fig. 7.
Total intensity of red claudin-4 (shaded bars) and green ZO-1 (solid bars) relative to the total control intensities for the respective fluorophores 1 h post-airway reopening under fast (UF), slow (US), or control conditions using a 37°C PBS occlusion fluid. Results are normalized by the total number of cells contained in each image field and presented as means ± SE (n = 8 flow chambers for all groups). Significant differences between results (P < 0.05): *claudin-4 and #ZO-1.
Model Analysis
To elucidate the mechanical mechanisms responsible for these experimentally induced changes in paracellular permeability and TJ protein distribution across the pulmonary epithelium, we carried out a supplemental computational simulation. In previous analytic studies [Jacob and Gaver (22) and components of Bilek et al. (4) and Kay et al. (24)], we have computed the fluid mechanical stresses that are exerted on the apical surface of the pulmonary epithelium, xapical, during airway reopening. However, the manner by which these apical stresses transmit through the cell body to the basolateral cell surface, xbasolateral, where the normally and tangentially directed stresses between the cells and underlying substrate affect mechanotransduction and paracellular permeability, remained to be determined. In this light, we carried out an instructional investigation that incorporated the mechanical properties of the cellular epithelium into our parallel plate flow chamber analysis using the finite-element method. This analysis thus illustrates the apical-to-basolateral transmission of interfacial stresses exerted during a reopening event.
There are several conceptual models that might be appropriate to simulate cell material responses to mechanical stimuli. In our approach, the cell is characterized as a hyperelastic solid. Implicit in this model definition is the assumption that the material is isotropic, nearly elastic and incompressible, as well as highly compliant under shear. However, our analysis neglects the spatiotemporal variations in internal hydrostatic pressure that arise as the cytosol flows through the dense actin cytoskeleton. Such intracellular flows have been well-characterized in recent studies of cellular poroelasticity to model deformations such as blebbing that might occur during reopening (11, 12, 32, 33). Nonetheless, the hyperelastic model, which ensures that the pulmonary epithelium resists changes in volume as it deforms during the airway reopening simulation (5), serves well for the purposes of this gedanken experiment whose goal is to investigate the stresses that must be resisted at intercellular junctions.
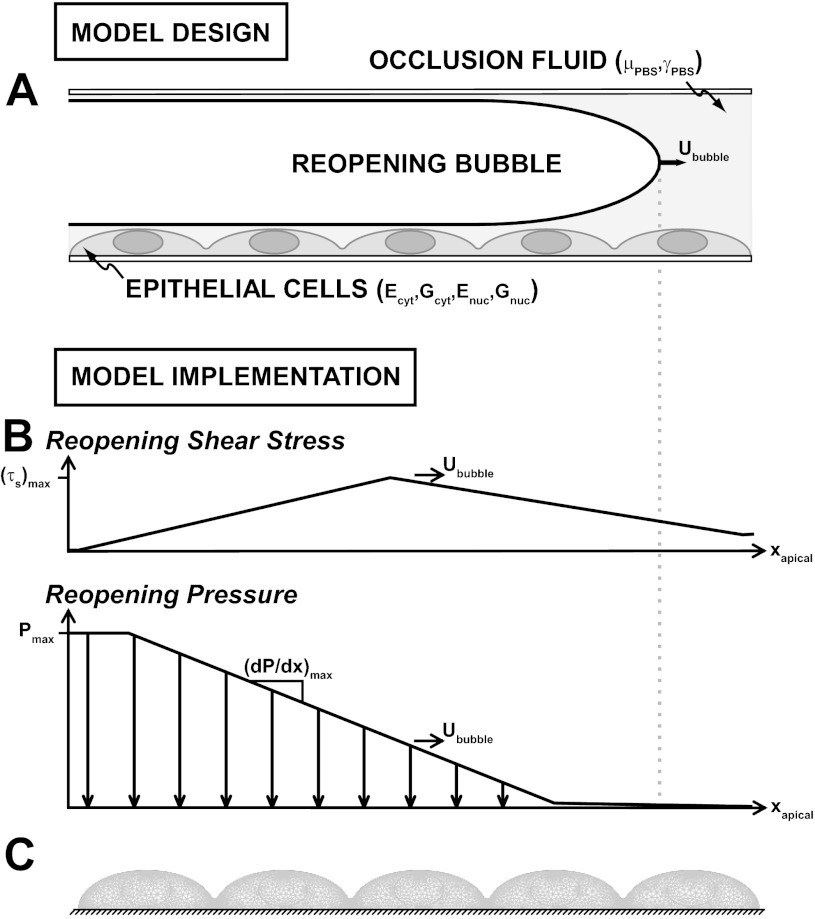
The model was designed to represent the experiments outlined in this paper, with a channel height of 2H = 1.7 mm; a fast or slow bubble velocity (i.e., “reopening velocity”), Ububble of UF = 2.7 cm/s or US = 0.27 cm/s, respectively; and fluid viscosity and surface tension magnitudes of μPBS = 8 × 10−3 g·cm−1·s−1 and γPBS = 70 dyn/cm. Cells were treated as hyperelastic protrusions, as depicted in Fig. 8A, with geometric and material properties that matched those of Caille et al. (5). The cell diameter and height were 31.25 μm and 8.75 μm, respectively; the cytoplasm and nucleus Young's moduli were Ecyt = 775 Pa and Enuc = 5,100 Pa, respectively; and the cytoplasm and nucleus shear moduli were Gcyt = 258 Pa and Gnuc = 1,700 Pa, respectively.
Fig. 8.
A: this computational study investigates the response of the incompressible, hyperelastic pulmonary epithelium to our parallel plate flow chamber model of airway reopening (not drawn to scale) using a neo-Hookean finite-element model. The cell cytoplasm and nucleus are treated as separate cell constituents with unique Young's and shear moduli (Ecyt, Enuc and Gcyt, Gnuc, respectively), and the saline (PBS) fluid occlusion is characterized by a viscosity and surface tension of μPBS and γPBS, respectively. B: the model was implemented by applying the reopening-induced shear stress, τs(xapical), and pressure, P(xapical), fields to the apical cell surface (i.e., the plasma membrane), xapical, as the reopening bubble swept through at a fast or slow velocity (Ububble = UF or US, respectively) using Eqs. 1–3. C: the meshed epithelial cell layer was free to deform in response to these reopening stresses over all but the basolateral cell surface, xbasolateral, which remained fixed to the rigid underlying substrate (i.e., glass coverslip). (dP/dx)max, maximal pressure gradient.
Pressure and shear stress were applied to the apical epithelial surface at either the UF or US using an idealized form of the computed mechanical stress fields of Jacob et al. (22) shown in Fig. 8B. During a single reopening event, an epithelial cell is exposed to this bubble tip-associated traveling mechanical stress field for a limited duration that ranges between ∼10 and 50 ms (for UF and US, respectively). Maximum values of the different components of fluid stress that develop in this airway reopening model (Fig. 8B, Table 1) were determined from the following regression equations (4):
| (1) |
| (2) |
| (3) |
where (dP/dx)max, (τs)max, and (dτs/dx)max are the maximum pressure gradient, shear stress, and shear stress gradient, respectively. The magnitude of the maximum pressure drop (Pmax ≈ γPBS/H), which develops far upstream of the bubble tip, is independent of the reopening velocity. This system was computationally simulated using COMSOL Multiphysics 3.2a (Burlington, MA) with quadratic Lagrangian elements of a global maximum element size of 0.5 μm, except near the highest elevation of each cell and the junctions between neighboring cells, where a local maximum element length of 0.1 μm was specified (as depicted in Fig. 8C). The epithelium was modeled using ∼7,000 body elements and ∼900 surface elements, with a total of 33,675 degrees of freedom.
Table 1.
| Ububble, cm/s | (τs)max, dyn/cm2 | Pmax, dyn/cm2 | (dP/dx)max, dyn/cm3 | |
|---|---|---|---|---|
| Fast (UF) | 2.7 | 31 | 820 | 35,200 |
| Slow (US) | 0.27 | 14 | 820 | 68,600 |
(τs)max, Pmax, and (dP/dx)max denote the maximum shear stress, pressure drop and pressure gradient, respectively, that develop in this model of airway reopening under fast or slow reopening velocity conditions (Ububble = UF or US, respectively).
The resulting vector field shown in Fig. 9A represents the pattern of surface stresses, T, between the basolateral cell membrane and underlying extracellular matrix that are generated when apically applied fluid stresses transmit through the body of the hyperelastic pulmonary epithelium during a single reopening event. This result demonstrates that T is minimal beneath the high-profile region of the relatively inelastic cell nucleus. Rather, the traveling apical fluid stresses distribute through the perinuclear cytoplasm toward the cell perimeter to substantially increase T in the region of cell-cell junctions. Biologically this could be advantageous for protecting the cell nucleus from the damaging reopening bubble front; on the other hand, it also should render the cell periphery more susceptible to plasma membrane rupture and the paracellular junctions more prone to excessive strain and disruption.
Fig. 9.
Results of the airway reopening simulation collected once the semi-infinite bubble had passed entirely through the channel. A: this image compares the initial epithelium (depicted in gray) and its final deformed shape (outlined in black, with displacement values amplified by a factor of 100) following US reopening. The basolateral vector field represents the relative magnitude and direction of surface stresses exerted by the pulmonary epithelium on the underlying rigid substrate during US reopening. The graphs below present the distribution of the normal, Ty (B) and tangential, Tx (C) components of the surface stresses acting on the basolateral cell membrane, xbasolateral, due to either slow, US (solid line), or fast, UF (dashed line), airway reopening. The vertical dotted lines indicate the location of the junctions between neighboring cells, and the abscissa is scaled by the diameter of a pulmonary epithelial cell in this study (Lcell = 31.25 μm).
Previous airway reopening analyses have shown that the fluid mechanics that govern airway reopening are dominated by a large interfacial (dP/dx)max that nearly doubles as the velocity is ratcheted down an order of magnitude (Table 1). Conversely, the relatively insignificant reopening-associated (τs)max remains well below accepted short-duration critical shear stress levels (26, 28) and only weakens with decreasing bubble velocity (4, 22, 24). It naturally follows that this mechanical stress-velocity relationship, which drives fluid flow over the apical cell surface, in this system translates to an inverse dependence on velocity of the reopening-induced basolateral stresses. This is demonstrated by Fig. 9, B and C, where airway reopening at US has considerably amplified the epithelial-substrate surface stresses over those that have developed from the UF scenario. This is most prominent in the junctional region between neighboring cells, where the magnitude of the normal component Ty (Fig. 9B), and the total jump in the tangential component, Tx (Fig. 9C), both increased by ∼70% when the velocity of reopening was decreased an order of magnitude to US.
DISCUSSION
Our system was designed to explore the relationship between cell wounding and barrier dysfunction that occurs during atelectrauma. As indicated by prior studies, the fluid-structure interactions that lead to epithelial wounding are dominated by a steep, transient pressure gradient near the bubble tip, (dP/dx)max, that is significantly greater when the bubble progresses more slowly. In contrast, tangential stresses at the apical surface of the cell are vanishingly small and are not implicated in cell wounding. However, our computations indicate that this apical stress field results in a steep gradient in the tangential component of the basolateral surface stresses, dTx/dx (i.e., the slope of Tx in Fig. 9C) that is greatest at cell-cell junctions. This could considerably strain TJs and other intercellular junctions, as well as basolateral integrins at focal adhesion sites. In fact, just as basolateral dTx/dx was amplified by increased apical (dP/dx)max during the slower reopening maneuver, particularly in the region of the thinner cell periphery, our corresponding in vitro studies have demonstrated an attendant inverse dependence on reopening velocity of paracellular permeability and TJ protein alteration. This suggests that increases in pulmonary epithelial basolateral dTx/dx imposed by interfacial flows enhance TJ disruption and paracellular permeability during more damaging reopening maneuvers. This mechanical stress could contribute to the cell and tissue stretch that has been shown in stretch-only VILI studies to increase pulmonary epithelial plasma membrane rupture and paracellular permeability and would exacerbate harmful basolateral stresses and contribute to escalating respiratory distress and pulmonary edema during mechanical ventilation (7–9, 13, 40, 47). In the subsections below, we discuss the nuances related to this study.
Paracellular Permeability
The paracellular permeability analysis described herein unequivocally shows that US reopening significantly enhances the leakiness of the pulmonary epithelium to the paracellular flux of liquids and solutes over all of other conditions investigated. In fact, Fig. 10A, which demonstrates the difference between Īouab due to US and UF reopening (i.e., Fig. 5A minus Fig. 5B), illustrates that the relative increases in paracellular permeability under the slower reopening condition dominate both near to and far from a damaged epithelial cell. This lends additional support to the conclusion that the predominant apical (dP/dx)max associated with slower interfacial velocities, as well as the corresponding basolateral dTx/dx, is the primary damaging mechanical stimulus during airway reopening (4, 22, 24, 53). It is now evident that this stimulus not only causes cell membrane disruption, but also increases paracellular permeability, with or without cell wounding, which could lead to pulmonary edema and the influx of proteinaceous infiltrates that may further deactivate pulmonary surfactant and exacerbate lung injury during mechanical ventilation.
While barrier disruption arises across the epithelial layer, regardless of flow rate, it is most prominent closest to wounded epithelial cells. To illustrate this point, Fig. 10B represents the cumulative amount of BODIPY-ouabain that has passed through the epithelium during the first hour following a reopening event as a function of the distance from the nearest wounded cell nucleus. This result clearly demonstrates that increases in paracellular permeability are greatest within one cell length of a wounded nucleus (∼40 μm, as demarcated by the vertical dotted line). The total BODIPY-ouabain content continues to build slowly beyond this point for several hundreds of micrometers. This reinforces our understanding that the presence of edema fluid in the lungs is not always symptomatic of more catastrophic forms of cellular injury such as plasma membrane rupture, although pulmonary epithelial plasma membrane rupture is highly likely to induce pulmonary edema. In addition, Fig. 10B shows that US reopening is far more destructive to barrier function than the UF scenario, in both the presence and absence of cell wounding, with the total difference between UF and US reopening-induced paracellular permeability approaching a factor of 2.7. Thus optimal mechanical ventilation strategies for reintroducing air into the lung should avoid decreasing flow rates to values equivalent to US under depleted surfactant conditions.
TJ Analysis
While we have shown that cell wounding considerably increases paracellular permeability, it is also important to examine reopening-induced changes in cellular TJs that could further disrupt the barrier properties of the pulmonary epithelium and significantly impact cellular recovery following airway reopening. We thus examined the cell layers for changes in two critical TJ proteins, ZO-1 and claudin-4, that under healthy conditions are responsible for impeding protein and fluid leakage across the pulmonary epithelium. While these investigations demonstrated an increase in the total content of both ZO-1 and claudin-4 (Fig. 4) within the first hour following a US reopening event, claudin-4 alone (stained red in Fig. 3) exhibited a distinctive and substantial increase, predominantly of the intracellular rather than membrane-bound content. Conversely, ZO-1 (stained green in Fig. 3) was more resistant to such macro-scale, observable changes and did not undergo a statistically significant increase or decrease relative to control conditions. We speculate that the stability of ZO-1 is a consequence of its direct interactions with the actin cytoskeleton as well as several other proteins, including those of the gap and adherens junctions (17, 23). In contrast, claudin 4 is solely involved with TJs and functionally resides in the plasma membrane. The multiprotein and cytoskeletal associations of ZO-1 may, therefore, provide additional structural support that is unavailable to the transmembranous claudin-4 and may render ZO-1 less susceptible to dislocation and damage during airway reopening.
These results are consistent with TJ and barrier function analyses performed elsewhere. For example, an internalization of claudin-4 from the cell periphery has been found to occur on the same hour-long timescale during Eph4 mammary epithelial cell remodeling (30). In addition, a recent investigation by Wray et al. (51) revealed an analogous counterintuitive increase in claudin-4 mRNA and protein expression in whole mouse lungs subjected to 3 h of high-volume ventilation, despite enhanced levels of pulmonary edema. In ex vivo perfused human lungs, this research group also found that alveolar fluid clearance rates are positively correlated with claudin-4 levels but do not change with varying ZO-1 quantities (37). They have attributed these changes in claudin-4 to an adaptation by the epithelium that could bolster alveolo-capillary defense against continued fluid flooding. Similarly, Mitchell et al. (31) have demonstrated a direct relationship between claudin-4 expression and barrier function in primary rat alveolar epithelial cells. Further investigation is necessary to better discern the precise mechanisms responsible for these alterations to pulmonary epithelial claudin-4 during airway reopening, e.g., does the cytosolic accumulation of this TJ protein result from its dissociation from the plasma membrane or, rather, from its upregulation by reopening-associated mechanotransduction? The answers to these questions may be key to developing improved modes of mechanical ventilation and supplemental therapies that fortify the TJs against the influx of pulmonary edema, minimize VILI, and enhance subsequent recovery of the pulmonary epithelium.
Conclusions
Mechanical ventilation of patients in respiratory distress can injure the pulmonary epithelium and heighten airway and alveolar susceptibility to infiltration by edema fluid. The proteins contained within this fluid exudate may deactivate pulmonary surfactant, counteracting its protective effects and intensifying the mechanical stresses experienced by the already compromised epithelial cell layer (18). As VILI continues and pulmonary edema progresses, lung function further diminishes. This may increase patient risk for developing ARDS and associated multiorgan dysfunction/failure syndrome. The present in vitro study was designed to examine the pulmonary epithelial injury response to airway reopening with the goal of gaining deeper insight into the underlying physical stressors that lead to pulmonary edema during mechanical ventilation to work toward improved treatment strategies for respiratory distress that minimize lung injury and the incidence and severity of ARDS.
In summary, we explored the process of airway reopening using a range of approaches, including 1) experimental assessment of the cell- and protein-level changes that arise as airflow is initiated in a fluid-occluded airway and 2) computational determination of the mechanical stresses that develop along the basolateral surface of the pulmonary epithelium. Our investigation indicates that decreasing the velocity of reopening from UF = 2.7 cm/s to US = 0.27 cm/s significantly augments pulmonary epithelial barrier dysfunction, as evidenced by an increase in paracellular permeability and TJ protein (ZO-1 and claudin-4) alteration. While cell wounding plays a certain role in determining barrier function, these permeability and TJ protein changes do not always coincide spatially with regions of increased plasma membrane rupture.
These cellular responses are induced by a large fluid pressure gradient, (dP/dx)max, that sweeps across the apical epithelial membrane with the traveling reopening air front. This imposes significant fore-aft pressure differences along the length of the pulmonary epithelial cell. Our analysis shows that this fluid stress transmits through the cell body to induce large tangential gradients in basolateral stresses, dTx/dx, particularly in the junctional region between neighboring cells. Heightened dTx/dx levels could significantly strain TJs and promote the breakdown of pulmonary epithelial barrier function during airway reopening.
It is evident from the present study that an attenuation of the fluid pressure gradient is necessary for both the reduction of cell wounding and the maintenance of barrier function during airway reopening. This reinforces the need for a higher velocity mechanical ventilation strategy to minimize pulmonary epithelial damage and manage respiratory distress under conditions where surfactant is inactive or nonexistent. Our investigation suggests that a temporary high-flow rate reopening maneuver could provide a favorable course of action for ensuring minimal atelectrauma while rapidly introducing air into fluid-filled, edematous airways and alveoli. However, under conditions where surfactant exists at low concentrations, an unsteady reopening scenario may be protective, because it can enhance surfactant concentrations and minimize pressure gradients (18, 34).
The development of ventilation scenarios that inflict minimal harm and can take into account the fluid structure and physicochemical interactions that exist in a diseased lung is a challenging endeavor. Furthermore, the nonuniform nature and distribution of cell wounding and barrier disruption demonstrate that natural biological variability exists on multiple scales in the pulmonary system. To design protective ventilation strategies, a computational approach based on systems biology must include both deterministic and stochastic components that can be used to predict the intensity and distribution of damage in the lung model and the feedback that can exist due to surfactant deactivation by proteinaceous edema fluid in affected air spaces. Furthermore, these models must include a network of interdependent patent and obstructed airways, each with their own lining fluid and tissue properties and unique mechanical interactions with surrounding airways and alveoli (3, 25, 27, 41, 44, 50). The present study provides insight into the constitutive relationships from which such a model could be based.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-81266 and National Science Foundation Grant CBET-1033619.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.-M.J. and D.P.G. conception and design of research; A.-M.J. performed experiments; A.-M.J. and D.P.G. analyzed data; A.-M.J. and D.P.G. interpreted results of experiments; A.-M.J. prepared figures; A.-M.J. and D.P.G. drafted manuscript; A.-M.J. and D.P.G. edited and revised manuscript; A.-M.J. and D.P.G. approved final version of manuscript.
REFERENCES
- 1. ARDSNet Ventilation with lower tidal volumes compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342: 1301–1308, 2000 [DOI] [PubMed] [Google Scholar]
- 2. ATCC Product Information Sheet for HTB-174. Manassas, VA: ATCC, 2009 [Google Scholar]
- 3. Bates JH. Stochastic model of the pulmonary airway tree and its implications for bronchial responsiveness. J Appl Physiol 75: 2493–2499, 1993 [DOI] [PubMed] [Google Scholar]
- 4. Bilek AM, Dee KC, Gaver DP. Mechanisms of surface-tension-induced epithelial cell damage in a model of pulmonary airway reopening. J Appl Physiol 94: 770–783, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Caille N, Thoumine O, Tardy Y, Meister JJ. Contribution of the nucleus to the mechanical properties of endothelial cells. J Biomech 35: 177–187, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, Golland P, Sabatini DM. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol 7: R100.101–R100.111, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cavanaugh KJ, Cohen TS, Margulies SS. Stretch increases alveolar epithelial permeability to uncharged micromolecules. Am J Physiol Cell Physiol 290: C1179–C1188, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cavanaugh KJ, Margulies SS. Measurement of stretch-induced loss of alveolar epithelial barrier integrity with a novel in vitro method. Am J Physiol Cell Physiol 283: C1801–C1808, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Cavanaugh KJ, Oswari J, Margulies SS. Role of stretch on tight junction structure in alveolar epithelial cells. Am J Respir Cell Mol Biol 25: 584–591, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Cereijido M, Ehrenfeld J, Fernàndez-Castelo S, Meza I. Fluxes, junctions, and blisters in cultured monolayers of epithelioid cells (MDCK). Ann N Y Acad Sci 372: 422–441, 1981 [DOI] [PubMed] [Google Scholar]
- 11. Charras GT, Coughlin M, Mitchison TJ, Mahadevan L. Life and times of a cellular bleb. Biophys J 94: 1836–1853, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Charras GT, Yarrow JC, Horton MA, Mahadevan L, Mitchison TJ. Non-equilibration of hydrostatic pressure in blebbing cells. Nature 435: 365–369, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cohen TS, Cavanaugh KJ, Margulies SS. Frequency and peak stretch magnitude affect alveolar epithelial permeability. Eur Respir J 32: 854–861, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Contreras RG, Avila G, Gutierrez C, Bolívar JJ, González-Mariscal L, Darzon A, Beaty G, Rodriguez-Boulan E, Cereijido M. Repolarization of Na+-K+ pumps during establishment of epithelial monolayers. Am J Physiol Cell Physiol 257: C896–C906, 1989 [DOI] [PubMed] [Google Scholar]
- 15. Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev 75: 519–560, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ghadiali SN, Gaver DP. Biomechanics of liquid-epithelium interactions in pulmonary airways. Respir Physiol Neurobiol 163: 232–243, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giepmans BN, Moolenaar WH. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr Biol 8: 931–934, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Glindmeyer HW, Smith BJ, Gaver DP. In situ enhancement of pulmonary surfactant function using temporary flow reversal. J Appl Physiol 112: 149–158, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gonzalez-Mariscal L, Chávez de Ramírez B, Cereijido M. Tight junction formation in cultured epithelial cells (MDCK). J Membr Biol 86: 113–125, 1985 [DOI] [PubMed] [Google Scholar]
- 20. Hermanns MI, Unger RE, Kehe K, Peters K, Kirkpatrick CJ. Lung epithelial cell lines in coculture with human pulmonary microvascular endothelial cells: development of an alveolo-capillary barrier in vitro. Lab Invest 84: 736–752, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Huh D, Fujioka H, Tung YC, Futai N, Paine Rr Grotberg JB, Takayama S. Acoustically detectable cellular-level lung injury induced by fluid mechanical stresses in microfluidic airway systems. Proc Natl Acad Sci U S A 104: 18886–18891, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jacob AM, Gaver DP. An investigation of the influence of cell topography on epithelial mechanical stresses during pulmonary airway reopening. Phys Fluids 17: 031502 (DOI:10.1063/1.1862642), 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kapus A, Szaszi K. Coupling between apical and paracellular transport processes. Biochem Cell Biol 84: 870–880, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Kay SS, Bilek AM, Dee KC, Gaver DP. Pressure gradient, not exposure duration, determines the extent of epithelial cell damage in a model of pulmonary airway reopening. J Appl Physiol 97: 269–276, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Kitaoka H, Suki B. Branching design of the bronchial tree based on a diameter-flow relationship. J Appl Physiol 82: 968–976, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Kretzmer G, Schügerl K. Response of mammalian cells to shear stress. Appl Microbiol Biotechnol 34: 613–616, 1991 [DOI] [PubMed] [Google Scholar]
- 27. Leary D, Bhatawadekar SA, Parraga G, Maksym GN. Modeling stochastic and spatial heterogeneity in a human airway tree to determine variation in respiratory system resistance. J Appl Physiol 112: 167–75, 2012 [DOI] [PubMed] [Google Scholar]
- 28. Ludwig A, Kretzmer G, Schügerl K. Determination of a “critical shear stress level” applied to adherent mammalian cells. Enzyme Microb Technol 14: 209–213, 1992 [DOI] [PubMed] [Google Scholar]
- 29. Martynowicz MA, Minor TA, Walters BJ, Hubmayr RD. Regional expansion of oleic acid-injured lungs. Am J Respir Crit Care Med 160: 250–258, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Matsuda M, Kubo A, Furuse M, Tsukita S. A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J Cell Sci 117: 1247–1257, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Mitchell LA, Overgaard CE, Ward C, Margulies SS, Koval M. Differential effects of claudin-3 and claudin-4 on alveolar epithelial barrier function. Am J Physiol Lung Cell Mol Physiol 301: L40–L49, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mitchison TJ, Charras GT, Mahadevan L. Implications of a poroelastic cytoplasm for the dynamics of animal cell shape. Semin Cell Dev Biol 19: 215–223, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oeckler RA, Hubmayr R. Cell wounding and repair in ventilator injured lungs. Respir Physiol Neurobiol 163: 44–53, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pillert JE, Gaver DP. Physicochemical effects enhance surfactant transport in pulsatile motion of a semi-infinite bubble. Biophys J 96: 312–327, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pitelka DR, Taggart BN, Hamamoto ST. Effects of extracellular calcium depletion on membrane topography and occluding junctions of mammary epithelial cells in culture. J Cell Biol 96: 613–624, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 282: 54–61, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Rokkam D, Lafemina MJ, Lee JW, Matthay MA, Frank JA. Claudin-4 levels are associated with intact alveolar fluid clearance in human lungs. Am J Pathol 179: 1081–1087, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rubenfeld GD. Epidemiology of acute lung injury. Crit Care Med 31: S276–S284, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Savla U, Waters CM. Mechanical strain inhibits repair of airway epithelium in vitro. Am J Physiol Lung Cell Mol Physiol 274: L883–L892, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Suki B. Fluctuations and power laws in pulmonary physiology. Am J Respir Crit Care Med 166: 133–137, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Sznajder JI. Alveolar edema must be cleared for the acute respiratory distress syndrome patient to survive. Am J Respir Crit Care Med 163: 1293–1294, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Tavana H, Zamankhan P, Christensen PJ, Grotberg JB, Takayama S. Epithelium damage and protection during reopening of occluded airways in a physiologic microfluidic pulmonary airway model. Biomed Microdevices 13: 731–742, 2011 [DOI] [PubMed] [Google Scholar]
- 44. Thorpe CW, Bates JH. Effect of stochastic heterogeneity on lung impedance during acute bronchoconstriction: a model analysis. J Appl Physiol 82: 1616–1625, 1997 [DOI] [PubMed] [Google Scholar]
- 45. Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 163: 1376–1383, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Ware LB, Matthay MA. Medical progress: the acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Waters CM, Savla U. Keratinocyte growth factor accelerates wound closure in airway epithelium during cyclic mechanical strain. J Cell Physiol 181: 424–432, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Weibel ER, Gomez DM. Architecture of the Human Lung. Science 137: 577–585, 1962 [DOI] [PubMed] [Google Scholar]
- 49. Wheatley SP, Wang YL. Indirect immunofluorescence microscopy in cultured cells. Methods Cell Biol 57: 313–332, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Winkler T, Suki B. Emergent structure-function relations in emphysema and asthma. Crit Rev Biomed Eng 39: 263–280, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wray C, Mao Y, Pan J, Chandrasena A, Piasta F, Frank JA. Claudin-4 augments alveolar epithelial barrier function and is induced in acute lung injury. Am J Physiol Lung Cell Mol Physiol 297: L219–L227, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yalcin HC, Hallow KM, Wang J, Wei MT, Ou-Yang HD, Ghadiali SN. Influence of cytoskeletal structure and mechanics on epithelial cell injury during cyclic airway reopening. Am J Physiol Lung Cell Mol Physiol 297: L881–L891, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Yalcin HC, Perry SF, Ghadiali SN. Influence of airway diameter and cell confluence on epithelial cell injury in an in vitro model of airway reopening. J Appl Physiol 103: 1796–1807, 2007 [DOI] [PubMed] [Google Scholar]