Abstract
When exposed to chronic hypoxia (CH), the pulmonary circulation responds with enhanced contraction and vascular remodeling, resulting in elevated pulmonary arterial pressures. Our work has identified CH-induced alterations in the expression and activity of several ion channels and transporters in pulmonary vascular smooth muscle that contribute to the development of hypoxic pulmonary hypertension and uncovered a critical role for the transcription factor hypoxia-inducible factor-1 (HIF-1) in mediating these responses. Current work is focused on the regulation of HIF in the chronically hypoxic lung and evaluation of the potential for pharmacological inhibitors of HIF to prevent, reverse, or slow the progression of pulmonary hypertension.
Keywords: hypoxia-inducible factor-1, pulmonary hypertension, chronic hypoxia
the lung is a unique organ in many respects. The pulmonary circulation is the only vasculature required to accommodate the entire cardiac output and does so at arterial pressures ∼10 times lower than the systemic circulation. The pulmonary vasculature is also unique in its response to hypoxia. In contrast to the systemic circulation, which dilates with hypoxia in an attempt to increase oxygen delivery to meet the metabolic demands of tissues, the pulmonary vessels constrict as oxygen tension falls. The first demonstration of this phenomenon can be attributed to Beutner [(7) and reviewed in (75)], working in the lab of Henry Pickering Bowditch's mentor, Carl Ludwig. In 1946, the first detailed study characterizing this response in the intact cat was published by von Euler and Liljestrand (81). Although the precise teleology of hypoxic pulmonary vasoconstriction remains an area of debate, it is widely held that when the hypoxic challenge is short in duration and localized, as can happen with a small embolism or pneumonia, the vasoconstriction serves to divert blood from oxygen-poor areas of the lung to optimize ventilation/perfusion matching. However, when the hypoxic stimulus is global and prolonged, as can occur with residence at high altitude or many lung diseases, widespread vasoconstriction results in increased pulmonary vascular resistance, elevated pulmonary arterial pressure, and when severe and long enough in duration, eventual right heart failure. Although many of the structural and functional changes that occur in the lung with exposure to prolonged hypoxia have been documented, the mechanisms underlying the pathogenesis of hypoxic pulmonary hypertension remain incompletely understood. The following sections will focus on our work identifying some of the changes in pulmonary arterial smooth muscle cells (PASMCs) that occur in response to chronic hypoxia (CH), the role of the transcription factor, hypoxia-inducible factor 1 (HIF-1), in mediating these changes and recent work describing the regulation of HIF-1 in the hypoxic lung.
CHRONIC HYPOXIA AND THE LUNG
There are numerous instances, both physiological and pathological, during which the lung experiences prolonged exposure to localized or global hypoxia. For example, during embryogenesis, lung development occurs in a hypoxic environment (27). With respect to pathological conditions, prolonged alveolar hypoxia is a consequence of chronic lung diseases such as emphysema, chronic bronchitis, and cystic fibrosis and results in the development of pulmonary hypertension, which can have a deletorius effect on patient mortality and morbidity with the potential for eventual right heart failure. With current treatment modalities limited primarily to supplemental oxygen, mechanical ventilation, and lung transplant, the development of new therapeutic approaches to prevent and/or reverse pulmonary hypertension requires an understanding of the cellular mechanisms underlying pulmonary vascular responses to CH.
To explore the impact of long-term hypoxic exposure on the pulmonary circulation, investigators have developed and utilized animal models, in particular rats or mice exposed to normobaric or hypobaric hypoxia (PiO2 = 10% O2) for 14–28 days in an environmental chamber. By using these rodent models, investigators have shown that pulmonary hypertension is attributable to both pulmonary vascular remodeling, characterized by smooth muscle cell proliferation, intimal thickening, and extension of smooth muscle into previously nonmuscular arterioles (10, 39, 71, 72, 80), and active contraction of vascular smooth muscle (21, 43, 48). Despite considerable advances in knowledge regarding the structural and functional changes that occur in the pulmonary vasculature in response to CH, the cellular mechanisms underlying the PASMC contraction, migration, hypertrophy, and hyperplasia that characterize pulmonary hypertension remain poorly understood.
ABNORMALITIES IN PULMONARY ARTERIAL SMOOTH MUSCLE CELLS
Although it is clear that the pathogenesis of hypoxia-induced pulmonary hypertension involves changes in circulating factors and hemodynamic forces, an abundance of data has accumulated demonstrating that both the sustained vasoconstriction and vascular remodeling associated with CH may be related to abnormalities in the PASMCs. In particular, a number of studies now suggest that changes in PASMC function may be related to changes in membrane transporter/channel expression and intracellular ion concentrations, with our lab and others reporting hypoxia-induced changes in K+ channels, Ca2+ channels, and pH regulation.
Effect of Chronic Hypoxia on K+ Channels
K+ channels are the primary regulators of resting membrane potential (Em) in PASMCs (3, 96). In turn, Em controls the activity of sarcolemmal voltage-dependent Ca2+ channels (VDCC) and, thus, calcium influx and intracellular Ca2+ homeostasis. Changes in intracellular calcium concentration ([Ca2+]i) are critical for modulating PASMC function, with increased [Ca2+]i required for PASMC contraction (37, 59, 60), proliferation (12, 24, 74), and migration (28). PASMCs express a variety of K+ channel family subtypes; however, in adult animals under normal conditions, members of the voltage-gated K+ (KV) channel family bear most of the responsibility for controlling basal Em. Inhibition of KV channels leads to membrane depolarization, activation of VDCC, and increased [Ca2+]i (3, 96), and initial experiments reported that PASMCs isolated from rats exposed to CH exhibited depolarization (73) and reduced KV channel activity (68), suggesting that prolonged exposure to hypoxia altered K+ channel regulation and/or expression. Given that the effects of hypoxia were maintained for several hours after return to normoxic conditions, coupled with the fact that most adaptations to CH are accomplished by changes in mRNA expression, a reasonable explanation was that the decrease in KV channel activity observed in these studies was mediated by transcriptional regulation of KV channel expression. This hypothesis was first tested in vitro, where culture of PASMCs under hypoxic conditions for 3 days decreased expression of mRNAs encoding several KV channel α (pore-forming)-subunits, including KV1.1, KV1.5, and KV2.1 (82). These results suggested that hypoxia could directly repress K+ channel expression; however, it could also be argued that the effect of short-term hypoxia in cultured cells may not accurately reflect the effects of CH on K+ channel expression in the intact animal, where the duration of exposure and level of hypoxia are likely to be different and where changes in hemodynamic stresses and/or locally produced or circulating factors may alter the response. To address this possibility, intact animal models of CH were used to test the effect of CH on pulmonary vascular smooth muscle KV channel expression in vivo (Fig. 1). The results from these studies (15, 52, 85) were similar to those obtained in vitro, suggesting that the reductions in KV current observed by our lab and others in PASMCs from chronically hypoxic rats could be attributable to a reduction in KV channel density.
Fig. 1.
Effect of chronic hypoxia (CH) on mRNA expression of voltage-gated K+ (KV) channel family members KV1.1, KV1.2, KV1.5, and KV2.1 in endothelium-denuded resistance pulmonary arteries (PA) and aorta isolated from normoxic (N) and chronically hypoxic (CH; 3 wk at FiO2 = 10% O2) rats. Levels of the gene of interest were normalized to β-actin levels within the sample. n = 3 or 4 animals per condition. *Significantly different from N (P < 0.05 via Student's t-test). Data modified with permission from Wang et al. (85).
Along with the pore-forming α-subunits, PASMCs also express several regulatory β-subunits that associate with α-subunits and exert an inhibitory influence by accelerating inactivation and/or shifting the voltage sensitivity of the α-subunits. Expression of inhibitory KV β-subunits was unaffected by in vitro (82) or in vivo (85) hypoxic exposure, suggesting that a reduction in KV channel number coupled with a maintained levels of inhibitory β-subunits (thereby increasing α- and β-subunit interactions) is the likely underlying cause of reduced KV current density. In stark contrast to the effects of CH on KV channel expression observed in pulmonary arteries, CH had no effect on KV channel expression in aortas from these animals (85), indicating that the regulation of KV channel expression by CH is a pulmonary-specific response. The mechanisms underlying differential effects of CH on pulmonary and systemic smooth muscle are still under investigation.
Effect of Chronic Hypoxia on Ca2+ Homeostasis
Alterations in Ca2+ homeostasis are associated with both hypoxic pulmonary vasoconstriction and PASMC proliferation and migration (12, 24, 28, 37, 59, 60, 74, 87). The finding that PASMCs from chronically hypoxic animals were depolarized, presumably secondary to decreased KV channel activity, gave rise to the hypothesis that increased [Ca2+]i attributable to activation of VDCC was the mechanism underlying hypoxic pulmonary hypertension (68, 73). However, this supposition was not supported by in vivo data, which demonstrated that voltage-gated Ca2+ channel antagonists did not prevent development of hypoxia-associated pulmonary hypertension (20), or by clinical data showing that acute administration of vasodilators (41) but not Ca2+ channel antagonists (20), reduced pulmonary artery pressure in patients with hypoxic pulmonary hypertension attributable to chronic obstructive pulmonary disease. In attempting to answer whether activation of VDCCs was a driving force in the development and progression of hypoxic pulmonary hypertension, we initially investigated the effects of CH on Ca2+ homeostasis in PASMCs (67), demonstrating that basal [Ca2+]i was increased and confirming profound changes in Ca2+ regulation. That this increase in [Ca2+]i was rapidly normalized by removal of extracellular Ca2+ was not surprising and supported the hypothesis that active Ca2+ entry was required to maintain elevated [Ca2+]i. However, the finding that VDCC blockers did not alter resting [Ca2+]i (67) was unexpected and ruled out influx through voltage-dependent enhancement of Ca2+ channel activity during CH. Similar findings came from isolated vessels studies, where removal of extracellular Ca2+ relaxed arteries from chronically hypoxic rats, whereas blockade of VDCCs had no effect on tone (67). Although these data confirmed that the increase in resting [Ca2+]i maintained sustained contraction of the pulmonary arteries during CH, they also eliminated a role for VDCCs.
Ca2+ influx in PASMCs can also occur via nonselective cation channels (NSCCs), which include a large family of proteins that appear to encode for both receptor-operated Ca2+ channels and store-operated Ca2+ channels. NSCCs are not activated by depolarization; rather, receptor-operated channels are activated by ligand binding to membrane receptors whereas store-operated Ca2+ channels are activated by depletion of intracellular stores. A main function of store-operated Ca2+ influx, or capacitative Ca2+ entry (CCE), is to replenish endoplasmic reticulum/sarcoplasmic reticulum stores. Functional studies have revealed that CCE is present in PASMCs (12, 83) and is involved in PASMC contraction and growth (12, 74). We (84) and others (29) found that CCE is greater in PASMCs from chronically hypoxic rats compared with PASMCs from normoxic rats and contributes to the maintenance of elevated basal [Ca2+]i during CH because inhibitors of these channels decreased resting [Ca2+]i in PASMCs from hypoxic, but not normoxic, animals (Fig. 2).
Fig. 2.
Effect of nonselective cation channel inhibitors on resting intracellular calcium concentration ([Ca2+]i) in pulmonary arterial smooth muscle cells from normoxic and chronically hypoxic rats. Data were collected from 10–30 cells per field and averaged to obtain a single value per experiment; each experiment was conducted on cells from different animals. [Ca2+]i levels were measured using fluorescent microscopy in Fura-2-loaded cells before (control) and 15 min after exposure to the nonselective cation channel inhibitors: A, SKF96365 (50 μM); B, NiCl2 (500 μM). n = 3 or 4 animals per condition. *Significantly different from normoxic control; †significantly from hypoxic control. Data modified with permission from Wang et al. (84).
Enhanced CCE following exposure to CH is attributable to increased expression of Ca2+-permeable NSCCs, which are believed to be comprised of mammalian homologs of transient receptor potential (TRP) proteins, particularly isoforms in the canonical TRP (TRPC) subfamily, alone or in a complex with Orai1 and stromal interacting protein 1 (STIM1) proteins. Several of these proteins are known to be expressed in PASMCs (12, 29, 31, 83, 84), and experiments using RNA interference techniques have revealed that TRPC1, TRPC6, STIM1, and Orai1 all contribute to CCE in PASMCs (30, 45, 95). Examination of the levels of TRPC proteins in pulmonary vascular smooth muscle from normoxic and chronically hypoxic rats revealed that the expression of TRPC1 and TRPC6, but not TRPC4, increased in both the rat and murine models of hypoxic pulmonary hypertension (84).
To determine whether the effect of CH exposure on TRPC expression could be attributable to stimuli other than hypoxia (i.e., increased mechanical forces or altered exposure to circulating factors), PASMCs isolated from normoxic rats were cultured under hypoxic conditions (4% O2; 60 h). PASMCs exposed to hypoxia ex vivo also exhibited increased TRPC1 and TRPC6 mRNA and protein levels and an increase in basal [Ca2+]i (84), indicating that CH upregulated TPRC expression through a direct effect on mRNA expression in PASMCs.
Effect of Chronic Hypoxia on Intracellular pH
Mammalian systems possess three primary mechanisms for maintaining intracellular pH (pHi) homeostasis: the Na+-dependent Cl−/HCO3− exchange, Na+-independent Cl−/HCO3− exchange and Na+/H+ exchange (NHE). All of these exchangers are present in vascular smooth muscle (32, 55), with NHE appearing to be the main mechanism responsible for regulating PASMC pHi (55). The Na+/H+ exchanger is a plasmalemmal protein that uses the transmembrane Na+ gradient to extrude protons. Tight control of NHE and pHi is critical for maintaining cell viability, volume regulation, and mediator release. Early studies indicated that an increase in pHi occurs during exposure of PASMCs to acute hypoxia (32) and during sustained hypoxic contraction (25). The importance of NHE in regulating cell function was underscored by studies showing that activation of NHE and alkalinization were required for growth factor-induced PASMC proliferation (53) and that hypoxia-induced pulmonary vascular remodeling was prevented by inhibitors of NHE (54).
In our initial studies examining the effects of CH on pH homeostasis, we found that basal pHi and NHE activity were elevated in PASMCs from chronically hypoxic animals (58). Because inhibitors of NHE reduced basal pHi in PASMCs from chronically hypoxic animals to near normal levels, we concluded that the alkaline shift in pHi was attributable to increased NHE activity. The fact that the change in pHi and NHE activity in PASMCs isolated from chronically hypoxic mice was observed for several days after returning to normoxic conditions suggested that the increase in NHE activity most likely resulted from an increase in NHE protein expression. Thus we examined the effect of CH on NHE expression in pulmonary vascular smooth muscle. Ten genes have been identified that encode different isoforms of the Na+/H+ exchanger (NHE1–10). NHEs 1–3 have been the most widely studied, with NHE1 ubiquitously expressed and NHE2 and NHE3 found predominately in the gastrointestinal epithelium, although low-level expression of NHE2 in the lung has been reported (9, 86). Little is known about the function and localization of NHE4 and NHE5, neither of which are present in the lung (4, 49) and NHE6–10 are thought to be localized in mitochrondria, other organelles, or nonpulmonary tissues (14, 40, 44, 46, 47). By using RT-PCR and immunoblot, we demonstrated the presence of NHE1, but not NHE2 or NHE3, in mouse pulmonary vascular smooth muscle (58, 65). Examination of tissue from chronically hypoxic mice revealed that NHE1 mRNA and protein expression were increased significantly, correlating with the effect of CH on NHE activity and basal pHi (58). A recent study using mice deficient for NHE1 revealed that these animals exhibited reduced pulmonary vascular remodeling and decreased pulmonary hypertension in response to CH (94), confirming the importance of NHE1 in pulmonary vascular changes induced by CH. These data indicate that CH has dramatic effects on PASMC function and pH homeostasis via induction of NHE1.
ROLE OF HYPOXIA-INDUCIBLE FACTOR
After demonstrating that induction of NHE1 and TRPC proteins and downregulation of KV channel expression during CH occurred at the level of transcription, we began to search for mechanisms that would meditate these responses. The hypoxia-inducible factors (HIFs), a family of oxygen-sensitive transcription factors, have been identified as critical mediators of adaptive responses to hypoxia, regulating the expression of dozens of genes important in growth, vascular development, and metabolism. The initial family member, hypoxia-inducible factor 1 (HIF-1), was identified as a basic helix-loop-helix protein bound to the hypoxia response element of the EPO gene during hypoxia (63). HIF-1 is highly conserved, tightly regulated by O2 availability, found in all nucleated cells, and has been demonstrated to regulate the expression of hundreds of genes (61, 62). HIF-1 is a heterodimer, composed of HIF-1α, which is found at very low levels under normoxic conditions, and HIF-1β, which is ubiquitously expressed. Thus it is the HIF-1α subunit that confers sensitivity and specificity for hypoxic induction of HIF-1 transcriptional activity.
The mechanism by which cells sense and transduce a drop in oxygen into increased HIF-1 activity was discovered in 2001 (Fig. 3) when several groups reported that HIF-1α ubiquitination required hydroxylation at two proline residues by prolyl hydroxylase domain (PHDs) proteins using molecular O2 as a substrate (11, 17, 19, 34, 93). As O2 levels decrease, PHD activity is reduced, hydroxylation at the proline residues ceases, and the protein is stabilized and translocates into the nucleus, where it binds HIF-1β and recruits coactivator proteins to the HIF binding site within the hypoxia response element. To date, four PHD isoforms have been identified, although only PHD1–3 appear to hydroxylate HIF-1, with evidence suggesting that PHD2 is the primary isoform responsible for HIF-1α hydroxylation in vivo (2, 6, 38). In general, HIF-1α protein accumulation correlates with increased transcriptional activity; however, transactivation of HIF-1 is regulated by factor inhibiting HIF-1 (FIH-1), which hydroxylates HIF-1α at an asparagine residue within the COOH-terminal transactivation domain and prevents binding of the transcriptional coactivators CBP and p300 (33).
Fig. 3.
Schematic illustrating the regulation of HIF-1α. Under normoxic conditions, prolyl hydroxylase domain (PHD) proteins use molecular oxygen as a substrate to hydroxylate HIF-1α. Once hydroxylated, HIF-1α binds von Hippel-Lindau (VHL) protein and becomes polyubiquitylated (Ub) and targeted for proteosomal degradation. Under hypoxic conditions, PHD activity is reduced and HIF-1α escapes hydroxylation, accumulating and translocating to the nucleus where it binds with HIF-1β and CBP/p300 at the hypoxia response element (HRE).
Regulation of Hypoxia-Inducible Factor in the Lung
As described in the preceding sections, we and others have shown that CH induces changes in PASMC ion channel/transporter expression. In the search for a possible mediator of hypoxic regulation of these genes, the transcription factor, HIF-1, became a leading candidate. Regulation of HIF-1α expression occurs at several levels. In mouse lung, HIF-1α mRNA is rapidly increased within 30 min of exposure to 7% oxygen (89). The oxygen-dependence of HIF-1α protein expression was initially demonstrated in an isolated, perfused ferret lung preparation, where oxygen levels could be precisely controlled and in the in vitro setting where acute exposure of cultured PASMCs and pulmonary endothelial cells to hypoxia increased HIF-1α protein levels (91). Accumulation of HIF-1α protein under these conditions was correlated with enhanced DNA binding activity, demonstrating both induction and activation of HIF-1.
In addition to hypoxia, HIF-1 can be activated by several physiological stimuli, including transforming growth factor-β, insulin, hydralazine, epidermal growth factor, platelet-derived growth factor, and angiotensin II (23, 36, 50, 57, 64, 77, 78, 97). Our studies have unexpectedly revealed an exciting new paradigm whereby the vasoconstrictor peptide, endothelin-1 (ET-1), itself a HIF target, regulates HIF-1 expression, leading to a positive feedback mechanism. Exposing PASMCs to exogenous ET-1, which mimics the release of ET-1 from endothelial cells, induced HIF-1α in the absence of hypoxia (51), findings that are in line with recent results in ovarian cancer and melanoma cells (69, 70). Moreover, we found that at moderate levels of hypoxia, which are at or near the threshold for HIF-1α induction (91) and are similar to those occurring in the pulmonary circulation with an FiO2 of 10% (76), ET-1 is required for accumulation of HIF-1α in PASMCs, because upregulation of HIF-1 was prevented by ET-1 subtype A (ETA) receptor antagonists (51). This feedforward mechanism of HIF-1 induction by ET-1 does not appear to be a general feature of all cells, because aortic smooth muscle cells do not exhibit HIF-1 induction in response to either moderate hypoxia or ET-1, raising the tantalizing possibility that the presence of this mechanism in PASMCs underlies the different functional response to hypoxia observed between these cell types.
Hypoxia Inducible Factor-1 and Ion Channels/Transporters
To evaluate the in vivo role of HIF-1, Iyer et al. (18) generated mouse embryonic stem cells homozygous or heterozygous for a null allele at the Hif1a locus exhibiting complete (Hif1a−/−) and partial deficiency (Hif1a+/−) for HIF-1α, respectively, that were subsequently used to generate transgenic Hif1a−/− and Hif1a+/− mice. Hif1a−/− embryos died midgestation, whereas Hif1a+/− mice were viable and phenotypically indistinguishable from their wild-type (Hif1a+/+) littermates (18). Hif1a+/+ mice exposed to 10% O2 for 3 wk exhibit right heart hypertrophy, elevated pulmonary artery pressure, polycythemia, and vascular remodeling (Fig. 4); however, these changes were markedly attenuated in chronically hypoxic Hif1a+/− mice (92), demonstrating that HIF-1α plays a pivotal role in development of hypoxic pulmonary hypertension. We then utilized Hif1a+/+ and Hif1a+/− mice to evaluate the role of HIF-1 in mediating the effect of CH on ion channels/transporters. With respect to KV channels, decreased KV current and channel expression, and concomitant depolarization were observed in PASMCs isolated from Hif1a+/+ mice, whereas the effects of CH were reduced or absent in PASMCs from Hif1a+/− mice (66, 88), indicating that full expression of HIF-1α was required for the hypoxia-induced reduction in PASMC KV channel activity. That HIF activation mediated repression of KV channels was further demonstrated by the finding that overexpression of HIF-1 under normoxic conditions using an adenovirus that encodes a constitutively active form of HIF-1α (22) was able to cause downregulation of KV1.5 and KV2.1 expression (88).
Fig. 4.
Reduced vascular remodeling in CH mice with partial deficiency for HIF-1α (Hif1a+/−). Mice were exposed to room air or FiO2 = 10% O2 for 3 wk. A: representative images show left lung sections stained for smooth muscle specific α-actin (SMA; brown) and counterstained with hematoxylin and eosin. Images are of vessels negative (left) and positive (right) for SMA. B: bar graph shows means ± SE data for the number of SMA-positive small diameter (<100 μm) vessels as a percentage of the total vessels counted per lung in normoxic (N) and CH wild-type (Hif1a+/+) and Hif1a+/− mice. n = 3 or 4 lungs per group. C: bar graph shows means ± SE data for PASMC proliferation measured using an ELISA for BrDU incorporation. Cells (5,000/well) isolated from normoxic Hif1a+/+ and Hif1a+/− mice were plated in basal media (Ham's F-12 with 0.5% serum) and exposed to control (20% O2; 5% CO2) or hypoxic (4% O2; 5% CO2) conditions for 72 h. BrDU was added for an additional 24 h and incorporation into proliferating cells measured via ELISA. Absorbance values were normalized to control Hif1a+/+ within an experiment. n = 3 or 4 animals for each condition.
The transcriptional regulation of TRPCs and NHEs is just beginning to be explored and much is yet to be learned with respect to the factors involved in the process. Because the genes encoding TRPC1, TRPC6, and NHE1 all contain putative HIF-1 binding sites, and previous work demonstrated a crucial role for HIF-1 in the pathogenesis of hypoxic pulmonary hypertension (92), we hypothesized that HIF-1 might be involved in the hypoxic induction of TRPC and NHE1 proteins. As anticipated based on data from chronically hypoxic rats, exposure to CH markedly increased TRPC1, TRPC6, and NHE1 expression in endothelium-denuded pulmonary arteries isolated from Hif1a+/+ mice (65, 84). Functionally, PASMCs isolated from these animals displayed elevated [Ca2+]i, an alkaline shift in pHi, and increased NHE activity. In mice with partial HIF-1α deficiency, the effects of CH on basal [Ca2+]i pHi and NHE activity and the hypoxic induction of TRPC1, TRPC6, and NHE1 were absent (84). To verify that the hypoxia-induced increase in protein expression was attributable to activation of HIF-1 and not an unrelated aspect of hypoxic exposure, HIF-1α was overexpressed in rat PASMCs isolated from normoxic animals and cultured under nonhypoxic conditions. Following expression of a constitutively active form of HIF-1α, TRPC1, TRPC6, and NHE1 expression were increased, as were resting pHi and NHE activity (65, 84).
Taken together, these data from loss-of-function and gain-of-function models provided strong evidence that HIF-1 was both necessary and sufficient for hypoxia-induced alterations in KV, TRPC, and NHE1 proteins and indicated that HIF-1 plays a critical role in the hypoxic regulation of K+, Ca2+, and pHi homeostasis during CH (Fig. 5). Although all of the genes encoding these ion channels/transporters contain putative HIF binding sites, it remains to be determined 1) whether HIF-1 directly binds to the genes encoding these channels/transporters to regulate the induction/repression observed with CH; 2) whether other HIF-dependent intermediates may be involved; and 3) whether these changes are regulated independently or if alterations in the expression/activity of one channel can then modulate the expression of others.
Fig. 5.
Schematic detailing the effects of CH on pulmonary arterial smooth muscle cell ion homeostasis. Exposure to CH induces HIF-1, which leads to upregulation of Na+/H+ exchange isoform 1 (NHE1) and transient receptor potential canonical family member 1 (TRPC1) and downregulation of voltage-gated K+ channel family member 1.5 (KV1.5). Alterations in channel/transporter expression cause depolarization and increased intracellular K+ concentration ([K+]i), an alkaline shift in intracellular pH (pHi), and elevated intracellular calcium concentration ([Ca2+]i), resulting in a cell phenotype that is more contractile, proliferative, and/or migratory, contributing to the development of pulmonary hypertension.
Ongoing Experiments: Treatments Targeting Hypoxia Inducible Factor
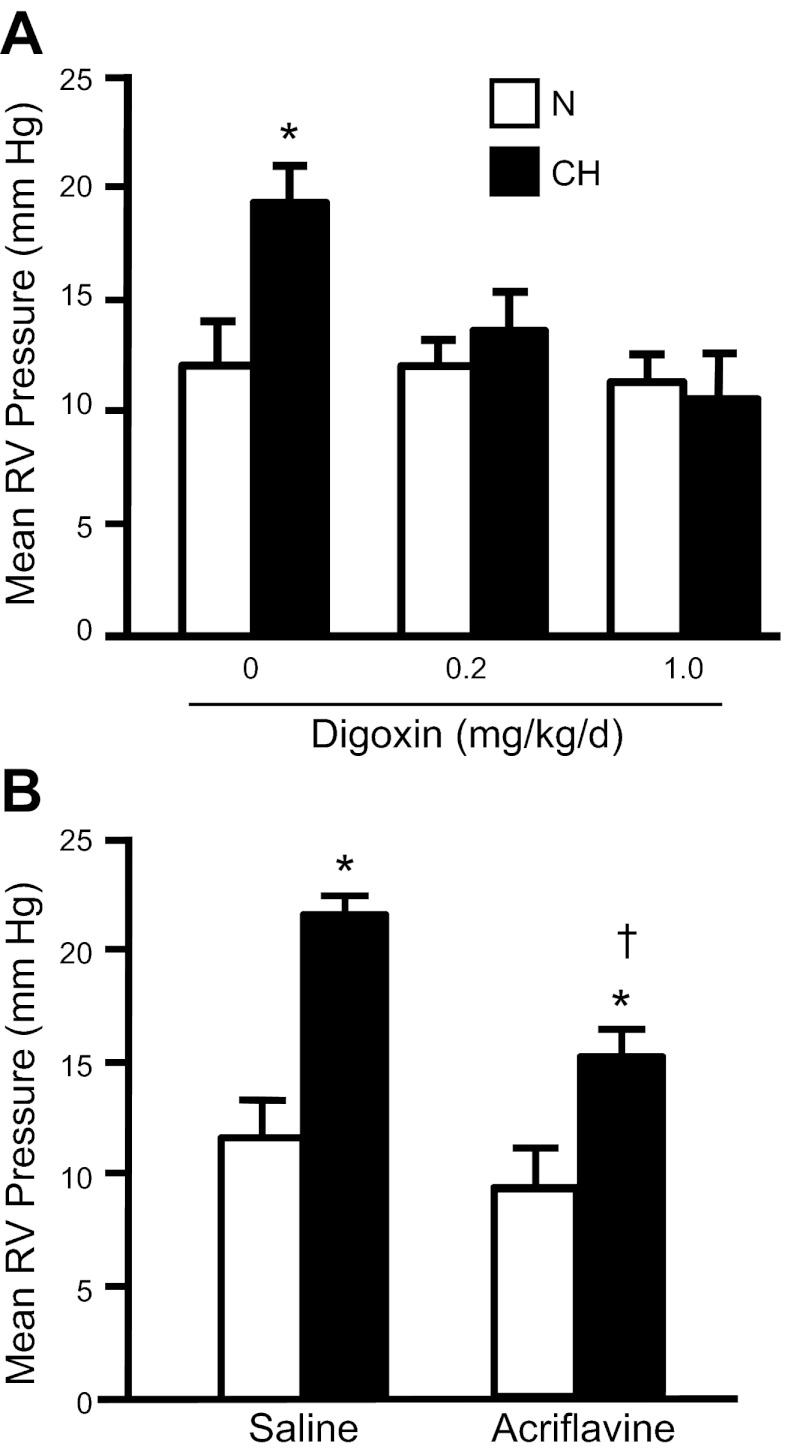
Given their wide distribution throughout the body and the general lack of isoform-selective inhibitors, targeting TRPC1/6 or NHE1 for inhibition as a treatment for pulmonary hypertension was not a clinically reasonable approach. In contrast, HIF-1 is generally expressed at very low levels in adult tissues and only increases under certain conditions, providing an attractive target for therapeutic intervention. Until recently, inhibiting HIF-1 was a daunting task; however, in 2008, screening of 3,120 clinically used compounds in the Johns Hopkins Drug Library revealed several drugs that inhibit HIF-1α (98), including 11 cardiac glycosides. Digoxin, which has been used for decades as an inotrope to treat heart failure, was found to inhibit HIF-1α protein translation and blocked HIF-1 activity in vivo (90, 98). In chronically hypoxic mice receiving daily injections of digoxin, we found that the changes in pulmonary vascular [Ca2+]i, pHi, remodeling, and pressure (Fig. 6) were absent (1). Similar effects were observed with acriflavine (1), an antiseptic that prevents HIF-1α/HIF-1β dimerization (26). When digoxin treatment was initiated after pulmonary hypertension was established, right ventricular systolic pressure was reduced and Ca2+ and pH homeostasis was normalized (1). These data provide further evidence that HIF-1 plays a critical role in the development of hypoxia-induced pulmonary hypertension and demonstrate the ability of digoxin to slow the progression of the process. That elevated levels of HIF-1α and similar defects in PASMC function were observed in the lung of patients with other forms of pulmonary hypertension (8, 16, 42, 79) raises the possibility that digoxin, or other HIF inhibitors, might reduce pulmonary vascular pressure and remodeling in pulmonary hypertension not associated with hypoxia. Although digoxin has been proposed to increase cardiac contractility and output in patients with right ventricular failure (35, 56), the use of this drug in the pulmonary hypertension population remains controversial on the basis of the small therapeutic window, potential toxicity in the COPD patient population (5, 13), and a lack of data supporting a positive survival effect. Nonetheless, these data provide “proof of concept” of the potential beneficial effects of HIF-1 inhibitors and provide a starting point for further investigation.
Fig. 6.
Effect of HIF inhibitors (digoxin and acriflavine) on mean right ventricular (RV) pressure in normoxic (N) and CH (10% O2 for 3 wk) animals. A: bar graph shows means ± SE values for mean RV pressure in adult male C57/B6 mice treated with digoxin (0.2 or 1.0 mg/kg daily ip injection, beginning 1 day prior to hypoxia) or saline (0 mg/kg digoxin). RV pressure was measured in lightly anesthetized animals via closed-chest puncture with a needle-tip pressure transducer. n = 4–6 animals per group. *Significant difference from N value of the same treatment. B: bar graphs show means ± SE values for mean RV pressure in adult male Wistar rats treated with acriflavine (2 mg·kg−1·day−1 ip) or saline. n = 5 per group. *Significant difference from normoxic value within treatment; †significant difference from hypoxic saline value by one-way ANOVA with Bonferroni post hoc test (P < 0.05).
CONCLUSIONS
Over the past two decades, we have endeavored to better understand the molecular signals that result in enhanced pulmonary arterial smooth muscle cell contraction, proliferation, and migration during the development of hypoxic pulmonary hypertension, with the hope that the information obtained could be used to interrogate novel therapeutic strategies. Relating the alterations in pulmonary arterial smooth muscle cell ion homeostasis that occur in chronic hypoxia models to the mechanistic underpinnings of different forms and severities of human pulmonary hypertension continues to pose a significant challenge. However, in a disease where treatment options are limited, our studies have pointed to possible cellular mechanisms involved in the pathogenesis of pulmonary hypertension, and although questions remain as to whether inhibitors of hypoxia-inducible factors will ultimately prove clinically beneficial in pulmonary hypertension patients, recent findings have demonstrated that old drugs can be taught new tricks. Clearly, much work is yet to be done, which will most certainly keep us occupied for the next two decades and beyond.
GRANTS
The author is appreciative of support and funding from the National Institutes of Health, the American Physiological Society, and the American Heart Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
Author contributions: L.A.S. conception and design of research; L.A.S. performed experiments; L.A.S. analyzed data; L.A.S. interpreted results of experiments; L.A.S. prepared figures; L.A.S. drafted manuscript; L.A.S. edited and revised manuscript; L.A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
This work would not have been possible without the talent and dedication of past and current members of the lab: Tish Weigand, Clark Undem, Sarah Pisarcik, Jian Wang, Michele Fallon, Eon Rios, E. Miles Whitman, Julie Maylor, and Wenju Lu. I have been fortunate to have had wonderful mentors during my training, including Jane Madden and James Sham. I am particularly grateful for ongoing collaboration with two individuals who have contributed significantly to the progression of my career: J. T. Sylvester, who has provided invaluable support, collegiality, and mentorship, and Gregg L. Semenza, who has generously provided advice and reagents for several of the studies described. I also wish to thank members of Dr. Semenza's lab who contributed to this work, including Aimee Yu, who was instrumental in jump-starting the line of investigation regarding HIF, and Dominador Manalo.
REFERENCES
- 1. Abud EM, Maylor J, Undem C, Punjabi A, Zaiman AL, Myers AC, Sylvester JT, Semenza GL, Shimoda LA. Digoxin inhibits development of hypoxic pulmonary hypertension in mice. Proc Natl Acad Sci USA 109: 1239–1244, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, Ratcliffe PJ, Gleadle JM. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem 279: 38458–38465, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Archer SL, Souil E, Dinh-Xuan AT, Schremmer B, Mercier JC, El Yaagoubi A, Nguyen-Huu L, Reeve HL, Hampl V. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv21, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest 101: 2319–2330, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Attaphitaya S, Park K, Melvin JE. Molecular cloning and functional expression of a rat Na+/H+ exchanger (NHE5) highly expressed in brain. J Biol Chem 274: 4383–4388, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Beller GA, Smith TW, Abelmann WH, Haber E, Hood WB., Jr Digitalis intoxication. A prospective clinical study with serum level correlations. N Engl J Med 284: 989–997, 1971 [DOI] [PubMed] [Google Scholar]
- 6. Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1α in normoxia. EMBO J 22: 4082–4090, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beutner A. Ueber die strom-und druckkrafte des blutes in der arteria pulmonalis. Z Rationelle Med 2: 97–138, 1852 [Google Scholar]
- 8. Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thebaud B, Bonnet S, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK, Archer SL. An abnormal mitochondrial-hypoxia inducible factor-1α-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation 113: 2630–2641, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Brant SR, Yun CH, Donowitz M, Tse CM. Cloning, tissue distribution, and functional analysis of the human Na+/H+ exchanger isoform, NHE3. Am J Physiol Cell Physiol 269: C198–C206, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Davies P, Maddalo F, Reid L. Effects of chronic hypoxia on structure and reactivity of rat lung microvessels. J Appl Physiol 58: 795–801, 1985 [DOI] [PubMed] [Google Scholar]
- 11. Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJC. Elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107: 43–54, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol 280: H746–H755, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Green LH, Smith TW. The use of digitalis in patients with pulmonary disease. Ann Intern Med 87: 459–465, 1977 [DOI] [PubMed] [Google Scholar]
- 14. Hofstetter W, Siegrist M, Simonin A, Bonny O, Fuster DG. Sodium/hydrogen exchanger NHA2 in osteoclasts: subcellular localization and role in vitro and in vivo. Bone 47: 331–340, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Hong Z, Weir EK, Nelson DP, Olschewski A. Subacute hypoxia decreases voltage-activated potassium channel expression and function in pulmonary artery myocytes. Am J Respir Cell Mol Biol 31: 337–343, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 43: 13S-24S, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292: 464–468, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev 12: 149–162, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Johnson DC, Joshi RC, Mehta R, Cunnington AR. Acute and long term effect of nifedipine on pulmonary hypertension secondary to chronic obstructive airways disease. Eur J Respir Dis 146: 495–502, 1986 [PubMed] [Google Scholar]
- 21. Jones K, Higenbottam T, Wallwork J. Pulmonary vasodilation with prostacyclin in primary and secondary pulmonary hypertension. Chest 96: 784–789, 1989 [DOI] [PubMed] [Google Scholar]
- 22. Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res 93: 1074–1081, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Knowles HJ, Tian YM, Mole DR, Harris AL. Novel mechanism of action for hydralazine: induction of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and angiogenesis by inhibition of prolyl hydroxylases. Circ Res 95: 162–169, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Kruse HJ, Bauriedel G, Heimerl J, Hofling B, Weber PC. Role of l-type calcium channels on stimulated calcium influx and on proliferative activity of human coronary smooth muscle cells. J Cardiovasc Pharmacol 24: 328–335, 1994 [PubMed] [Google Scholar]
- 25. Leach RM, Sheehan DW, Chacko VP, Sylvester JT. Energy state, pH, and vasomotor tone during hypoxia in precontracted pulmonary and femoral arteries. Am J Physiol Lung Cell Mol Physiol 278: L294–L304, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Lee K, Zhang H, Qian DZ, Rey S, Liu JO, Semenza GL. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc Natl Acad Sci USA 106: 17910–17915, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27. Lee YM, Jeong CH, Koo SY, Son MJ, Song HS, Bae SK, Raleigh JA, Chung HY, Yoo MA, Kim KW. Determination of hypoxic region by hypoxia marker in developing mouse embryos in vivo: a possible signal for vessel development. Dev Dyn 220: 175–186, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Leggett K, Maylor J, Undem C, Lai N, Lu W, Schweitzer KS, King LS, Myers AC, Sylvester JT, Sidhaye VK, Shimoda LA. Hypoxia-induced migration in pulmonary arterial smooth muscle cells requires calcium-dependent upregulation of aquaporin 1. Am J Physiol Lung Cell Mol Physiol 303: L343–L353, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 95: 496–505, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Lu W, Wang J, Peng G, Shimoda LA, Sylvester JT. Knockdown of stromal interaction molecule 1 attenuates store-operated Ca2+ entry and Ca2+ responses to acute hypoxia in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 297: L17–L25, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lu W, Wang J, Shimoda LA, Sylvester JT. Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol 295: L104–L113, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Madden JA, Ray DE, Keller PA, Kleinman JG. Ion exchange activity in pulmonary artery smooth muscle cells: the response to hypoxia. Am J Physiol Lung Cell Mol Physiol 280: L264–L271, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev 15: 2675–2686, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ. Independent function of two destruction domains in hypoxia-inducible factor-α chains activated by prolyl hydroxylation. EMBO J 20: 5197–5206, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McLaughlin VV, Rich S. Pulmonary hypertension. Curr Probl Cardiol 29: 575–634, 2004 [DOI] [PubMed] [Google Scholar]
- 36. McMahon S, Charbonneau M, Grandmont S, Richard DE, Dubois CM. Transforming growth factor β1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J Biol Chem 281: 24171–24181, 2006 [DOI] [PubMed] [Google Scholar]
- 37. McMurtry IF, Davidson AB, Reeves JT, Grover RF. Inhibition of hypoxic pulmonary vasoconstriction by calcium antagonists in isolated rat lungs. Circ Res 38: 99–104, 1976 [DOI] [PubMed] [Google Scholar]
- 38. Metzen E, Berchner-Pfannschmidt U, Stengel P, Marxsen JH, Stolze I, Klinger M, Huang WQ, Wotzlaw C, Hellwig-Burgel T, Jelkmann W, Acker H, Fandrey J. Intracellular localisation of human HIF-1α hydroxylases: implications for oxygen sensing. J Cell Sci 116: 1319–1326, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Meyrick B, Reid L. The effect of continued hypoxia on rat pulmonary arterial circulation. An ultrastructural study. Lab Invest 38: 188–200, 1978 [PubMed] [Google Scholar]
- 40. Miyazaki E, Sakaguchi M, Wakabayashi S, Shigekawa M, Mihara K. NHE6 protein possesses a signal peptide destined for endoplasmic reticulum membrane and localizes in secretory organelles of the cell. J Biol Chem 276: 49221–49227, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Moinard J, Manier G, Pillet O, Castaing Y. Effect of inhaled nitric oxide on hemodynamics and VA/Q inequalities in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 149: 1482–1487, 1994 [DOI] [PubMed] [Google Scholar]
- 42. Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 54: S20–31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nagaoka T, Fagan KA, Gebb SA, Morris KG, Suzuki T, Shimokawa H, McMurtry IF, Oka M. Inhaled Rho kinase inhibitors are potent and selective vasodilators in rat pulmonary hypertension. Am J Respir Crit Care Med 171: 494–499, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Nakamura N, Tanaka S, Teko Y, Mitsui K, Kanazawa H. Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J Biol Chem 280: 1561–1572, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Ng LC, Ramduny D, Airey JA, Singer CA, Keller PS, Shen XM, Tian H, Valencik M, Hume JR. Orai1 interacts with STIM1 and mediates capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 299: C1079–C1090, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Numata M, Orlowski J. Molecular cloning and characterization of a novel (Na+,K+)/H+ exchanger localized to the trans-Golgi network. J Biol Chem 276: 17387–17394, 2001 [DOI] [PubMed] [Google Scholar]
- 47. Numata M, Petrecca K, Lake N, Orlowski J. Identification of a mitochondrial Na+/H+ exchanger. J Biol Chem 273: 6951–6959, 1998 [DOI] [PubMed] [Google Scholar]
- 48. Oka M, Morris KG, McMurtry IF. NIP-121 is more effective than nifedipine in acutely reversing chronic pulmonary hypertension. J Appl Physiol 75: 1075–1080, 1993 [DOI] [PubMed] [Google Scholar]
- 49. Orlowski J, Kandasamy RA, Shull GE. Molecular cloning of putative members of the Na/H exchanger gene family. cDNA cloning, deduced amino acid sequence, and mRNA tissue expression of the rat Na/H exchanger NHE-1 and two structurally related proteins. J Biol Chem 267: 9331–9339, 1992 [PubMed] [Google Scholar]
- 50. Phillips RJ, Mestas J, Gharaee-Kermani M, Burdick MD, Sica A, Belperio JA, Keane MP, Strieter RM. Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1α. J Biol Chem 280: 22473–22481, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Pisarcik S, Sylvester JT, Semenza GL, Shimoda LA. Endothelin-1 (ET-1) induces hypoxia-inducible factor 1 (HIF-1) in pulmonary arterial smooth muscle cells (PASMCs). FASEB J 22: 1209–1222., 2008 [Google Scholar]
- 52. Pozeg ZI, Michelakis ED, McMurtry MS, Thebaud B, Wu XC, Dyck JR, Hashimoto K, Wang S, Moudgil R, Harry G, Sultanian R, Koshal A, Archer SL. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation 107: 2037–2044, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Quinn DA, Dahlberg CG, Bonventre JP, Scheid CR, Honeyman T, Joseph PM, Thompson BT, Hales CA. The role of Na+/H+ exchange and growth factors in pulmonary artery smooth muscle cell proliferation. Am J Respir Cell Mol Biol 14: 139–145, 1996 [DOI] [PubMed] [Google Scholar]
- 54. Quinn DA, Du HK, Thompson BT, Hales CA. Amiloride analogs inhibit chronic hypoxic pulmonary hypertension. Am J Respir Crit Care Med 157: 1263–1268, 1998 [DOI] [PubMed] [Google Scholar]
- 55. Quinn DA, Honeyman TW, Joseph PM, Thompson BT, Hales CA, Scheid CR. Contribution of Na+/H+ exchange to pH regulation in pulmonary artery smooth muscle cells. Am J Respir Cell Mol Biol 5: 586–591, 1991 [DOI] [PubMed] [Google Scholar]
- 56. Rich S, Seidlitz M, Dodin E, Osimani D, Judd D, Genthner D, McLaughlin V, Francis G. The short-term effects of digoxin in patients with right ventricular dysfunction from pulmonary hypertension. Chest 114: 787–792, 1998 [DOI] [PubMed] [Google Scholar]
- 57. Richard DE, Berra E, Pouyssegur J. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1α in vascular smooth muscle cells. J Biol Chem 275: 26765–26771, 2000 [DOI] [PubMed] [Google Scholar]
- 58. Rios EJ, Fallon M, Wang J, Shimoda LA. Chronic hypoxia elevates intracellular pH and activates Na+/H+ exchange in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 289: L867–L874, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Rodman DM, Yamaguchi T, O'Brien RF, McMurtry IF. Hypoxic contraction of isolated rat pulmonary artery. J Pharmacol Exp Ther 248: 952–959, 1989 [PubMed] [Google Scholar]
- 60. Salvaterra CG, Goldman WF. Acute hypoxia increases cytosolic calcium in cultured pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 264: L323–L328, 1993 [DOI] [PubMed] [Google Scholar]
- 61. Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell 148: 399–408, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology 24: 97–106, 2009 [DOI] [PubMed] [Google Scholar]
- 63. Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12: 5447–5454, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Seshadri V, Fox PL, Mukhopadhyay CK. Dual role of insulin in transcriptional regulation of the acute phase reactant ceruloplasmin. J Biol Chem 277: 27903–27911, 2002 [DOI] [PubMed] [Google Scholar]
- 65. Shimoda LA, Fallon M, Pisarcik S, Wang J, Semenza GL. HIF-1 regulates hypoxic induction of NHE1 expression and alkalinization of intracellular pH in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 291: L941–L949, 2006 [DOI] [PubMed] [Google Scholar]
- 66. Shimoda LA, Manalo DJ, Sham JS, Semenza GL, Sylvester JT. Partial HIF-1α deficiency impairs pulmonary arterial myocyte electrophysiological responses to hypoxia. Am J Physiol Lung Cell Mol Physiol 281: L202–L208, 2001 [DOI] [PubMed] [Google Scholar]
- 67. Shimoda LA, Sham JS, Shimoda TH, Sylvester JT. l-type Ca2+ channels, resting [Ca2+]i, and ET-1-induced responses in chronically hypoxic pulmonary myocytes. Am J Physiol Lung Cell Mol Physiol 279: L884–L894, 2000 [DOI] [PubMed] [Google Scholar]
- 68. Smirnov SV, Robertson TP, Ward JP, Aaronson PI. Chronic hypoxia is associated with reduced delayed rectifier K+ current in rat pulmonary artery muscle cells. Am J Physiol Heart Circ Physiol 266: H365–H370, 1994 [DOI] [PubMed] [Google Scholar]
- 69. Spinella F, Rosano L, Di Castro V, Decandia S, Nicotra MR, Natali PG, Bagnato A. Endothelin-1 and endothelin-3 promote invasive behavior via hypoxia-inducible factor-1α in human melanoma cells. Cancer Res 67: 1725–1734, 2007 [DOI] [PubMed] [Google Scholar]
- 70. Spinella F, Rosano L, Di Castro V, Natali PG, Bagnato A. Endothelin-1 induces vascular endothelial growth factor by increasing hypoxia-inducible factor-1α in ovarian carcinoma cells. J Biol Chem 277: 27850–27855, 2002 [DOI] [PubMed] [Google Scholar]
- 71. Stenmark KR, McMurtry IF. Vascular remodeling versus vasoconstriction in chronic hypoxic pulmonary hypertension: a time for reappraisal? Circ Res 97: 95–98, 2005 [DOI] [PubMed] [Google Scholar]
- 72. Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 297: L1013–L1032, 2009 [DOI] [PubMed] [Google Scholar]
- 73. Suzuki H, Twarog BM. Membrane properties of smooth muscle cells in pulmonary hypertensive rats. Am J Physiol Heart Circ Physiol 242: H907–H915, 1982 [DOI] [PubMed] [Google Scholar]
- 74. Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol 283: L144–L155, 2002 [DOI] [PubMed] [Google Scholar]
- 75. Sylvester JT, Shimoda LA, Aaronson PI, Ward JP. Hypoxic pulmonary vasoconstriction. Physiol Rev 92: 367–520, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tagaito Y, Polotsky VY, Campen MJ, Wilson JA, Balbir A, Smith PL, Schwartz AR, O'Donnell CP. A model of sleep-disordered breathing in the C57BL/6J mouse. J Appl Physiol 91: 2758–2766, 2001 [DOI] [PubMed] [Google Scholar]
- 77. Treins C, Giorgetti-Peraldi S, Murdaca J, Monthouel-Kartmann MN, Van Obberghen E. Regulation of hypoxia-inducible factor (HIF)-1 activity and expression of HIF hydroxylases in response to insulin-like growth factor I. Mol Endocrinol 19: 1304–1317, 2005 [DOI] [PubMed] [Google Scholar]
- 78. Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza GL, Van Obberghen E. Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J Biol Chem 277: 27975–27981, 2002 [DOI] [PubMed] [Google Scholar]
- 79. Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, Bishop AE, Geraci M, Semenza GL, Yacoub M, Polak JM, Voelkel NF. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol 195: 367–374, 2001 [DOI] [PubMed] [Google Scholar]
- 80. Voelkel NF, Tuder RM. Hypoxia-induced pulmonary vascular remodeling: a model for what human disease? J Clin Invest 106: 733–738, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. von Euler U, Liljestrand G. Observations on the pulmonary arterial blood pressure of the cat. Acta Physiol (Oxf) 12: 301–320, 1946 [Google Scholar]
- 82. Wang J, Juhaszova M, Rubin LJ, Yuan XJ. Hypoxia inhibits gene expression of voltage-gated K+ channel α subunits in pulmonary artery smooth muscle cells. J Clin Invest 100: 2347–2353, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang J, Shimoda LA, Sylvester JT. Capacitative calcium entry and TRPC channel proteins are expressed in rat distal pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 286: L848–L858, 2004 [DOI] [PubMed] [Google Scholar]
- 84. Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res 98: 1528–1537, 2006 [DOI] [PubMed] [Google Scholar]
- 85. Wang J, Weigand L, Wang W, Sylvester JT, Shimoda LA. Chronic hypoxia inhibits Kv channel gene expression in rat distal pulmonary artery. Am J Physiol Lung Cell Mol Physiol 288: L1049–L1058, 2005 [DOI] [PubMed] [Google Scholar]
- 86. Wang Z, Orlowski J, Shull GE. Primary structure and functional expression of a novel gastrointestinal isoform of the rat Na/H exchanger. J Biol Chem 268: 11925–11928, 1993 [PubMed] [Google Scholar]
- 87. Weigand L, Foxson J, Wang J, Shimoda LA, Sylvester JT. Inhibition of hypoxic pulmonary vasoconstriction by antagonists of store-operated Ca2+ and nonselective cation channels. Am J Physiol Lung Cell Mol Physiol 289: L5–L13, 2005 [DOI] [PubMed] [Google Scholar]
- 88. Whitman EM, Pisarcik S, Luke T, Fallon M, Wang J, Sylvester JT, Semenza GL, Shimoda LA. Endothelin-1 mediates hypoxia-induced inhibition of voltage-gated K+ channel expression in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 294: L309–L318, 2008 [DOI] [PubMed] [Google Scholar]
- 89. Wiener CM, Booth G, Semenza GL. In vivo expression of mRNAs encoding hypoxia-inducible factor 1. Biochem Biophys Res Commun 225: 485–488, 1996 [DOI] [PubMed] [Google Scholar]
- 90. Yoshida T, Zhang H, Iwase T, Shen J, Semenza GL, Campochiaro PA. Digoxin inhibits retinal ischemia-induced HIF-1α expression and ocular neovascularization. FASEB J 24(6): 1759–1767, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yu AY, Frid MG, Shimoda LA, Wiener CM, Stenmark K, Semenza GL. Temporal, spatial, and oxygen-regulated expression of hypoxia-inducible factor-1 in the lung. Am J Physiol Lung Cell Mol Physiol 275: L818–L826, 1998 [DOI] [PubMed] [Google Scholar]
- 92. Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, Semenza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. J Clin Invest 103: 691–696, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yu F, White SB, Zhao Q, Lee FS. HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci USA 98: 9630–9635, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yu L, Quinn DA, Garg HG, Hales CA. Deficiency of the NHE1 gene prevents hypoxia-induced pulmonary hypertension and vascular remodeling. Am J Respir Crit Care Med 177: 1276–1284, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, Yuan JX. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol 284: C316–C330, 2003 [DOI] [PubMed] [Google Scholar]
- 96. Yuan XJ. Voltage-gated K+ currents regulate resting membrane potential and [Ca2+]i in pulmonary arterial myocytes. Circ Res 77: 370–378, 1995 [DOI] [PubMed] [Google Scholar]
- 97. Zelzer E, Levy Y, Kahana C, Shilo BZ, Rubinstein M, Cohen B. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1α/ARNT. EMBO J 17: 5085–5094, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhang H, Qian DZ, Tan YS, Lee K, Gao P, Ren YR, Rey S, Hammers H, Chang D, Pili R, Dang CV, Liu JO, Semenza GL. Digoxin and other cardiac glycosides inhibit HIF-1α synthesis and block tumor growth. Proc Natl Acad Sci USA 105: 19579–19586, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]