Summary
Background
The Two layer method (TLM) has been extremely successful in the preservation of the pancreas. However, this has not been thoroughly investigated in other organs or in clinically relevant large animal models. The aim of this study was to assess the effects of TLM in a large animal model of kidney preservation.
Material/Methods
Porcine kidneys were retrieved after 10 minutes of warm ischaemic injury and flushed with 300 ml UW solution at 4°C. Kidneys were then either placed in University of Wisconsin solution (UW) or TLM using pre-oxygenated perfluorodecalin and UW. Kidneys were stored for 18 hours at 4°C then reperfused with oxygenated autologous blood to assess renal function.
Results
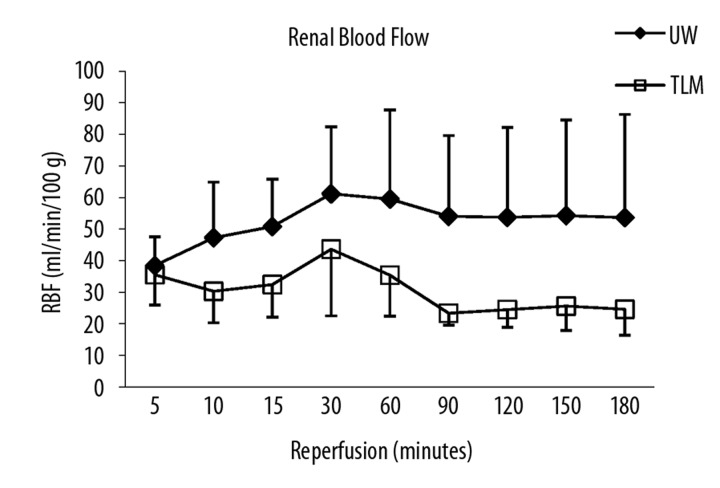
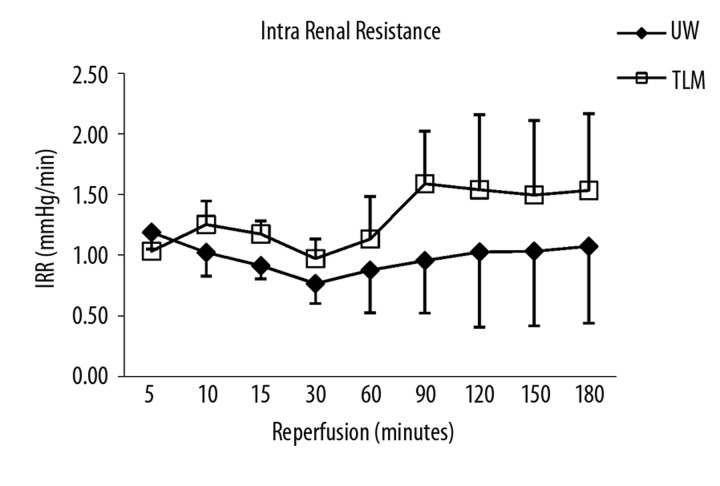
Renal blood flow (RBF) was significantly lower and intra-renal resistance (IRR) higher in TLM compared to UW group [Area under the curve (AUC) RBF, UW; 427±168 vs TLM; 247±55 ml/min/100g.h; P=0.041, AUC IRR, UW; 7.7±2.2 vs TLM; 10.5±1.9 ml/min/mmHg; P=0.041]. Levels of creatinine clearance (CrCl) were significantly lower in TLM group [AUC CrCl, UW; 1.8±1.0 vs TLM; 0.6±0.4 ml/min/100 g.h; P=0.034]. Levels of lipid peroxidation were significantly lower in TLM group [8-isoprostane/Cr ratio 3h; UW 3338±896 vs TLM 2072±886 pg/ml/mmol/L; P=0.04]. Levels of total nitric oxide were significantly higher in TLM group (P=0.009).
Conclusions
TLM did not improve the preservation condition of porcine kidneys. Furthermore, there appeared to be increased inflammation, endothelial injury and reduced renal function compared to preservation with UW. Further experimental work is needed to determine the role of PFC in kidney preservation.
Keywords: kidney, hypothermic preservation, two layer method, perfluorocarbon, preservation solution
Background
Organ preservation traditionally on relies on hypothermic conditions to maintain tissue viability [1]. Static cold storage is without doubt the simplest and most practical means of organ preservation. Flushing the organ with cold preservation solution removes the blood and rapidly reduces the temperature. The reduction in temperature lowers the metabolic rate and requirement for oxygen [1,2]. Nonetheless, the anaerobic environment causes substantial tissue injury which exacerbates the level of ischaemia reperfusion (I/R) injury after transplantation [3].
The addition of oxygen with the use of perfluorocarbons (PFC) has been shown to support a low level of metabolism and reduce tissue injury during preservation [4]. PFC are hydrocarbons in which all or most of the hydrogen atoms are replaced with fluorine. They have twice the density of water and a high capacity for dissolving respiratory gases. The solubility of dissolved oxygen in PFC is approximately 25 times greater than in blood or water [5]. Their ability to release oxygen following Henry’s linear law is not significantly influenced by temperature making them an attractive means of delivering oxygen during organ preservation.
PFCs can be used in combination with a preservation solution as a method of delivering oxygen to the organ during static storage. The two layer method (TLM) has been used to successfully preserve the pancreas in experimental models and in clinical practice [6–8]. The density of the PFC allows two layers to be formed, with the pancreas suspended in between. The PFC can be pre-oxygenated before preservation or continuously oxygenated throughout the preservation period [9–11]. Nonetheless, there is still controversy over how effectively the pancreas or indeed other solid organs can be oxygenation using this method. There also appears to be some discrepancy between animal models, particularly concerning the size and density of the organ [12]. The method is dependent on oxygen diffusing through the organ to provide adequate tissue oxygenation. It is therefore reasonable to suggest, that with larger more dense organs, oxygen penetration may not be as efficient as with the smaller organs.
TLM has not been thoroughly investigated in organs other than the pancreas and in particular there are few studies in clinically relevant large animal models. This study aimed to assess the effects of TLM in a porcine kidney model.
Material and Methods
Kidneys were retrieved from Landrace pigs (approx 60Kg) sacrificed by electrical stunning and then exsanguination [Schedule 1 Method Home Office (Scientific act) 1986] after 10 minutes of in situ warm ischemia (WI). 2 litres of blood was collected into a sterile receptacle containing 25,000 units of heparin (Multiparin®; CP Pharmaceuticals, Wrexham, UK). The blood was then transferred into CPDA-1 blood bags (Baxter Healthcare, Thetford UK) for storage at 4°C.
The kidneys were flushed with 300 ml of University of Wisconsin (UW) preservation solution at 4°C infused at a hydrostatic pressure of 100 cmH2O. Kidneys were then placed in a sterile bag containing either 300 ml of UW solution or 250ml of pre-oxygenated perfluorodecalin (F2 chemicals, Preston. UK) and 300ml of UW solution at 4°C. Kidneys were then stored in crushed ice for a period of 18 hours. There were 3 animals in each group (6 kidneys per group).
The PFC was pre-oxygenated with 95% O2/5% CO2 at 2 litres/min for 45 minutes at 4°C before use.
Reperfusion
After the preservation period kidneys were prepared for ex-vivo reperfusion. The renal artery, vein and ureter were cannulated with soft silastic catheters (Pennine, UK). Kidneys were then placed on an isolated organ preservation system (IOPS). The system is based on cardiopulmonary bypass technology (Medtronic, Watford, UK) incorporating a paediatric centrifugal pump, flow and pressure transducers, heat exchanger, venous reservoir, membrane oxygenator and temperature probe. The system was primed with 500 ml Ringer’s solution, (Baxter Healthcare, UK), 15 ml Sodium Bicarbonate 8.4% (Fresenius Kabi), 5 g Mannitol 10% (Sigma-Aldrich) and 750 mcg Cefuroxime (Stragen, Reigate, UK). This system has no ability to metabolically produce creatinine, 1000 μmol/L creatinine (Sigma-Aldrich, Steinheim, Germany) was added to the circuit so that serum creatinine fall and creatinine clearance could be measured as a marker of renal function. 500 ml of whole autologous heparized blood was then added to the priming solution and allowed to re-circulate through the system at a temperature of 38–39°C (within the normal range of a pig). Kidneys were placed on the circuit and reperfused at a set mean arterial pressure of 85 mmHg for a period of 3 hours. These conditions have previously been found to be the optimal perfusion pressure and duration of perfusion using this system [14–16]. Dilution of the blood with an isotonic solution reduces the viscosity of the perfusate and improves capillary blood distribution [17]. The haematocrit level was measured before and after reperfusion.
A nutrient solution containing a balance of electrolytes and amino acids (Nutriflex infusion (B Braun, Sheffield, UK) with 100 iu of insulin (Novo Nordisk, Denmark) and 25ml of sodium bicarbonate 8.4% (Fresenius Kabi) added, was infused into the arterial arm of the circuit throughout reperfusion at a rate of 20 ml per hour. 5% glucose solution (Baxter healthcare) was also continually infused at a rate of 7 ml per hour. Continual infusion of these solutions ensured that a constant level was maintained in the perfusate.
Renal blood flow (RBF) and mean arterial pressure (MAP) were recorded continuously and intra-renal resistance (IRR) calculated (MAP/RBF). Urine output was also measured during reperfusion.
Biochemical analysis of serum and urine samples was carried out at hourly intervals. Creatinine clearance (urinary creatinine × urinary volume / plasma creatinine), fractional excretion of sodium [(urinary sodium × urine volume)/(glomerular filtration rate x plasma sodium) ×100)] and the urinary total protein (mg/L) to creatinine (mmol/L) ratio were calculated.
Blood gas analysis (Blood Gas Analyser; Rapidlab 248, Bayer Corp, East Walpole, MA. USA) was used to record PaO2, PaCO2, PvO2, and acid-base homeostasis. Oxygen consumption [(PaO2 – venous PvO2) x flow rate/weight] was also calculated.
Urine samples were taken > after 1 and 3 hours of reperfusion and stored at −80ºC until analysis.
8-Isoprostane
Urine levels of 8-isoprostane were determined by ELISA (Cayman Chemical Co, MI, USA). Samples were centrifuged at 10,000 g for 2 minutes and the supernatant taken for analysis. Urine samples were diluted 10 fold prior to analysis. The sample and standards were added in duplicate to the ELISA plate together with an 8-isoprostane-acetylcholinesterase (AChE) conjugate and incubated for 18 hours at 4°C. During incubation 8-isoprostane present in the sample competed with the 8-isoprostane AChE conjugate for the 8-isoprostane rabbit antiserum binding sites on the pre-coated plate. The plate was then washed and developed by the addition of the substrate to AChE. The plate was read at 405nm after colour development for 90 minutes [18].
Protein carbonyl
Urine levels of protein carbonyls were determined by ELISA (Biocell; AXXORA, Nottingham, UK). Standards and samples were reacted with dinitrohenylhydrazine (DNP) for 45 minutes before addition to the plate in duplicate and incubated for 2 hours at 37°C. The unconjugated DNP and non-protein constituents were washed and the absorbed protein incubated with biotinylated anti-DNP antibody for 1 hour at 37°C. The plate was then washed and incubated with streptavidin-linked horseradish peroxidase for 1 hour at room temperature. After the final wash the chromatin reagent was applied. The reaction was stopped after 7 minutes and the absorbance determined at 450 nm [18].
Total nitric oxide
Urine levels of nitric oxide were quantified using the total nitric oxide test kit (Assay Designs, MI, USA). This assay is based on the conversion of nitric oxide to nitrate and the subsequent conversion of nitrate to nitrite by the enzyme nitrate reductase. Nitrite is then detected colourimetrically at 540 nm as an azodye product of the Griess reaction. Briefly, urine sample were centrifuged at 10,000 g and the supernatant withdrawn. 50 μl of each sample were added in duplicate to a micro titre test plate. 25 μl NADH and 25 μl nitrate reductase were added to each well and incubated at 37°C for 30 minutes. 100 μl Griess reagents (sulphanilamide and N-(l-Naphthyl) ethylenediamine in 2M HCl) were then added and incubated at room temperature for 10 minutes. Optical density was then read at 540 nm using a spectrophotometer and the concentration calculated using standards.
Histology
Wedge biopsies were taken post cold storage and then after reperfusion from each kidney. The tissue was then fixed in 10 percent formal saline, dehydrated and embedded in paraffin wax. Sections of 4 μm were cut and stained with Periodic Acid-Schiff stain for evaluation using light microscopy. Sections were scored by a blinded assessor over ten fields, assessing changes in eight morphological variables; Brush border loss, Tubular dilation, Tubular debris, Vaculolation, Interstitial oedema, Basement membrane, Tubular epithelial loss and glomeruli shrinkage. Samples were scored from 0 to 3 according to the level of damage; 0 representing normal, 1 mild, 2 moderate and 3 severe morphological changes. The mean ±SD of the total score over 10 fields per kidney in each of the groups (n=60) was calculated and analysed.
Statistical analysis
Values are presented as mean ±S.D. Levels of continuous variables such as RBF were plotted against time and the area under the curve (AUC) for individual perfusion experiments was calculated using Excel® software (Microsoft, Reading, UK) and Graphpad Prism (GraphPad Software, San Diego California USA).
Mean values and mean AUC values were compared using the Mann Whitney U test (GraphPad InStat version 3.00 for Windows 95, GraphPad Software, California USA). P<0.05 was taken as statistically significant.
Results
Preservation
Immediately after oxygenation the pO2 of the PFC was above the range of the blood gas analyser (>749 mmHg) and pCO2 54.9±0.1 mmHg. Levels of pO2 and pCO2 fell after 18 hours of preservation to 392.4±49.2 mmHg (P=0.002) and 12.4±2.0 mmHg (P=0.009) respectively.
All kidneys typically floated above the PFC layer remaining in the UW solution.
Renal function
Renal blood flow was significantly lower and intra-renal resistance higher in TLM group compared to UW (AUC RBF: UW; 427±168 vs TLM; 247±55 ml/min/100 g.h; P=0.041: AUC IRR: UW; 7.7±2.2 vs TLM; 10.5±1.9 mmHg/ml/min.h; P=0.041; Figure 1A, B).
Figure 1A.
Mean renal blood flow over 3 hours of reperfusion. UW and TLM group. Area under the curve (AUC) renal blood flow (P=0.041) Mann Whitney U test.
Figure 1B.
Mean intra renal resistance over 3 hours of reperfusion. UW and TLM groups. Area under the curve (AUC) (P=0.041) Mann Whitney U test.
Oxygen consumption was also significantly lower in TLM group after 3 hours of reperfusion (3 h; TLM; 15.7±5.1 vs UW; 32.7±17.9 ml/min/g; P=0.026).
Arterial pH levels fell in both groups throughout reperfusion with no significant difference between groups at 3 hours (pH: TLM; 7.21±0.05 vs UW; 7.32±0.10; P=0.078). However, levels of potassium were significantly higher after 3 hours in TLM group (3 h; TLM 14.5±0.61 vs UW; 12.6±1.3 mmol/L; P=0.041).
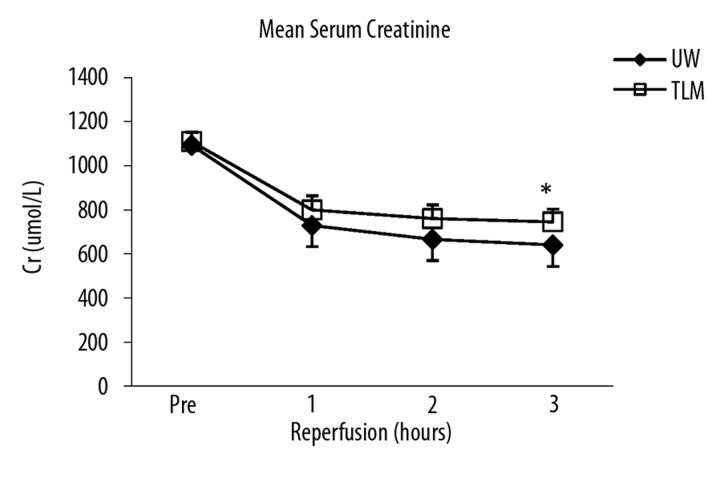
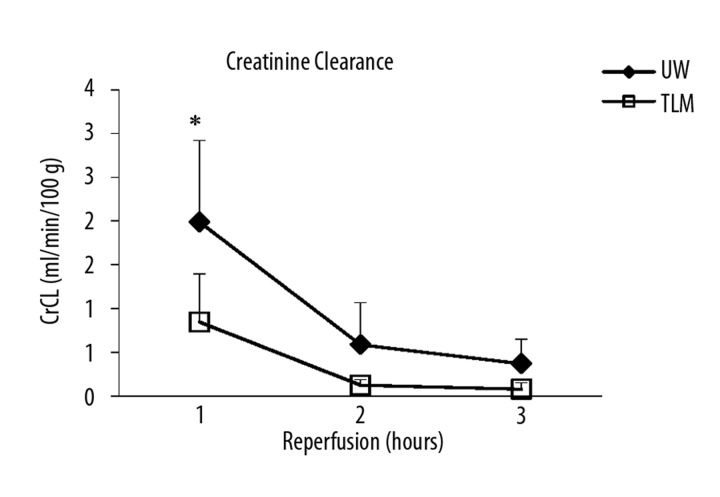
Serum creatinine levels fell in both groups and were lower in the UW group at 3 hours (P=0.053; Figure 2A). However, there was no significant difference in AUC Cr (UW; 2262±246 vs TLM; 2488±162 μmol/L.h; P=0.093). Creatinine clearance was significantly lower at 1 hour in the TLM group compared to UW (P=0.033; Figure 2B) and overall AUC CrCl significantly higher in the UW group (P=0.034).
Figure 2A.
Mean serum creatinine over 3 hours of reperfusion UW and TLM groups (3h; P=0.053) Mann Whitney U test.
Figure 2B.
Mean creatinine clearance over 3 hours of reperfusion. UW and TLM groups (1h; P=0.033). Mann Whitney U test.
Levels of urine output were numerically higher in the UW group, however this did not reach statistical significance (Total urine output UW; 240±153 vs TLM 106±62 ml; P=0.132). There was also no significant difference in the level of fractional excretion of sodium (AUC UW; 117.5±21.4 vs TLM; 135.5±12.3%.h; P=0.119) or in the levels of protein/creatinine ratio after 3 hours of reperfusion (3 h Pr/Cr, UW; 7528±3558 vs TLM; 8801±4420 mg/L/mmol/L; P=0.620).
Oxidative damage and inflammation
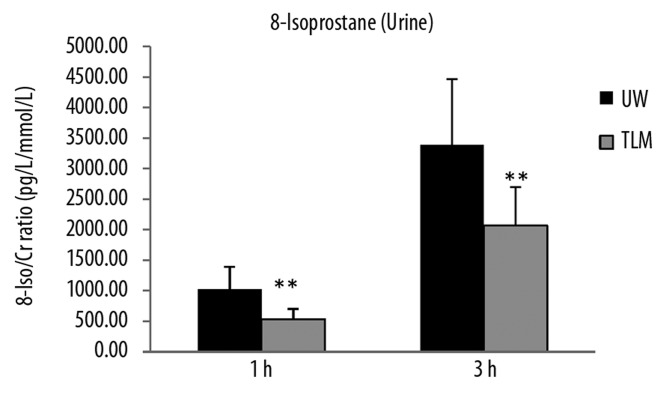
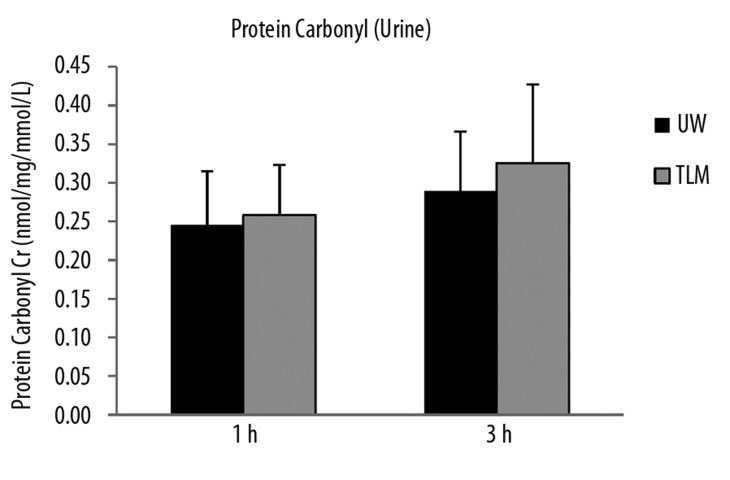
Urinary levels of 8-isoprostane were significantly lower in TLM group at 1 and 3 hours of reperfusion (P=0.026, 0.041; Figure 3A). Although, there was no significant difference in urinary levels of protein carbonyl at 1 or 3 hours of reperfusion (P=0.114, 0.601; Figure 3B).
Figure 3A.
Urinary levels of 8-iosprostane at 1 and 3 hours of reperfusion in the UW and TLM groups. (P=0.026, 0.041) Mann Whitney U test.
Figure 3B.
Urinary levels of protein carbonyl at 1 and 3 hours of reperfusion in the UW and TLM groups. (P=0.114, 0.601). Mann Whitney U test.
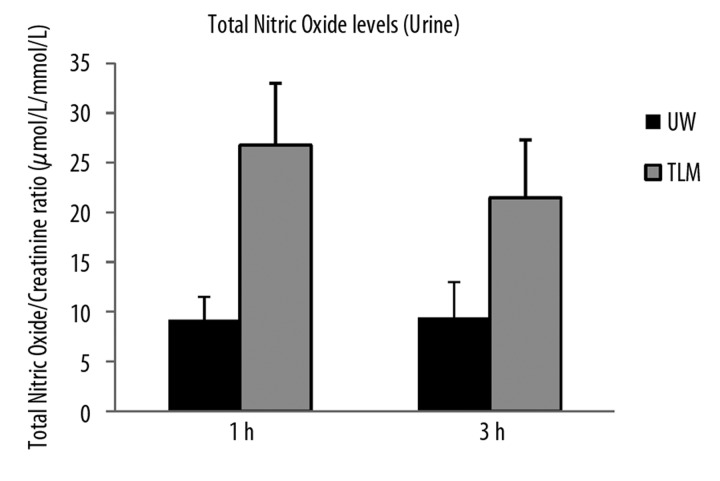
Urinary levels of nitric oxide were significantly higher in the TLM at both 1 and 3 hours of reperfusion (P=0.002, 0.009; Figure 3C).
Figure 3C.
Urinary levels of total nitric oxide/creatinine ratio at 1 and 3 hours of reperfusion in the UW and TLM groups. (P=0.002, 0.009) Mann Whitney U test.
Histology
Baseline post cold storage biopsies showed a significant increase in tubular dilatation, vacuolation, basement membrane loss and glomeruli shrinkage in TLM group compared to UW (P<0.05; Table 1). Post reperfusion biopsies showed a significant degree of morphological change across all parameters in both groups compared post cold storage (P<0.0001; Table 1). However, there was a significant increase in brush border loss, tubular dilatation, interstitial oedema and glomeruli shrinkage in TLM group compared kidneys stored in UW (P<0.05; Table 1).
Table 1.
Histology: PAS stain Light Microscopy ×400. Post cold storage and post reperfusion.
| UW | TLM | |||
|---|---|---|---|---|
| Post CS | Post Rep | Post CS | Post Rep | |
| Brush Border | 1.05±0.62 | 2.22±0.67* | 1.07±0.45 | 2.57±0.53*,** |
| Tub dilatation | 0.00±0.00 | 0.66±0.54* | 0.83±0.56** | 1.80±0.48*,** |
| Oedema | 0.15±0.36 | 0.88±0.53* | 0.33±0.48 | 1.77±0.46*,** |
| Vacuolation | 0.05±0.22 | 1.95±1.04* | 0.62±0.74** | 2.05±0.70* |
| Tub debris | 1.27±0.75 | 2.22±0.74* | 1.07±0.31 | 2.10±0.48* |
| Base membrane | 0.42±0.59 | 2.29±0.62* | 0.90±0.48** | 2.38±0.49* |
| Tub Epithelial | 0.97±0.58 | 2.43±0.65* | 1.17±0.38 | 2.68±0.47* |
| Glomeruli | 0.18±0.38 | 1.24±0.60* | 1.04±0.33** | 2.12±0.43*,** |
P<0.0001 between time points post reperfusion and post cold storage;
P<0.05 between groups TLM versus UW.
Discussion
TLM did not improve kidney preservation in this porcine model. Renal blood flow and function were severely impaired with increased tubular cell inflammation and morphological change compared to kidneys stored in UW solution.
Perfluorocarbon (PFC) with the addition of oxygen during static storage has been used to improve and prolong the preservation of the pancreas for several decades [4,6,11]. Experimental studies have demonstrated that TLM can adequately resuscitate the pancreas after warm ischaemic injury with sufficient generation of ATP to maintain tissue viability [19]. The technique has been adopted clinically by a number of centres and has been particularly beneficial in the allogenic islet transplant program, improving islet yields after preservation [7]. A recent study in the rat kidney using a modified TLM incorporating a PFC emulsion (Pher-O2, emulsion 88%) mixed with UW suggested that this method of preservation could be translated to the kidney [20]. The authors found increased expression of mRNA HO-1 and down regulation of mRNA iNOS after preservation. Furthermore, Marada et al. recently demonstrated increased survival and improved renal function after preservation using the standard TLM (PFC+UW) again in rat kidneys [21].
In the present study we found that porcine kidneys stored by TLM had a higher level of injury compared to kidneys stored in UW alone with reduced renal function and significant deterioration of cellular viability during reperfusion. In contrast, TLM was associated with a reduction in the level of oxidative damage. 8-isoprostane is product of the free radical mediated oxidation of arachidonic acid and can be used as a marker of lipid peroxidation. It is associated with membrane damage and is known to instigate pro-inflammatory mediators and stimulate the release of cytokines and chemokines causing tissue injury [22]. Lower urinary levels after TLM suggest a reduction of proximal tubular cell injury. This effect may be due to the addition of oxygen during preservation, preventing some of the detrimental effects of anaerobic metabolism. However, further investigation is needed to elucidate the mechanisms of protection.
There have been several attempts to translate TLM for the preservation of other organs. An early study by Sumimoto et al. found no benefit in the preservation of rat liver [23] and Lui et al. reported increased levels of inflammation and lower levels of PaO2 in the rat lung after 6 hours of preservation using TLM [24]. Direct contact with perfluorodecalin was also found to cause significant endothelial alteration and increased pulmonary inflammation when it was administered endobronchially [25]. We cannot rule out that perfluorodecalin caused an adverse inflammatory reaction in the kidney also. In addition, there have been reports of difficulties in maintaining the position of the organ. The pancreas is reported to remain between the two layers however, as in this present study Lui et al. found that the rat lung typically floated in the UW solution above the PFC [24]. Forcibly immersing an organ in PFC to optimize oxygen delivery has been investigated and warrants further investigation in the kidney [26]. Immersion of the pancreas was shown to improve islet yield [27] and also prolong the preservation period in studies of the rat heart when high levels of CO2 were used in combination with O2 [28].
Despite the success of TLM in the preservation of the pancreas, the effectiveness of oxygen diffusion through the organ is still questionable. Papas et al. measured oxygen penetration by introducing fiber optic sensors into cubes of porcine pancreas. The PO2 was found to be zero at the core of the tissue during TLM preservation, suggesting inadequate oxygenation [13]. Brandhorst et al. reported only 15% oxygenation of the total volume of the pancreas using TLM. Interestingly, this was enough to maintain ATP levels in islet cells damaged by warm ischaemic injury [29]. The exact mechanism by which oxygen can penetrate through an organ and reduce preservation injury is undetermined. There also appears to be some discrepancy between large and small animal experimental models, with evidence of improved oxygenation and deeper penetration of oxygen in organs from small animal models [12].
In attempting to translate a simple TLM to the kidney, this study has several limitations. The PFC was pre-oxygenated rather than using continuous oxygenation which may have proved more beneficial. In addition, the kidney was allowed to float above the PFC layer. The technique could be adapted to ensure that the kidney remains in the PFC or positioned between the two layers to ensure adequate oxygenation. The PO2 of the UW solution was also not measured during preservation which again questions the effectiveness of oxygen delivery to the kidney in this study. More conclusive evidence is needed to determine whether the TLM can be adapted for kidney preservation in the clinical setting. Measurement of tissue oxygenation and cellular metabolism would provide evidence on the mechanism and ability of oxygen to penetrate through the organ. UW solution is generally the solution of choice for TLM. However, other solutions such as Celsior and M-Koyoto have also been used with successful results [8,30]. In addition, Kuroda et al. found that adding adenosine improved ATP synthesis within the pancreas [31]. Therefore, modification of TLM may prove to be beneficial for the kidney.
To summarize, evidence so far suggests that TLM is relatively ineffective in organs other than the pancreas. Possible explanations include; inadequate oxygen delivery due to the density and robust capsules of organs such as the liver and kidney, which questions the suitability of large animal models and clinical relevance of the technique; difficulty in maintaining the position of the organ in the solution; the inflammatory response possibly caused by perfluorodecalin or depletion of substrates due to maintaining a level of aerobic metabolism during preservation.
Conclusions
In conclusion, TLM did not improve the preservation condition of porcine kidneys in this experimental model. Furthermore, there appeared to be increased inflammation, endothelial injury and reduced renal function compared to preservation with UW. However, there was some indication of a reduction in oxidative injury with lower levels of lipid peroxidation.
Footnotes
Source of support: This study was funded by The University Hospitals of Leicester, Department of Renal & Urology
References
- 1.Fuller BJ, Lee CY. Hypothermic perfusion preservation: The future of organ preservation revisited? Cryobiology. 2007;54(2):129–45. doi: 10.1016/j.cryobiol.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Goujon JM, Vandewalle A, Baumert H, et al. Influence of cold-storage conditions on renal function of autotransplanted large pig kidneys. Kidney Int. 2000;58(2):838–50. doi: 10.1046/j.1523-1755.2000.00233.x. [DOI] [PubMed] [Google Scholar]
- 3.Brinkkoetter PT, Song H, Losel R, et al. Hypothermic injury: The mitochondrial calcium, ATP and ROS love-hate triangle out of balance. Cell Physiol Biochem. 2008;22(1–4):195–204. doi: 10.1159/000149797. [DOI] [PubMed] [Google Scholar]
- 4.Atias S, Mizrahi SS, Shaco-Levy R, Yussim A. Preservation of pancreatic tissue morphology, viability and energy metabolism during extended cold storage in two-layer oxygenated University of Wisconsin/perfluorocarbon solution. Isr Med Assoc J. 2008;10(4):273–76. [PubMed] [Google Scholar]
- 5.Clark MC, Weiman DS, Pate JW, Gir S. Perfluorocarbons. future clinical possibilities. J Invest Surg. 1997;10(6):357–65. doi: 10.3109/08941939709099599. [DOI] [PubMed] [Google Scholar]
- 6.Kuroda Y, Kawamura T, Suzuki Y, et al. A new, simple method for cold storage of the pancreas using perfluorochemical. Transplantation. 1988;46(3):457–60. doi: 10.1097/00007890-198809000-00027. [DOI] [PubMed] [Google Scholar]
- 7.Lakey JR, Tsujimura T, Shapiro AM, Kuroda Y. Preservation of the human pancreas before islet isolation using a two-layer (UW solution-perfluorochemical) cold storage method. Transplantation. 2002;74(12):1809–11. doi: 10.1097/00007890-200212270-00031. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto S. Clinical application of perfluorocarbons for organ preservation. Artif Cells Blood Substit Immobil Biotechnol. 2005;33(1):75–82. doi: 10.1081/bio-200046698. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto S, Kuroda Y. Perfluorocarbon for organ preservation before transplantation. Transplantation. 2002;74(12):1804–9. doi: 10.1097/00007890-200212270-00030. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto S, Kuroda Y, Hamano M, et al. Direct evidence of pancreatic tissue oxygenation during preservation by the two-layer method. Transplantation. 1996;62(11):1667–70. doi: 10.1097/00007890-199612150-00023. [DOI] [PubMed] [Google Scholar]
- 11.Kin S, Stephanian E, Gores P, et al. 96-Hour Cold-Storage Preservation of the Canine Pancreas with Oxygenation using Perfluorochemical. Transplantation. 1993;55(1):229–30. [PubMed] [Google Scholar]
- 12.Avgoustiniatos ES, Hering BJ, Papas KK. The rat pancreas is not an appropriate model for testing the preservation of the human pancreas with the two-layer method. Transplantation. 2006;81:1471–72. doi: 10.1097/01.tp.0000215389.64186.3f. author reply 1472. [DOI] [PubMed] [Google Scholar]
- 13.Papas KK, Hering BJ, Guenther L, et al. Pancreas oxygenation is limited during preservation with the two-layer method. Transplant Proc. 2005;37:3501–4. doi: 10.1016/j.transproceed.2005.09.085. Erratum in: Transplant Proc, 2006; 38: 1205. [DOI] [PubMed] [Google Scholar]
- 14.Hosgood SA, Bagul A, Yang B, Nicholson ML. The relative effects of warm and cold ischemic injury in an experimental model of nonheartbeating donor kidneys. Transplantation. 2008;85(1):88–92. doi: 10.1097/01.tp.0000296055.76452.1b. [DOI] [PubMed] [Google Scholar]
- 15.Hosgood S, Harper S, Kay M, et al. Effects of arterial pressure in an experimental isolated haemoperfused porcine kidney preservation system. Br J Surg. 2006;93:879–84. doi: 10.1002/bjs.5381. [DOI] [PubMed] [Google Scholar]
- 16.Harper S, Hosgood S, Kay M, Nicholson M. Leucocyte depletion improves renal function during reperfusion using an experimental isolated haemoperfused organ preservation system. Br J Surg. 2006;5:623–29. doi: 10.1002/bjs.5324. [DOI] [PubMed] [Google Scholar]
- 17.Dittrich S, Schuth A, Aurich H, et al. Haemodilution improves organ function during normothermic cardiopulmonary bypass: Investigations in isolated perfused pig kidneys. Perfusion. 2000;15:225–29. doi: 10.1177/026765910001500307. [DOI] [PubMed] [Google Scholar]
- 18.Waller HL, Harper SJ, Hosgood SA, et al. Biomarkers of oxidative damage to predict ischaemia-reperfusion injury in an isolated organ perfusion model of the transplanted kidney. Free Radic Res. 2006;40(11):1218–25. doi: 10.1080/10715760600907368. [DOI] [PubMed] [Google Scholar]
- 19.Kuroda Y, Morita A, Fujino Y, et al. Successful extended preservation of ischemically damaged pancreas by the two-layer (University of Wisconsin solution/perfluorochemical) cold storage method. Transplantation. 1993;56(5):1087–90. doi: 10.1097/00007890-199311000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Maluf DG, Mas VR, Yanek K, et al. Molecular markers in stored kidneys using perfluorocarbon-based preservation solution: Preliminary results. Transplant Proc. 2006;38(5):1243–46. doi: 10.1016/j.transproceed.2006.02.109. [DOI] [PubMed] [Google Scholar]
- 21.Marada T, Zacharovova K, Saudek F. Perfluorocarbon Improves Post-Transplant Survival and Early Kidney Function following Prolonged Cold Ischemia. Eur Surg Res. 2010;44(3–4):170–78. doi: 10.1159/000280438. [DOI] [PubMed] [Google Scholar]
- 22.Milne GL, Musiek ES, Morrow JD. F2-isoprostanes as markers of oxidative stress in vivo: an overview. Biomarkers. 2005;10(Suppl 1):S10–23. doi: 10.1080/13547500500216546. [DOI] [PubMed] [Google Scholar]
- 23.Sumimoto R, Jamieson NV, Kamada N. Attempted application of the two-layer storage method to liver preservation. Transplantation. 1990;49(5):1027–28. doi: 10.1097/00007890-199005000-00048. [DOI] [PubMed] [Google Scholar]
- 24.Liu CC, Hsu PK, Huang WC, et al. Two-layer method (UW solution/perfluorochemical plus O2) for lung preservation in rat lung transplantation. Transplant Proc. 2007;39(10):3019–23. doi: 10.1016/j.transproceed.2007.06.085. [DOI] [PubMed] [Google Scholar]
- 25.Loehe F, Mueller C, Bittmann I, et al. Influence of long-term preservation with endobronchially administered perfluorodecalin on pulmonary graft function. Transplantation. 2000;70(10):1417–24. doi: 10.1097/00007890-200011270-00004. [DOI] [PubMed] [Google Scholar]
- 26.Kuroda Y, Kawamura T, Tanioka Y, et al. Heart preservation using a cavitary two-layer (University of Wisconsin solution/perfluorochemical) cold storage method. Transplantation. 1995;59(5):699–701. doi: 10.1097/00007890-199503150-00010. [DOI] [PubMed] [Google Scholar]
- 27.Brandhorst D, Iken M, Brendel MD, et al. Successful pancreas preservation by a perfluorocarbon-based one-layer method for subsequent pig islet isolation. Transplantation. 2005;79(4):433–37. doi: 10.1097/01.tp.0000151765.96118.1b. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida Y, Hatayama N, Sekino H, Seki K. Heterotopic transplant of an isolated rat heart preserved for 72 h in perfluorocarbon with CO2. Cell Transplant. 2008;17(1–2):83–89. doi: 10.3727/000000008783907017. [DOI] [PubMed] [Google Scholar]
- 29.Brandhorst D, Iken M, Bretzel RG, Brandhorst H. Pancreas storage in oxygenated perfluorodecalin does not restore post-transplant function of isolated pig islets pre-damaged by warm ischemia. Xenotransplantation. 2006;13(5):465–70. doi: 10.1111/j.1399-3089.2006.00340.x. [DOI] [PubMed] [Google Scholar]
- 30.Noguchi H, Ueda M, Nakai Y, et al. Modified two-layer preservation method (M-Kyoto/PFC) improves islet yields in islet isolation. Am J Transplant. 2006;6(3):496–504. doi: 10.1111/j.1600-6143.2006.01223.x. [DOI] [PubMed] [Google Scholar]
- 31.Kuroda Y, Hiraoka K, Tanioka Y, et al. Role of adenosine in preservation by the two-layer method of ischemically damaged canine pancreas. Transplantation. 1994;57(7):1017–20. [PubMed] [Google Scholar]