Summary
Empathy allows us to internally simulate the affective and cognitive mental states of others. Neurobiological studies suggest that empathy is a complex phenomenon, which can be described using a model that includes 2 modes of processing: bottom-up and top-down. Bottom-up neural processing is achieved via the mirroring representation systems that play a key role in the direct sharing of the emotional states of others. Top-down processing, known as cognitive perspective-taking or theory of mind, where the feelings of others are fully imagined and understood, is based on control and inhibition mechanisms. Available evidence indicates that empathic brain responses are likely to be influenced by several different modulating factors.
Keywords: empathy, mirror neurons, perspective taking, theory of mind, anterior cingulate cortex, anterior insula
Background
Empathy (Gr. empatheia-passion) has been the subject of much study in social and developmental psychology, sociology and philosophy, and it has been defined in many different ways. The perception of the emotional state of others can result in emotional empathy, that is, elicitation of corresponding emotions in the observer, and moreover, sharing an emotional state with others [1,2]. However, empathy allows us to internally simulate not only the affective states of others, but also their cognitive mental states. Thus, empathy can also refer to our ability to take the cognitive perspective of other people, which helps us to understand their experiences, intentions, and needs [3,4].
In recent years, work in social neuroscience has begun to shed light on the neural underpinnings of empathy. The aim of this article is to review the findings of recent studies investigating how we empathize with others from a neurobiological perspective. The nature of individual differences in empathy is an important issue that could be considerate from both a scientific and a therapeutic point of view. Therefore, several factors that modulate the level of empathy, measured by changes in the activation of relevant brain areas, are discussed.
The Role of Bottom-Up Processing of Affective Sharing – Social Mirroring
For many years, researchers have undertaken efforts to explain the automatic mode of our perception of the emotional states of others and understanding of their feelings, behaviors, intentions, and needs. Recent studies have suggested that empathy may be based on so-called mirroring systems or the mirror neuron system (MNS). The mirror neurons found in the central promoter (area F5) and parietal (area PF) cortex were originally discovered in a monkey brain [5–7]. These cells fire during goal-directed actions (holding, grasping, or manipulating objects), and when a monkey observes the same actions performed by others, either monkeys or humans.
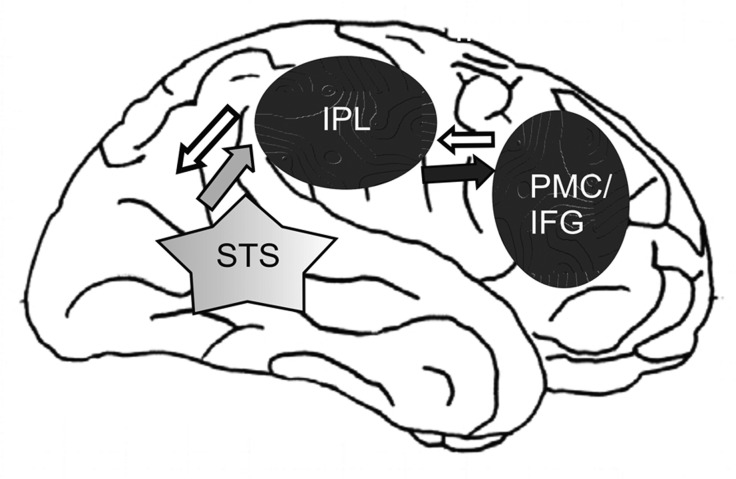
There are 2 classes of visuomotor neurons in monkey area F5: mirror neurons, which respond when the monkey sees an object-directed action, and canonical neurons, which respond to the presentation of an object. The object significance for a monkey has no obvious influence on the mirror-neuron response. They fire with the same intensity responding to grasping a piece of food or a geometric solid [6]. It has been argued that the functional role of the MNS understands the behavior of others based on direct mapping of a motor or somatosensory representation of the observed action in the observer brain [8,9]. Data from neuroimaging and electrophysiology studies in humans support this notion and indicate that the MNS involves the inferior parietal lobule (IPL) and the inferior frontal gyrus (IFG) [10] (Figure 1). These 2 areas are anatomically connected and form an integrated frontoparietal MNS [11]. The MNS operates according to the principle of “a mirror” – if one raises one’s right hand, we observe and understand this action by activating our own neural representation of this action even when we do not perform the action ourselves but observe others doing it [6]. This mirroring process is automatic [12].
Figure 1.
Neuronal basis of imitation (after [11], modified). The Figure shows the frontoparietal mirror neuron system (MNS) (black ovals) and visual input (grey star) in the human brain. The anterior area of the MNS involves the posterior inferior frontal gyrus (IFG) and the ventral premotor cortex (PMC), and the rostral area involves the inferior parietal lobule (IPL). The grey arrow indicates input to the MNS from the STS. The black arrow shows the information flow from the IPL to the PMC/IFG. The white arrows show the information flow from PMC/IFG to the IPL and to the STS (based on [11]).
Mirror neurons are activated when we observe or imagine some movement [13] and when we imitate others [11,14]. A schematic representation of the neural circuitry for imitation based on the MNS is shown in Figure 1.
For example, functional MRI studies on the imitation of simple movements or complex guitar fingering have shown that frontoparietal MNS is active in both cases. The MNS exhibits the highest activity during complex tasks [11,15].
Developmental behavioral data show that imitative behavior is crucial for developing social cognitive skills [11]. The behavioral links between imitation and social cognition suggest a key role for the MNS not only in understanding the intentions of others but also in sharing the emotions of others [11]. An fMRI study on the possible role of the MNS in emotional processes has shown that when people observe or imitate facial expressions of different emotions, structures connected with the representation of emotional states and facial movements are activated: the superior temporal sulcus (STS), the anterior insula (AI), the amygdala, and the premotor cortex (PMC) [16]. These data indicate that a mechanism using the same affective neurons is connected both with generating our own emotional states and with the MNS emotional operation.
Influenced by the concept of the MNS involved in understanding of motor behavior and imitation, Preston and de Waal [4] proposed a neuroscientific model of empathy. Their perception-action model suggests that the observation or imagination of another person in a particular emotional state automatically activates a representation of that state in the observer, with its associated autonomic and somatic responses [4]. Based on this inner representation, we can recognize the emotions of others and express them with gestures or facial expressions. The shared affective neural activation between self and others explains how we can feel the emotions of others [17].
Decety and Lamm [18] proposed a model in which bottom-up information processes (direct matching between perception and action) are fundamentally intertwined in the generation of empathy [19]. In these processes, the activated sensory transformation system in the temporal cortex (STS) “switches on” the MNS in the limbic system, and this neural information is transmitted to higher cortex structures responsible for executive functions. Imaging studies using fMRI have revealed that both the observation of pictures showing disgusted faces and the actual smelling of disgusting odors elicit similar brain activity: in the anterior cingulate cortex (ACC) and the AI, structures associated with empathic response in the domain of smell [20]. The assumed presence of affective mirroring in empathy is derived from studies on the empathy of pain [21–29]. In most fMRI studies on the observation or imagining of the pain of others in adults and children, activity has been predominately found in ACC and AI [24,28–36]. It has been suggested that these regions play a crucial role in representing one’s own subjective feeling states and affective processing of pain [37] in nonempathy conditions of “first hand” experience of the emotion, that is, when we experience pain ourselves [38]. This supports the notion that empathizing with others activates the neural network underlying this specific emotion in the empathizer [39]. In other words, when we want to understand how others feel when in pain, we activate the same neural networks, which are crucial for our own feelings of pain.
A question remains of how much the empathic reaction of the empathizer is isomorphic to the observed affective process of others. Most research on the empathy of pain conducted using the fMRI indicates that emphatic reactions are connected with the affective component of pain, namely with activation of the ACC and the AI, rather than with the sensory component of pain associated with activation of somatosensory cortex [24,25]. However, other studies using transcranial magnetic stimulation (TMS) [22], magnetoencephalography (MEG) [40] or somatosensory-evoked potentials (SEPs) [41], revealed that while empathizing with the pain of others, somatosensory cortices (SI) and (SII) can also be activated in areas related to pain signal transmission pathways, which indicates “direct mirroring of feeling pain.” These findings suggest that both sensory (SI and SII) and affective components (ACC and AI) of pain are likely to be involved in the process of empathizing.
The Role of Top-Down Processing of Cognitive Perspective-Taking
Witnessing others undergoing various emotions is a frequent occurrence. Taking MNS for granted, it could be assumed that we constantly share emotions of others in an unconscious way. In this situation, empathy would resemble mimicry – that is, a tendency to automatically synchronize affective expressions, vocalizations, postures, and movements with those of another person [42,43]. Automatic sharing the emotions of others might imply continuously being in some form of emotional chaos owing to an inability to distinguish between our own emotion and that of others, yet such a situation does not occur. When empathizing with the emotions or sensations of another person (affective empathy), cognitive perspective-taking (cognitive empathy) takes place which supports our ability to understand the intentions, desires, and beliefs of others [39]. The first step of cognitive perspective-taking is to distinguish between ourselves and others. In the next step, we imagine how another person feels and understand his or her intentions, desires, and beliefs [44]. This cognitive inference of the mental state of the other person is known as mentalizing [45] or having a theory of mind (ToM) [46,47]
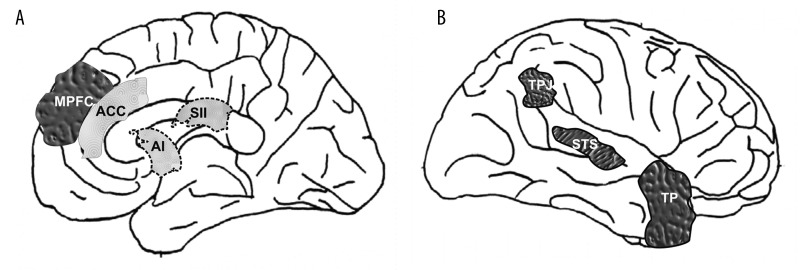
It has been proposed that top-down processing in which mainly prefrontal cortex areas are engaged [48] could be responsible for cognitive perspective-taking which might “protect” from automatic execution of mimicrylike processing. At the moment, when we are trying to understand what another person feels, the autonomic and somatic neuronal circuits responsible for direct sharing his/her emotional states might be inhibited. Studies using fMRI have shown that when participants were asked to consider the emotional state of a person shown in a cartoon or described in a story, the following brain regions were activated: the medial prefrontal cortex (MPFC), the temporo-parietal junction (TPJ), the STS, and the temporal pole (TP) [39,49,50]. The brain regions that participate in cognitive perspective-taking are shown in Figure 2.
Figure 2.
Key brain structures involved in empathy connected with the 2 modes of processing information: bottom-up (light grey) and top-down (dark grey) interoception. MPFC – medial prefrontal cortex; TPJ – temporo-parietal junction; STS – superior temporal sulcus; TP – temporal pole; ACC – anterior cingulate cortex; AI – anterior insula; SII – somatosensory cortex (after [39], modified).
Medial prefrontal cortex around BA 9 has been implicated in both sharing empathy and theory of mind [50]. Thus, it is likely that neural networks involved in mentalizing or ToM constitute the extended system supporting empathy. In addition, there is evidence that the region around paracingulate sulcus in MPFC contains spindle cells, a class of large projection neurons found only in great apes and humans, which are thought to be involved in coordinating neural activity relating to emotion and cognition [3,51]. In this area, neuronal circuits involved in sharing emotional states (affective empathy) could intertwine with those taking part in perspective-taking (cognitive empathy).
In a study by Ruby and Decety [52], the participants were presented with short written sentences depicting real-life situations likely to induce social emotions, and were asked to imagine how their mothers would feel if they were in such situations. It was shown that the MPFC and the ventromedial prefrontal cortex (VMPFC) as well as the right IPL were activated in these individuals.
Functional brain imaging studies in individuals with autism have found evidence of abnormal brain activation in VMPFC, ACC, TPJ, and TP during tasks aimed at eliciting social cognitive responses [53–55]. Interestingly, studies of people with autism spectrum disorders (ASD) indicate that VMPFC, anterior cingulate gyri, and TPJ exhibit reduced fractional anisotropy (FA) values, which is an indicator of the diameter and density of fibers, myelination, and macrostructural features of white matter fibers [56,57]. All those structures are implicated in social cognitive processes, such as ToM. Moreover, these individuals have a dysfunction of the MNS associated with a behavioral deficiency in recognizing and sharing emotions with others [11]. This impairment may reflect a dysfunction of both bottom-up and top-down processing in people with ASD.
There is also clinical evidence that frontal damage (the frontopolar cortex) can result in impaired perspective-taking ability [58]. In such cases, top-down regulation through executive functions is no longer active. However, the MNS could still be active, which may lead to mimicry or the chameleon effect [2,42]. On the other hand, the level of anxiety and discomfort of such people could be higher, which may lead to personal distress. Similar effects are also observed in small children in whom prefrontal cortex is immature [19,59,60]. For example, babies start crying when they hear other babies crying. An atypical pattern of activation in empathy-related brain areas is also observed in some mental disorders.
It has been reported that adolescents with childhood-onset aggressive conduct disorder (CD), show no activation in neural regions that contribute to self-regulation and metacognition (including moral reasoning), such as the MPFC, the TPJ, and the lateral orbitofrontal cortex (OFC), and exhibit activation in insula and precentral gyrus when watching situations in which pain was intentionally inflicted [61]. It has been proposed that adolescents with CD may be more likely to respond aggressively because their empathic mimicry might produce high levels of distress. Their deficiencies in the reactions to painful situations also suggest a lack of cognitive perspective-taking. These findings indicate that CD adolescents might be dysfunctional in top-down processing of empathy-inducing information.
Factors Modulating Empathy
Most fMRI studies have shown that the empathic brain is activated when participants watch video-films featuring situations in which pain is experienced [62], observe faces expressing pain [27], or observe cartoon images of painful situations, for example, trapping one’s finger in a door or crushing one’s toe under a heavy object [28]. These empathic brain responses vary depending on modulating factors such as the intensity of the stimulation or the displayed emotion [17]. Stronger activations in the AI and the ACC were recorded in situations where participants watched pictures showing the faces of patients having acute rather than chronic pain [29], or when they observed a needle deeply penetrating body parts (rated as high pain intensity), rather than just scratching the surface of the skin (rated as low pain intensity) [22]. The greater the intensity of the stimulation of pain or its facial expression, the higher the level of empathic brain activation observed [39]. Another factor modulating the empathy level is the relationship between the subject observed and the person empathizing. It was found that when the person empathizing was related to the individual in pain or when their relationship was of an emotional nature, the level of activity in the ACC and in the AI was greater [33,63]. Nevertheless, it should be noted that activation of the aforementioned areas also takes place when we empathize with a stranger or a person to whom we are not related [62,64].
The human response to pain of others can also be modulated by situation and its context. Lamm and associates [32] showed participants video clips featuring the faces of patients with neurologic disease (Tinnitus aurium) as they – within the framework of therapy – listened to unpleasant sounds. The brain empathic response was much smaller when the participants were convinced that the pain was inflicted with a therapeutic purpose [32]. In another fMRI experiment, participants were shown pictures of a hand or hands being pierced with a needle. Next, to divert their attention from the painful situation, they were asked to count the number of hands. The activation rate of particular brain areas (ACC, AI) was significantly lower in the second case, showing that attention processes affect the level of empathy, with distraction reducing it [31].
Interestingly, the characteristics of the person empathizing, and their experience or profession also affect the process of empathizing. The level of pain empathy was found to be lower in an acupuncturist than in people from a control group, which indicates diminished pain sensitivity in those involved in pain therapy [30].
Another significant factor modulating empathy is sex – both of the person being empathized with and of the person empathizing. A higher level of activity was noted in amygdala, the ACC, and in the somatosensory cortex when participants observed pain expressed on the faces of men rather than on those of women [65]. Strong activation was observed in the amygdala of both men and women. It could be assumed that the observation of an expression of pain on a man’s face is a distinctive signal of a threat that can lead to the conditioning of fear, for which amygdala is mainly responsible [27]. Owing to the stereotype of a woman’s role in inspiring harmony or creating a loving home, women are perceived as being more empathic than men. The results of several studies seem to confirm this assumption. For example, studies on the reactive cry of babies (baby starts to cry because other babies cry) as a primitive manifestation of empathy show that female infants behave in this way more often than do males [39].
Reactions of men and women can vary depending on the characteristics of the person they are empathizing with. Both men and women have been found to empathize with the pain of individuals whom they watched playing fair in a monetary investment game before the fMRI study. Receiving mild electric shocks by those individuals evoked activations in brain areas associated with pain and empathy in both sexes. However, when the shock was delivered to individuals who played unfair, men’s brains showed no increased activity in the empathy-related pain areas. Furthermore, high levels of activity have been observed in the brain regions associated with reward, namely the ventral tegmental area (VTA) and the nucleus accumbens (NA). Interestingly, the magnitude of this effect correlated positively with the intensity of the desire for revenge, admitted in a questionnaire filled after the experiment [25]. This suggests that empathic reactions in men are shaped by perceived fairness of others, and they could even derive a satisfaction from seeing the unfair individual being punished. In contrast to this, a woman’s brain reaction to viewing the unfair person being shocked was similar (though slightly weaker) to that displayed toward the fair player.
Recordings of EEG [66] and MEG [40], and recently, the use of voxel-based morphometry (VBM) have demonstrated neuroanatomic and neurophysiological differences between the sexes in the MNS [67]. Voxel-based morphometry revealed that pars opercularis of prefrontal cortex and parietal lobe, that is, the areas in which mirror neurons are located, contain more grey matter in women than in men. Moreover, changes in the activity of these neurons measured by MEG in the mu frequency band (~20 Hz) were greater for women than they were for men when observing situations connected with pain [68], which may indicate that in terms of neuroanatomic features and neurophysiological mechanisms, women are adapted to strongly empathize with others. It may be speculated that this adaptation is connected with their role of being a mother and allows them to quickly recognize and empathize with the emotions of children, and consequently, to react in a more-rapid and precise manner, especially in threatening situations. This ability is colloquially referred to as women’s insight. Mirror neuron activity in women may be of such magnitude that its inhibition by the prefrontal area is insufficient, leading to more-effective bottom-up processing than in men.
Conclusions
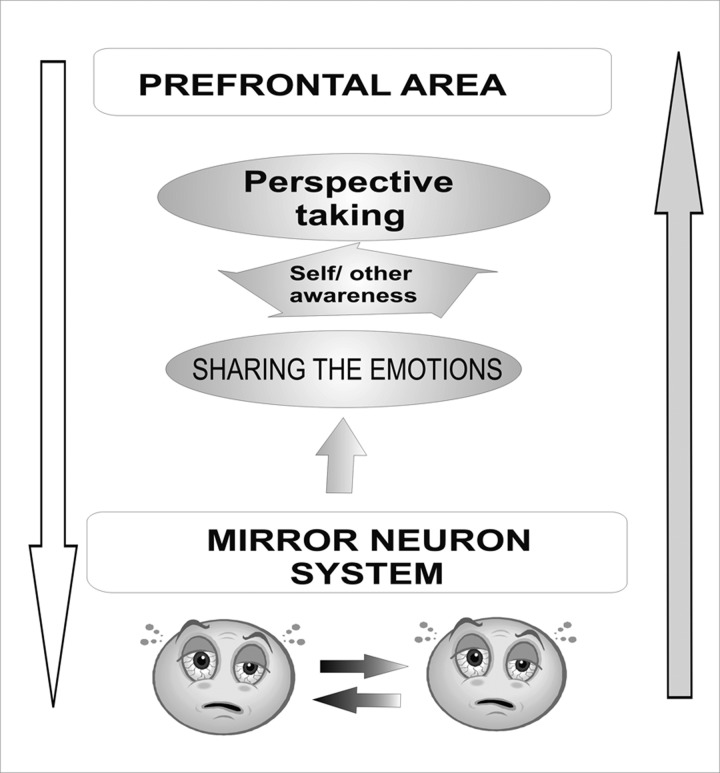
Neurobiological studies suggest that there are at least 2 modes of processing information in empathy: bottom-up and top-down. The mirror neuron system is probably engaged in the former, automatic processing mode. Neuroimaging studies indicate that the same areas of the brain are activated when people experience their own emotions and when they observe such emotions in others. Sharing an emotional state with others is, thus, an important aspect of empathizing. The ACC, the AI, and the somatosensory cortex take part in this process. Understanding of others’ feelings by taking their perspective is another vital factor in empathizing. Therefore, when we try to understand what others feel, autonomic and somatic neural pathways responsible for empathizing with the emotional state of others’ can be inhibited by top-down circuits involving mainly prefrontal areas of the brain (Figure 3). Various modulating factors affect the level of empathic response. It increases when the pain observed is greater, occurs suddenly, or when the person we empathize with is close or similar to us, and when the pain is inflicted for a nontherapeutic purpose. In addition, the sex of the observer is also important; women usually have a greater level of empathy than men, regardless of whether they like or dislike the person they empathize with. The empathic responses in men depend on the perceived fairness of others.
Figure 3.
Two modes of processing information engaged in empathy: bottom-up (grey arrow) and top-down (white arrow) (after [18], modified).
Footnotes
Source of support: Grant WB/BST/04/09 from Warsaw School of Social Sciences and Humanities and from the State Committee for Scientific Research (NN 106 042034)
References
- 1.Eisenberg N. Emotion, regulation, and moral development. Annu Rev Psychol. 2000;51:665–97. doi: 10.1146/annurev.psych.51.1.665. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman ML. Empathy and moral development. Gdańsk: GWP; 2006. [Google Scholar]
- 3.Decety J, Jackson PL. The functional architecture of human empathy. Behav Cogn Neurosci Rev. 2004;3:71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- 4.Preston SD, de Waal FB. Empathy: Its ultimate and proximate bases. Behav Brain Sci. 2002;25:1–20. doi: 10.1017/s0140525x02000018. [DOI] [PubMed] [Google Scholar]
- 5.Rizzolatti G, Fadiga L, Gallese V, et al. Premotor cortex and the recognition of motor actions. Brain Res Cogn Brain Res. 1996;3:131–41. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- 6.Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–92. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 7.Gallese V, Rochat M, Cossu G, et al. Motor cognition and its role in the phylogeny and ontogeny of action understanding. Dev Psychol. 2009;45:103–13. doi: 10.1037/a0014436. [DOI] [PubMed] [Google Scholar]
- 8.Brass M, Heyes C. Imitation: is cognitive neuroscience solving the correspondence problem? Trends Cogn Sci. 2005;9:489–95. doi: 10.1016/j.tics.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Nummenmaa L, Hirvonen J, Parkkola R, et al. Is emotional contagion special? An fMRI study on neural systems for affective and cognitive empathy. Neuroimage. 2008;43:571–80. doi: 10.1016/j.neuroimage.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci. 2001;2:661–70. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- 11.Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci. 2006;7:942–51. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- 12.Iacoboni M, Molnar-Szakacs I, Gallese V, et al. Grasping the intentions of others with one’s own mirror neuron system. PLoS Biol. 2005;3:e79. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruby P, Decety J. Effect of subjective perspective taking during simulation of action: a PET investigation of agency. Nat Neurosci. 2001;4:546–50. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- 14.Blakemore SJ, Decety J. From the perception of action to the understanding of intention. Nat Rev Neurosci. 2001;2:561–67. doi: 10.1038/35086023. [DOI] [PubMed] [Google Scholar]
- 15.Buccino G, Vogt S, Ritzl A, et al. Neural circuits underlying imitation learning of hand actions: an event-related fMRI study. Neuron. 2004;42:323–34. doi: 10.1016/s0896-6273(04)00181-3. [DOI] [PubMed] [Google Scholar]
- 16.Carr L, Iacoboni M, Dubeau MC, et al. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci USA. 2003;100:5497–502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Vignemont F, Singer T. The empathic brain: how, when and why? Trends Cogn Sci. 2006;10:435–41. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Decety J, Lamm C. Human empathy through the lens of social neuroscience. ScientificWorldJournal. 2006;6:1146–63. doi: 10.1100/tsw.2006.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Decety J, Michalska KJ. Neurodevelopmental changes in the circuits underlying empathy and sympathy from childhood to adulthood. Developmental Science. 2010:1–14. doi: 10.1111/j.1467-7687.2009.00940.x. [DOI] [PubMed] [Google Scholar]
- 20.Wicker B, Keysers C, Plailly J, et al. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–64. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- 21.Minio-Paluello I, Avenanti A, Aglioti SM. Left hemisphere dominance in reading the sensory qualities of others’ pain? Soc Neurosci. 2006;1:320–33. doi: 10.1080/17470910601035954. [DOI] [PubMed] [Google Scholar]
- 22.Avenanti A, Minio-Paluello I, Bufalari I, et al. Stimulus-driven modulation of motor-evoked potentials during observation of others’ pain. Neuroimage. 2006;32:316–24. doi: 10.1016/j.neuroimage.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Morrison I, Peelen MV, Downing PE. The sight of others’ pain modulates motor processing in human cingulate cortex. Cereb Cortex. 2007;17:2214–22. doi: 10.1093/cercor/bhl129. [DOI] [PubMed] [Google Scholar]
- 24.Singer T, Seymour B, O’Doherty J, et al. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 25.Singer T, Seymour B, O’Doherty JP, et al. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–69. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avenanti A, Bueti D, Galati G, et al. Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nat Neurosci. 2005;8:955–60. doi: 10.1038/nn1481. [DOI] [PubMed] [Google Scholar]
- 27.Botvinick M, Jha AP, Bylsma LM, et al. Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. Neuroimage. 2005;25:312–19. doi: 10.1016/j.neuroimage.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 28.Jackson PL, Rainville P, Decety J. To what extent do we share the pain of others? Insight from the neural bases of pain empathy. Pain. 2006;125:5–9. doi: 10.1016/j.pain.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Saarela MV, Hlushchuk Y, Williams AC, et al. The compassionate brain: humans detect intensity of pain from another’s face. Cereb Cortex. 2007;17:230–37. doi: 10.1093/cercor/bhj141. [DOI] [PubMed] [Google Scholar]
- 30.Cheng Y, Lin CP, Liu HL, et al. Expertise modulates the perception of pain in others. Curr Biol. 2007;17:1708–13. doi: 10.1016/j.cub.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Gu X, Han S. Attention and reality constraints on the neural processes of empathy for pain. Neuroimage. 2007;36:256–67. doi: 10.1016/j.neuroimage.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 32.Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. J Cogn Neurosci. 2007;19:42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- 33.Singer T. The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neurosci Biobehav Rev. 2006;30:855–63. doi: 10.1016/j.neubiorev.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Ogino Y, Nemoto H, Inui K, et al. Inner experience of pain: imagination of pain while viewing images showing painful events forms subjective pain representation in human brain. Cereb Cortex. 2007;17:1139–46. doi: 10.1093/cercor/bhl023. [DOI] [PubMed] [Google Scholar]
- 35.Decety J, Michalska KJ, Akitsuki Y. Who caused the pain? An fMRI investigation of empathy and intentionality in children. Neuropsychologia. 2008;46:2607–14. doi: 10.1016/j.neuropsychologia.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 36.Lamm C, Meltzoff AN, Decety J. How do we empathize with someone who is not like us? A functional magnetic resonance imaging study. J Cogn Neurosci. 2010;22:362–76. doi: 10.1162/jocn.2009.21186. [DOI] [PubMed] [Google Scholar]
- 37.Peyron R, Frot M, Schneider F, et al. Role of operculoinsular cortices in human pain processing: converging evidence from PET, fMRI, dipole modeling, and intracerebral recordings of evoked potentials. Neuroimage. 2002;17:1336–46. doi: 10.1006/nimg.2002.1315. [DOI] [PubMed] [Google Scholar]
- 38.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 39.Hein G, Singer T. I feel how you feel but not always: the empathic brain and its modulation. Curr Opin Neurobiol. 2008;18:153–58. doi: 10.1016/j.conb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Cheng Y, Yang CY, Lin CP, et al. The perception of pain in others suppresses somatosensory oscillations: a magnetoencephalography study. Neuroimage. 2008;40:1833–40. doi: 10.1016/j.neuroimage.2008.01.064. [DOI] [PubMed] [Google Scholar]
- 41.Bufalari I, Aprile T, Avenanti A, et al. Empathy for pain and touch in the human somatosensory cortex. Cereb Cortex. 2007;17:2553–61. doi: 10.1093/cercor/bhl161. [DOI] [PubMed] [Google Scholar]
- 42.Hatfield ECJ, Rapson R. Emotional contagion. New York: Cambridge University Press; 1994. [Google Scholar]
- 43.Singer T, Lamm C. The social neuroscience of empathy. Ann NY Acad Sci. 2009;1156:81–96. doi: 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- 44.Decety J, Grezes J. The power of simulation: imagining one’s own and other’s behavior. Brain Res. 2006;1079:4–14. doi: 10.1016/j.brainres.2005.12.115. [DOI] [PubMed] [Google Scholar]
- 45.Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci. 2003;358:459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behav Brain Sci. 1978;1:515–26. [Google Scholar]
- 47.Markiewicz K, MacQueen BD. The autistic mind: a case study. Med Sci Monit. 2009;15(1):CS5–13. [PubMed] [Google Scholar]
- 48.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 49.Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. J Cogn Neurosci. 2005;17:1306–15. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- 50.Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends Cogn Sci. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 51.Allman JM, Hakeem A, Erwin JM, et al. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann NY Acad Sci. 2001;935:107–17. [PubMed] [Google Scholar]
- 52.Ruby P, Decety J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emotions. J Cogn Neurosci. 2004;16:988–99. doi: 10.1162/0898929041502661. [DOI] [PubMed] [Google Scholar]
- 53.Baron-Cohen S, Ring HA, Wheelwright S, et al. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. 1999;11:1891–98. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- 54.Castelli F, Frith C, Happe F, et al. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–49. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- 55.Happe F, Ehlers S, Fletcher P, et al. ‘Theory of mind’ in the brain. Evidence from a PET scan study of Asperger syndrome. Neuroreport. 1996;8:197–201. doi: 10.1097/00001756-199612200-00040. [DOI] [PubMed] [Google Scholar]
- 56.Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 57.Barnea-Goraly N, Kwon H, Menon V, et al. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–26. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 58.Decety J, Sommerville JA. Shared representations between self and other: a social cognitive neuroscience view. Trends Cogn Sci. 2003;7:527–33. doi: 10.1016/j.tics.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 59.Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry. 2002;41:1231–38. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- 60.Bunge SA, Dudukovic NM, Thomason ME, et al. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–11. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Decety J, Michalska KJ, Akitsuki Y, et al. Atypical empathic responses in adolescents with aggressive conduct disorder: a functional MRI investigation. Biol Psychol. 2009;80:203–11. doi: 10.1016/j.biopsycho.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24:771–79. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 63.Singer T, Fehr E. The neuroeconomics of mind reading and empathy. Am Econ Rev. 2005;95:340–45. doi: 10.1257/000282805774670103. [DOI] [PubMed] [Google Scholar]
- 64.Morrison I, Lloyd D, di Pellegrino G, et al. Vicarious responses to pain in anterior cingulate cortex: is empathy a multisensory issue? Cogn Affect Behav Neurosci. 2004;4:270–78. doi: 10.3758/cabn.4.2.270. [DOI] [PubMed] [Google Scholar]
- 65.Simon D, Craig KD, Miltner WH, et al. Brain responses to dynamic facial expressions of pain. Pain. 2006;126:309–18. doi: 10.1016/j.pain.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 66.Yang CY, Decety J, Lee S, et al. Gender differences in the mu rhythm during empathy for pain: an electroencephalographic study. Brain Res. 2009;1251:176–84. doi: 10.1016/j.brainres.2008.11.062. [DOI] [PubMed] [Google Scholar]
- 67.Cheng Y, Chou KH, Decety J, et al. Sex differences in the neuroanatomy of human mirror-neuron system: a voxel-based morphometric investigation. Neuroscience. 2009;158:713–20. doi: 10.1016/j.neuroscience.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 68.Han S, Fan Y, Mao L. Gender difference in empathy for pain: an electrophysiological investigation. Brain Res. 2008;1196:85–93. doi: 10.1016/j.brainres.2007.12.062. [DOI] [PubMed] [Google Scholar]