Summary
Background
Glycemic variability is increasingly recognized as an important issue in diabetes management. However, the lack of normative values may limit its applicability in the clinical setting.
The objective of this study was to establish preliminary normal reference ranges for glycemic variability by analyzing continuous glucose monitoring (CGM) data obtained from healthy Chinese adults.
Material/Methods
Three-day CGM data were obtained from 434 healthy adults at 10 academic hospitals throughout China. Glycemic variability was calculated as the 24-hour mean amplitude of glycemic excursions (MAGE) and standard deviations (SD) of blood glucose readings.
Results
434 healthy subjects (male 213, female 221; age 43±14, 20–69 years old; BMI 21.8±1.7 kg/m2, 18.5–24.9 kg/m2) completed the study. MAGE and SD values for the 434 healthy subjects were 1.73 (1.08) mmol/L and 0.75 (0.42) mmol/L [median (interquartile range)], respectively. In both men and women, MAGE and SD tended to increase with age. Neither MAGE nor SD showed a significant difference between men and women. Values for both parameters were non-normally distributed within the population. The 95th percentiles of MAGE and SD were 3.86 and 1.40 mmol/L, respectively. These values were adopted as the upper limits of normal.
Conclusions
MAGE <3.9 mmol/L and SD <1.4 mmol/L are recommended as the normal reference ranges for glycemic variability in Chinese adults. The values established in this study may facilitate the adoption of glycemic variability as a metric of overall glycemic control in diabetes.
Keywords: glycemic variability, continuous glucose monitoring, reference ranges
Background
The well-known Diabetes Control and Complications Trials (DCCT) and United Kingdom Prospective Diabetic Studies (UKPDS) have established glycosylated hemoglobin (HbA1c) as a standard measure of average glucose control because of its strong correlation with the development of diabetes-related complications. However, in both basic and clinical studies in recent years, glycemic variability has become increasingly recognized as a risk factor for chronic diabetes complications. Evidence is accumulating that high levels of glycemic variability have deleterious effects beyond those of sustained chronic hyperglycemia in terms of oxidative stress, diabetes complications, and cardiovascular outcomes [1–6], although some contradictory reports also exist [7]. Low levels of glycemic variability are therefore recommended as a component of overall glycemic control [8–11]. Currently, efforts to reduce glycemic variability are largely frustrated by the lack of reference values [11]. In our previous multi-center studies, we established normal reference ranges for continuous blood glucose parameters (the 24-h mean blood glucose and the percentage of time that subjects’ blood glucose levels were ≥7.8 mmol/L and ≤3.9 mmol/L within 24 h) through analysis of continuous glucose monitoring (CGM) data obtained from healthy Chinese adults [12]. The current paper reports on further analyses on these data, aiming to generate reference ranges for glycemic variability in normal Chinese adults, which may facilitate clinical adoption.
Material and Methods
A total of 445 (out of 588 screened subjects) healthy subjects without related metabolic disorders were enrolled from 10 academic hospitals in China between October 2007 and July 2008. The inclusion/exclusion criteria and study methods have been described in detail in previous publication [10]. The inclusion criteria were the following: 1) clinically stable condition with no previous medical history of diabetes, hypertension, dyslipidemia, coronary artery diseases or cerebral stroke; 2) fasting plasma glucose <5.6 mmol/L and 2-h plasma glucose <7.8 mmol/L in 75 g oral glucose tolerance test; 3) normal body mass index between 18.5 and 24.9 kg/m2; 4) triglycerides <1.7 mmol/L and high density lipoprotein cholesterol ≥1.04 mmol/L; and 5) systolic pressure <140 mmHg and diastolic pressure <90 mmHg. The exclusion criteria were the following: 1) use of medications that may affect glucose metabolism, such as glucocorticoids, thyroid hormones and thiazide diuretics, 1 month before the study; and 2) hepatic or renal dysfunctions (>1.5-fold elevation of alanine aminotransferase, aspartate aminotransferase or direct bilirubin, or serum creatinine >115 μmol/L). This study was independently approved by the ethics committee of each participating hospital. All subjects gave written informed consent before study initiation. No medications known to affect glucose tolerance were allowed during the study.
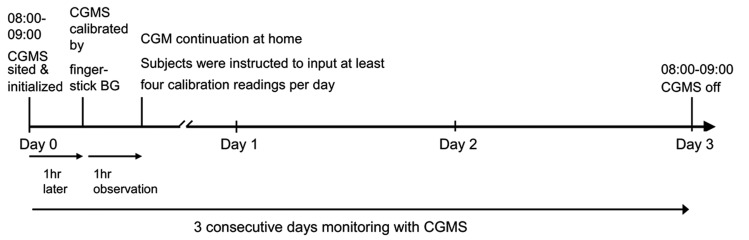
As described in a previous publication [12], the CGMS sensor was inserted into all subjects by the same specialist nurse at Day 0 around 8:00–9:00 AM in hospital. The first CGMS calibration by finger-stick BG was performed after 1 hour of initialization. If no abnormal CGMS situation was observed, the subjects was dismissed and they continued with CGM at home for 3 consecutive days. Subjects were instructed to input at least 4 calibration readings per day. At Day 3, around 8:00–9:00 AM, subjects came to the hospital and had the CGMS removed. All subjects completed a 3-day period of glucose monitoring using a continuous glucose monitoring system (CGMS) (Medtronic Inc, Northridge, CA), and 434 subjects had valid CGM data (Figure 1). The parameters of glycemic variability included the mean amplitude of glycemic excursions (MAGE) and the standard deviation (SD) of blood glucose readings. MAGE was obtained by measuring the arithmetic mean of the differences between consecutive peaks and nadirs, with measurement in the peak-to-nadir direction; only excursions >1 SD were considered [13]. For SD determination, the standard deviations of a total of 288 values collected during a 24-hour CGM period for each study subject were calculated. For 434 subjects, final values of both MAGE and SD were based on the mean values taken on Day 1 and Day 2. Data are expressed as the mean ±SD, except for skewed variables, which are presented as the median (interquartile range). CGM parameters were analyzed using CGMS Software 3.0. Statistical analyses were performed using SPSS software (version 13.0). Summary statistics were presented as χmacr;±s. The Wilcoxon rank test was used for inter-group comparisons. Spearman correlation analysis and linear regression analysis were employed for correlation analysis. The Kolmogorv-Smirnov test was used to determine the normality of the derived parameter’s distribution within the population.
Figure 1.
Schematic timeline of each study day.
A subgroup of 20 subjects was used to evaluate the CGMS reproducibility. To balance the sex and age of these 20 subjects, a stratified sample scheme was adopted: subjects were divided into 10 ages by sex strata (i.e., 20–29, 30–39, 40–49, 50–59 and 60–69; male and female). Within each stratum, 2 subjects were randomly selected.
Results
As reported in a previous publication [12], a total of 434 healthy subjects (male 213, female 221; age 43±14, 20–69 years old; BMI 21.8±1.7 kg/m2, 18.5–24.9 kg/m2) completed the study.
MAGE and SD levels in relation to age and sex
Calculated from the 434 healthy subjects, MAGE and SD were 1.73 (1.08) mmol/L and 0.75 (0.42) mmol/L [median (interquartile range)], respectively. Spearman correlation analysis indicated a significant positive correlation between MAGE and SD (r=0.90, P<0.001). In both men and women, both MAGE (r=0.18 and 0.17, respectively, P<0.05) and SD (r=0.17 and 0.16, respectively, P<0.05) were weakly positively correlated to age. Generally, the MAGE and SD positively correlated to age after adjustment for 24-MBG (r=0.18 and 0.17, respectively, both P<0.05, Figure 2). The analysis among different age groups revealed significantly higher values for both MAGE and SD levels in subjects above 60 years age (P<0.05). No significant difference in MAGE or SD value was observed between men and women in any single age group (P>0.05, Table 1).
Figure 2.
Correlation analysis of MAGE/SD levels with age in 434 healthy subjects. A significant positive correlation was shown between (A) MAGE with age (r=0.18, P<0.05), and (B) SD with age (r=0.17, P<0.05).
Table 1.
MAGE and SD levels by gender and age for 434 healthy adults.
| Age (years) | Men | Women | All | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 20–39 | 40–59 | 60–69 | All | 20–39 | 40–59 | 60–69 | All | ||
| n | 93 | 80 | 40 | 213 | 99 | 86 | 36 | 221 | 434 |
| MAGE (mmol/l) | |||||||||
| Mean | 1.80 | 1.99 | 2.16 | 1.94 | 1.84 | 1.95 | 2.37 | 1.97 | 1.96 |
| SD | 0.76 | 0.90 | 0.84 | 0.86 | 0.87 | 0.94 | 1.14 | 0.96 | 0.91 |
| P5 | 0.76 | 0.83 | 0.84 | 0.82 | 0.97 | 0.72 | 0.81 | 0.84 | 0.84 |
| P10 | 0.97 | 1.04 | 0.92 | 1.00 | 1.06 | 0.87 | 1.16 | 1.01 | 1.01 |
| P50 | 1.62 | 1.74 | 2.03 | 1.76 | 1.60 | 1.72 | 2.10 | 1.68 | 1.73 |
| P90 | 2.91 | 3.28 | 3.37 | 3.12 | 2.79 | 3.41 | 3.82 | 3.49 | 3.26 |
| P95 | 3.35 | 3.60 | 3.69 | 3.67 | 3.73 | 4.08 | 4.23 | 4.01 | 3.86 |
| SD of blood glucose readings (mmol/l) | |||||||||
| Mean | 0.75 | 0.79 | 0.89 | 0.79 | 0.76 | 0.76 | 0.94 | 0.79 | 0.79 |
| SD | 0.32 | 0.33 | 0.33 | 0.33 | 0.29 | 0.31 | 0.36 | 0.32 | 0.32 |
| P5 | 0.35 | 0.30 | 0.35 | 0.35 | 0.40 | 0.32 | 0.34 | 0.36 | 0.35 |
| P10 | 0.40 | 0.40 | 0.40 | 0.40 | 0.45 | 0.38 | 0.48 | 0.45 | 0.40 |
| P50 | 0.70 | 0.75 | 0.88 | 0.75 | 0.70 | 0.75 | 0.98 | 0.75 | 0.75 |
| P90 | 1.20 | 1.29 | 1.30 | 1.25 | 1.15 | 1.20 | 1.44 | 1.29 | 1.25 |
| P95 | 1.38 | 1.44 | 1.59 | 1.43 | 1.30 | 1.43 | 1.60 | 1.40 | 1.40 |
MAGE, mean amplitude of glycaemic excursions; P5, P10, P50, P90, and P95 indicate percentile values.
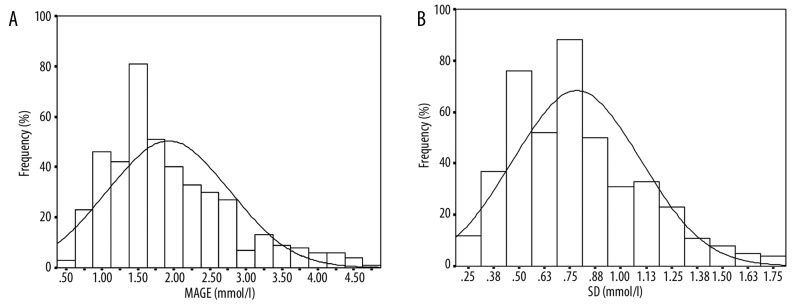
Distribution and normal reference values for MAGE and SD
MAGE and SD values were not normally distributed (P<0.001). The coefficients of skewness for these parameters were 1.18 and 0.79, respectively (Figure 3). The 95th percentile values of 3.86 mmol/L for MAGE and 1.40 mmol/L for SD represent the upper limit of normal (Table 1).
Figure 3.
Subject distribution according to (A) different MAGE levels, and (B) SD in 434 healthy subjects.
Reproducibility of CGM evaluation
Two men and 2 women were randomly selected from each of the 5 age groups (20–29, 30–39, 40–49, 50–59 and 60–69) for the evaluation of reproducibility. A total of 20 subjects with a mean age of 43±16 (range, 22–68) years and body mass index (BMI) of 22.2±1.8 kg/m2 underwent a second 3-day CGM evaluation 8–12 weeks after the initial monitoring. The values obtained for the 2 measurements were: 1.70 (0.82) mmol/L vs. 1.78 (0.87) mmol/L (first vs. second monitoring) for MAGE, and 0.75 (0.32) mmol/L vs. 0.82 (0.37) mmol/L [median (interquartile range)] (first vs. second monitoring) for SD. No significant difference was observed in MAGE or SD between the first and second monitoring periods (both P>0.05).
Discussion
CGM is an evolving technology that greatly facilitates measurement of glycemic variability [14,15]. Many different summary statistics have been proposed for accurate assessment of glycemic variability, including SD, coefficient of variation (%CV), interquartile range (IQR), percentage of glucose values within specified ranges, MAGE, M-value, mean of the daily differences (MODD), the average daily risk range (ADRR) [16], and continuous overlapping net glycemic action over an n-hour period (CONGA-n) [17]. Notwithstanding their apparent diversity, all of these parameters are derived from statistical interconversion and manipulation of sequential blood glucose levels. Accordingly, various assessment parameters for glycemic variability, with different intrinsic characteristics and scopes of applicability, should be selected in specific clinical settings [18]. In addition to SD, which is one of the most frequently used measurements of glycemic variability in statistics [19,20], MAGE obtained from CGM is currently generally regarded as the “gold standard” metric of glycemic variability [11]. Many ongoing studies use MAGE to investigate the relationship between glycemic variability in patients with diabetes and oxidative stress, chronic complications and pancreatic islet functions, and to evaluate the impact of treatment regimen on glycemic variability [2–4,21,22]. Therefore, both SD and MAGE were analyzed in the current study.
As seen in the current results, age seemed to have an impact on glucose homeostasis. Glycemic variability increased along with increased age in healthy people. Previous studies showed that insulin secretion decreased with aging in people with normal glucose tolerance (NGT) [23]. Moreover, glycemic variability (measured by MAGE) was found to be associated with postprandial beta-cell function in a study by Kohnert et al. [21,22]. It could therefore be assumed that the deterioration of beta-cell function with aging contributes to the association of age with glycemic variability observed in the current study.
Since the conduct of prospective follow-up studies on glycemic variability remains rather challenging, CGMS data analyses in healthy subjects provide a feasible approach to establish normal reference values for these measurements. The population mean value of MAGE in this study was 1.96 mmol/L, comparable to the previous findings of Monnier et al. [24]. They reported that at the mean oxidative stress level (represented as 24-hour 8-isoprostane F2α excretion rate in urine) of 275 pg/mg creatinine, the corresponding glycemic variability (represented as MAGE) was 2.2 mmol/L (40 mg/dl) in healthy subjects. Since distributions for both MAGE and SD values departed from normality, the 95th percentiles of the 2 parameters were used for defining normality in our study, and were set as the upper limits of the normal reference values (MAGE <3.9 mmol/L and SD <1.4 mmol/L, respectively). Analyses of the relationship between these parameters and demographic characteristics revealed that MAGE and SD were independent of sex, but increased with age. Both parameters increased significantly when age reached 60 years and older. We also recommend a unified cut-off point for the normal reference values of the 2 parameters for glycemic variability, similar to the normal glucose tolerance cutoff points recommended by ADA or WHO, without regard for age or sex. Further investigations are warranted to verify the normal reference values of the glycemic variability parameters established in this study.
Conclusions
Overall, the current concept of BG management calls for reduction of glycemic variability in patients with diabetes. This requires close monitoring of blood glucose levels with CGM, as well as judicious pharmacotherapy to minimize the risks of both postprandial hyperglycemia and hypoglycemia. The reference values of MAGE and SD parameters established in this study provide the preliminary targets for normalization of glycemic variability. Its clinical application depends on validation in other populations, such as those with different glycemic metabolism. Moreover, the impact of BMI on the MAGE and SD reference values needs further investigation.
Acknowledgments
We would like to thank all the involved clinicians, nurses and technicians at all the participating centers for their dedication to the study: Shanghai Jiaotong University Affiliated Sixth People’s Hospital (Kunsan Xiang, Yuqian Bao, Xiaojing Ma, Wei Lu, Cheng Hu, Huijuan Lu), Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University (Fenping Zheng), West China Hospital, Sichuan University (Liping He), China-Japan Friendship Hospital (Jinping Zhang, Na Wang), The Second Affiliated Hospital of Harbin Medical University (Lili Chen), Shanghai Jiaotong University Affiliated First People’s Hospital (Yufan Wang), The First Affiliated Hospital of Sun Yat-Sen University (Juan Liu), Fudan University Affiliated Zhongshan Hospital (Zhiqiang Lu, Ran You), and Ruijin Hospital, Shanghai Jiaotong University School of Medicine (Shouyue Sun).
Footnotes
Source of support: This work was supported by the Shanghai United Developing Technology Project of Municipal Hospitals (SHDC12006101), and Shanghai Key Laboratory of Diabetes Mellitus (08DZ2230200)
References
- 1.Muggeo M, Zoppini G, Bonora E, et al. Fasting plasma glucose variability predicts 10-year survival of type 2 diabetic patients: the Verona Diabetes Study. Diabetes Care. 2000;23:45–50. doi: 10.2337/diacare.23.1.45. [DOI] [PubMed] [Google Scholar]
- 2.Monnier L, Colette C. Glycemic Variability: Should we and can we prevent it? Diabetes Care. 2008;31:S150–54. doi: 10.2337/dc08-s241. [DOI] [PubMed] [Google Scholar]
- 3.Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57:1349–54. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 4.Pflueger A, Abramowitz D, Calvin AD. Role of oxidative stress in contrast-induced acute kidney injury in diabetes mellitus. Med Sci Monit. 2009;15(6):RA125–36. [PubMed] [Google Scholar]
- 5.Gordon L, Morrison EY, McGrowder DA, et al. Changes in clinical and metabolic parameters after exercise therapy in patients with type 2 diabetes. Arch Med Sci. 2008;4:427–37. [Google Scholar]
- 6.Wiersma JJ, Meuwese MC, van-Miert JN, et al. Diabetes mellitus type 2 is associated with higher levels of myeloperoxidase. Med Sci Monit. 2008;14(8):CR406–10. [PubMed] [Google Scholar]
- 7.Kilpatrick ES, Rigby AS, Atkin SL. Effect of glucose variability on the long-term risk of microvascular complications in type 1 diabetes. Diabetes Care. 2009;32:1901–3. doi: 10.2337/dc09-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch IB, Brownlee M. Should minimal blood glucose variability become the gold standard of glycemic control? J Diabetes Complications. 2005;19:178–81. doi: 10.1016/j.jdiacomp.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA. 2006;295:1707–8. doi: 10.1001/jama.295.14.1707. [DOI] [PubMed] [Google Scholar]
- 10.Monnier L, Colette C, Boegner C, et al. Continuous glucose monitoring in patients with type 2 diabetes: Why? When? Whom? Diabetes Metab. 2007;33:247–52. doi: 10.1016/j.diabet.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Monnier L, Colette C, Owens DR. Integrating glycaemic variability in the glycaemic disorders of type 2 diabetes: a move towards a unified glucose tetrad concept. Diabetes Metab Res Rev. 2009;25:393–402. doi: 10.1002/dmrr.962. [DOI] [PubMed] [Google Scholar]
- 12.Zhou J, Li H, Ran X, et al. Reference values for continuous glucose monitoring in Chinese subjects. Diabetes Care. 2009;32:1188–93. doi: 10.2337/dc09-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Service FJ, Molnar GD, Rosevear JW, et al. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19:644–55. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 14.Fiallo-Scharer R -Diabetes RiCNSG. Eight-point glucose testing versus the continuous glucose monitoring system in evaluation of glycemic control in type 1 diabetes. J Clin Endocrinol Metab. 2005;90:3387–91. doi: 10.1210/jc.2004-2510. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch IB, Armstrong D, Bergenstal RM, et al. Clinical application of emerging sensor technologies in diabetes management: consensus guidelines for continuous glucose monitoring (CGM) Diabetes Technol Ther. 2008;10:232–44. doi: 10.1089/dia.2008.0016. [DOI] [PubMed] [Google Scholar]
- 16.Kovatchev BP, Otto E, Cox D, et al. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care. 2006;29:2433–38. doi: 10.2337/dc06-1085. [DOI] [PubMed] [Google Scholar]
- 17.McDonnell CM, Donath SM, Vidmar SI, et al. A novel approach to continuous glucose analysis utilizing glycemic variation. Diabetes Technol Ther. 2005;7:253–63. doi: 10.1089/dia.2005.7.253. [DOI] [PubMed] [Google Scholar]
- 18.Rodbard D. Interpretation of continuous glucose monitoring data: glycemic variability and quality of glycemic control. Diabetes Technol Ther. 2009;11(Suppl 1):S55–67. doi: 10.1089/dia.2008.0132. [DOI] [PubMed] [Google Scholar]
- 19.Egi M, Bellomo R, Stachowski E, et al. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology. 2006;105:244–52. doi: 10.1097/00000542-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch IB. Glycemic variability: it’s not just about A1C anymore! Diabetes Technol Ther. 2005;7:780–83. doi: 10.1089/dia.2005.7.780. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J, Jia W, Bao Y, et al. Glycemic variability and its responses to intensive insulin treatment in newly diagnosed type 2 diabetes. Med Sci Monit. 2008;14(11):CR552–58. [PubMed] [Google Scholar]
- 22.Kohnert KD, Augstein P, Zander E, et al. Glycemic variability correlates strongly with postprandial beta-cell dysfunction in a segment of type 2 diabetic patients using oral hypoglycemic agents. Diabetes Care. 2009;32:1058–62. doi: 10.2337/dc08-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szoke E, Shrayyef MZ, Messing S, et al. Effect of aging on glucose homeostasis: accelerated deterioration of beta-cell function in individuals with impaired glucose tolerance. Diabetes Care. 2008;31:539–43. doi: 10.2337/dc07-1443. [DOI] [PubMed] [Google Scholar]
- 24.Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–87. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]