Summary
Background
When heat is applied to the skin, it is dissipated due to conductive heat flow in the tissue and the blood. While heat flow has been studied after applying a single heat exposure, the physiology of repeated exposures to local heat has not been well investigated.
Material/Methods
Twenty male and female subjects in the age range of 20–65 years old participated in a series of experiments during which a thermode was placed on their leg above the quadriceps muscle for 20 minutes, and on 3 sequential days, to see the effect of repeated local heat on skin blood flow, skin temperature, and on caloric transfer from a thermode used to raise skin temperature.
Results
The results of the experiment showed that, for young subjects, to raise skin temperature to 40 degrees C required more than double the calories required in older subjects. Further, in the younger subjects, the blood flow response in the first 20 minutes of heat exposure was over 30% higher than that seen in the older subjects. However, on the 2nd and 3rd day, the blood flow response of the younger subjects, was not significantly different between day 2 and 3, but was significantly less than day 1. There was no statistical difference in the blood flow response between day 1, 2 and 3 in the older subjects. In the younger subjects, in the 2 and 3rd day, the number of calories needed to warm the skin was also significantly less than that seen in the first day.
Conclusions
In younger subjects but not older subjects, there appears to be some degree of acclimatization with an enhanced blood flow response in the first day that was protective to the skin which was not seen in repeated heat exposure.
Keywords: heat, age, circulation, blood flow
Background
When heat above 42°C is applied to the skin, there is a two phase response of the skin circulation [1–3]. In the first phase, a rapid increase in circulation is mediated by sensory nerves just after heat is applied [1,3]. The initial vasodilation is believed to be mediated by substance P and/or calcitonin gene-related peptide (CGRP) or both [4–6]. This protects the skin from rapid changes in temperature that might cause damage [7–10]. The neuropeptides are released when TRPV1 voltage gated calcium channels in skin tactile sensory receptors become active [11]. A sequence of amino acids near the C terminus of this receptor changes calcium permeability in a graded response with local tissue temperature[11]. The release of neuropeptides then causes relaxation of vascular smooth muscle. The sustained skin vasodilation that occurs after the first few minutes of heat exposure is mediated by nitric oxide released by activation of the enzyme endothelial nitric oxide synthetase due to calcium released by temperature sensitive TRPV4 channels in vascular endothelial cells [1,3,12].
But ageing has an impact on skin blood flow. Numerous studies have shown that skin blood flow at rest is diminished with ageing [13–19]. Thus, it is of no surprise that heat tolerance is reduced with age [20,21]. To understand the interaction between skin temperature and applied local heat, Pennes first modeled the influence of circulation on thermal transfer from the skin [22]. He noted that for a rapid application of heat, such as touching a scalding hot piece of iron, circulation cannot increase fast enough to protect the skin and so, the skin is only protected by the ability to conduct heat passively [22,23]. Heat conduction in the skin depends on the water content of the skin, thickness of subcutaneous fat, and dermal epidermal thickness [22–24]. Associated with ageing, the skin dermal layer thickness thins due to the ageing process [25,26]. Further, associated with ageing, subcutaneous fat thickens and thus, it is more difficult for heat to transfer out of the skin due to the high insulative capacity of fat [16,26,27]. But for sustained heat, complicating matters further, skin circulation is also impaired with ageing [16,28–30]. Further, whereas the sustained response of the skin circulation to heat can be mediated by several compounds in younger individuals, in older individuals it is mainly nitric oxide that mediates vasodilatation in the skin [26]. The effect of age on the neuropeptide response to local heat application has not been investigated. It is of no surprise that older individuals are much more susceptible to skin damage, burns, and show slower healing [12].
However, almost all studies on exposure of the skin to local heat have dealt with a single thermal exposure for both young and older people. The effect of daily exposure to local heat to see if there is any adaptation in the skin has not been published. This is in sharp contrast to studies of whole body heating. When an individual is placed in a hot environment either at exercise or at rest, there is whole body adaptation to heat including a reduction in the sodium content of sweat, increase in skin circulation in response to global heating and numerous other mechanisms mediated through the hypothalamic adrenal axis [31].
Therefore, in the present investigation, we tested the hypothesis that there is acclimatization to the local application to heat which will be diminished with age. The study was conducted applying heat to the skin above the quadriceps muscle on 3 successive days in younger and older individuals. The parameters measured were skin temperature, skin blood flow, and to understand the thermal transfer of skin in younger and older individuals and on a day to day basis, a thermode was used to deliver a heat load to the skin. The thermode was calibrated such that the total caloric transfer to the skin could be assessed.
Material and Methods
Subjects
Twenty male and female subjects participated in these experiments. Their demographics are shown in Table 1 below. In the younger group, there were 5 men and 5 women while in the older group there were 6 men and 5 women. There was no statistical difference in the height. However, the weight and age were significantly higher in the older group than the younger group. The subjects were roughly equally divided between men and women in both the younger and older groups. Subjects were excluded if any individuals were taking alpha or beta antagonists or agonists, calcium channel blockers, or had any type of cardiovascular disease. All subjects were diagnosed free of diabetes. All experimental methods and procedures were explained to each subject who then signed a statement of informed consent as approved by the Institutional Review Board of Loma Linda University.
Table 1.
Subject demographics.
| Weight (Kg) | Height (cm) | Age (years) | BMI | Body fat (%) | Skin moisture (mg/cm2) | Skin thickness (cm) | Fat thickness (cm) | ||
|---|---|---|---|---|---|---|---|---|---|
| Young | Mean | 68.9 | 1.7 | 25.1 | 24.6 | 31.3 | 39.3 | 0.066 | 0.080 |
| SD | 19.6 | 0.1 | 2.2 | 4.6 | 7.7 | 6.7 | 0.012 | 0.011 | |
| Old | Mean | 73.6 | 1.6 | 59.9 | 27.1 | 27.3 | 29.2 | 0.054 | 0.106 |
| SD | 15.8 | 0.1 | 6.1 | 4.7 | 6.9 | 13.9 | 0.011 | 0.034 |
Methods
Blood flow
Skin blood flow was measured with a Moor LDF Laser Doppler Imager. The Laser Doppler Imager was placed in single point mode such that a continuous blood flow recording could be made through the thermode and on the skin. The laser Doppler imager (Moor Instruments Oxford, England) was calibrated prior to the experiments and provided blood flows in the range of 0–1400 flux. Blood flow data was analyzed over 5 second periods.
Skin temperature
Skin temperature was measured with a thermistor manufactured by BioPac systems (BioPac Inc., Goleta, CA). The thermistor output was amplified with a SKT 100 Thermistor amplifier (BioPac systems, Goleta, CA) and the output was then digitized at 1000 samples per second on a BioPac MP150 data collection system (BioPac Systems, Goleta, CA). The output was digitized with 24 bits resolution and stored on an IBM computer for later analysis with the Acknowledge 9.1 computer software. Data analysis was done over 5 second periods as the mean temperature. Two other thermistor sensors were used to measure the inflow and outflow temperature of the thermode described below. The thermistors were calibrated at 30 and 45 degrees C before the series of experiments and after the series of experiments to ensure linearity and accuracy with the series of water baths at different temperatures.
Thermode
The device to warm and measure heat exchange into the skin was milled from a Plexiglas block which was 4×5×2 cm thick. The center was milled from the plastic block so that there was a final wall thickness in the center of 0.5 cm around the outside edges in the bottom of the chamber. A small area in the center which was 2×1 cm was not milled and a hole was drilled through this center section such that with two water ports on the inlet and outlet of the chamber, water would traverse the chamber while a laser would go through the center of the chamber and measure blood flow in the skin. The hole through the center of the chamber was 0.4 cm in diameter. The inlet and outlet mills were on opposite sides of the rectangular chamber and were 0.4 cm in diameter. A thermistor was inserted in the inlet and outlet valves such that the temperature of the water in and out of the chamber could be measured. Flow diverters were used inside the chamber to keep linear flow across the entire chamber. A thin brass membrane 0.1 mm thick was applied to the open section at the bottom of the chamber so that when in contact with the skin, heat would flow through the thin brass layer into the skin. The thin brass plate was glued with silicone rubber to the outside edge of the chamber and allowed to cure for 48 hours before use.
Subcutaneous fat and skin thickness
These were determined with a Sonasite 180 (Seattle Washington) using a linear L35 126 element probe at 10 Megahertz. A 0.5cm standoff was used under the probe and the probe was held vertical to avoid false echoes in the measurement of skin and subcutaneous fat thickness.
Measurement of skin moisture
Skin moisture was measured with a Corneometer 810 capacitance skin moisture meter (Courage Khazaka Electronics, Cologne, Germany). It determines the humidity level of the stratum corneum by measuring electrical capacitance. Alterations in skin hydration lead to a change in capacitance. The probe is applied to the skin for one second at a pressure of 7·1 N/cm2. The degree of skin capacitance is indicated from 1 to 100 units. One unit represents a water content of the stratum corneum of 0·02 mg/cm2, at a measuring depth of 20 nm. Very dry skin is <30; dry 30–45; sufficiently moisturized >45 units. Tissue capacitance is measured by applying electromagnetic waves at a frequency of 100,000 cycles per second to image the skin surface. It has been used extensively to evaluate the effect of different treatments on skin conditions.
Procedures
All subjects followed the same procedures. On a similar time of the day, subjects entered the lab and rested for 20 minutes in a thermally neutral room. Following this period of time, skin temperature and skin blood flow was measured above the quadriceps muscle (medial head). After a period of 1 minute, the thermode with inlet water temperature of 44 degrees C was placed on the leg for 20 minutes. During that period of time, two bubble levels were used to make sure that thermode was kept perfectly horizontal on the skin such that the laser would go through the center and record skin blood flow. After 20 minutes, the thermode was removed and post data was collected for an additional 5 minutes. The procedures were repeated on 3 consecutive days at approximately the same time of the day on a group of 10 younger and 10 older subjects.
Statistical analysis
Statistical analysis involved the calculation of means, standard deviations, and related and unrelated t tests. In addition, on some analysis, ANOVA was used. The level of significance was p=0.05.
Results
Response on the first day
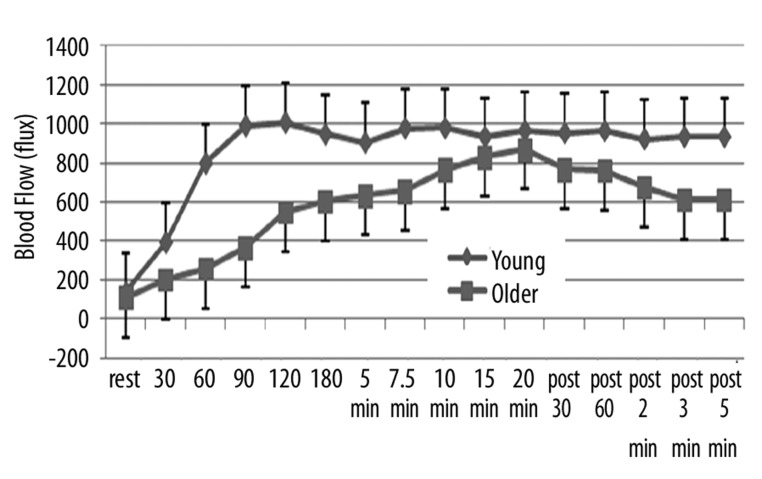
During the 20 minutes of heat exposure and for 5 minutes after, there was a clear difference in the blood flow in the younger vs. older group of subjects as shown in Figure 1. As shown here, the average blood flow for the younger group was 136.27±48.1 flux compared to 106.1±20.3 flux for the older group, significantly lower blood flow (p<0.05). This difference was maintained throughout the heat exposure. In the younger group, as shown in this figure, blood flow began to immediately increase such that it peaked within 2 minutes of exposure to heat. After that point, blood flow was essentially stable at an average of about 1000 flux throughout the remainder of the exposure period. After the thermode was removed, there was a slight reduction in blood flow, such that after 5 minutes, the average blood flow was 933.1±184 flux. In contrast, in older subjects, blood flow increased much more slowly and was only at its peak after 20 minutes of heat exposure averaging, at that point, 866.5±193 flux. As soon as the heat was removed, blood flow began to rapidly decrease and after 5 minutes, averaged 609.6±150.2 flux. Thus, at all points during heat exposure and rest, blood flow was significantly higher than in the older group (p<0.05).
Figure 1.
Illustrated here is the blood flow (in flux) measured at rest and at 30, 60, 90, 120, 180 seconds after exposure to heat and at 5, 7.5, 10, 15, and 20 minutes after exposure to heat and for 5 minutes after the heat was removed with measurements at 30 sec, 60 sec, 2 min, 3 min and 5 min as the average of 10 younger (diamonds) and 10 older (squares). Each point is the mean ± the respective standard deviation.
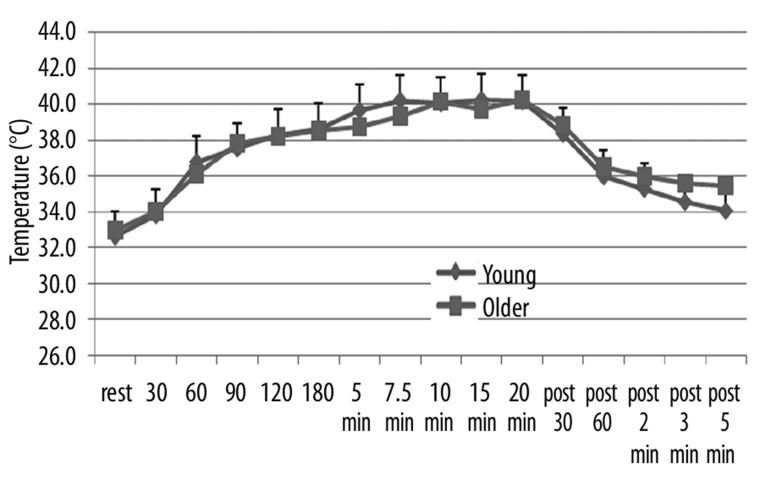
In contrast, skin temperature, which started at an average in both groups at 33.1±0.9 degrees C, increased steadily after exposure to heat to a final average temperature for the two groups of 40.3±1.8°C. There was no statistical difference at any point during exposure to heat or recovery in the skin temperature between the two groups (p>0.05).
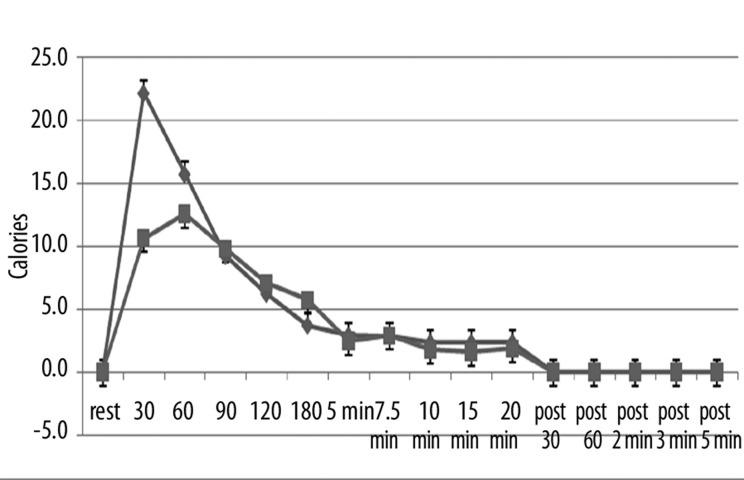
However, whereas skin temperature was the same, there was a difference in the number of calories necessary to increase skin temperature between the two groups. As shown in Figure 3, the greatest number of calories consumed by the skin was in the first 90 seconds in both groups of subjects. However, the younger subjects required an average of 22.2±4.3 calories in the first 30 seconds whereas the older subjects only consumed 10.6±1.9 calories to warm the skin. Integrating the area under the curve for the entire exposure period in the younger subjects, the average number of calories over the 20 minute period was 7.1 calories per minute whereas for the older group of subjects it was 5.66 calories. This difference was statistically significant (p<0.01).
Figure 3.
Illustrated here is the thermal heat transfer (in calories) measured at rest and at 30, 60, 90, 120, 180 seconds after exposure to heat and at 5, 7.5, 10, 15, and 20 minutes after exposure to heat and for 5 minutes after the heat was removed with measurements at 30 sec, 60 sec, 2 min, 3 min and 5 min as the average of 10 younger (diamonds) and 10 older (squares). Each point is the mean ± the respective standard deviation.
Effect of repeated heat
Younger group
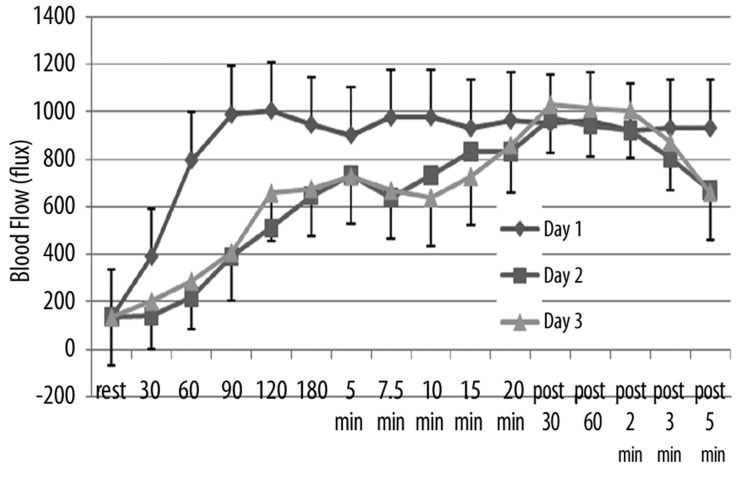
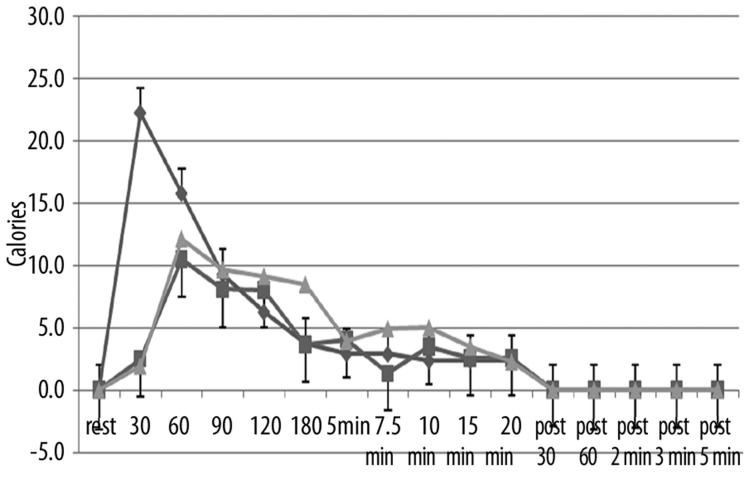
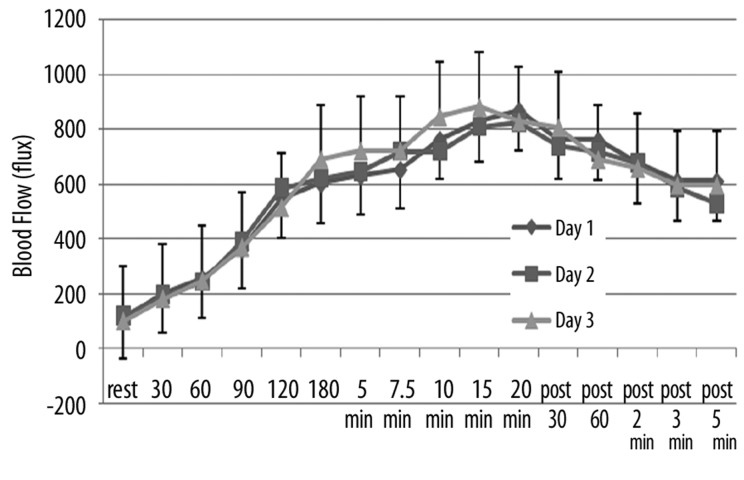
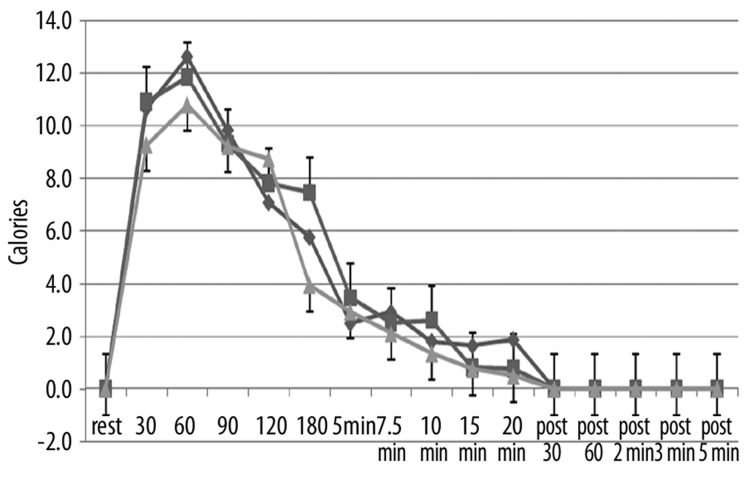
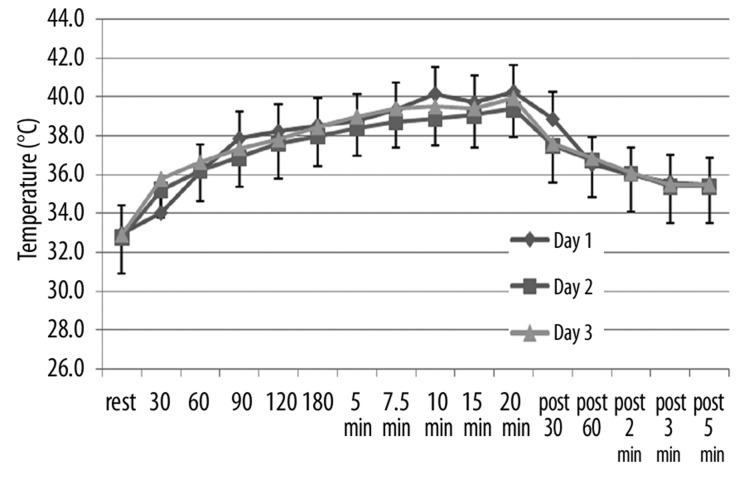
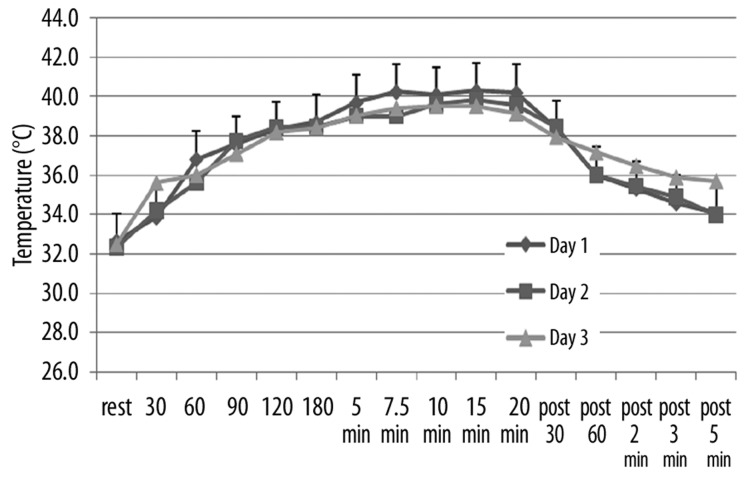
The effects of repeated heat on the same three parameters as in Figures 1–3 are shown in Figures 4–6 respectively for the younger groups of subjects. In Figure 4, for example, blood flow, which increased rapidly during the 1st day, increased much more slowly in the 2nd and 3rd day after heat exposure as can be seen in Figure 4. There is no statistical difference between the blood flow response in days 2 and 3 whereas, during the first 15 minutes, blood flow was significantly higher in the 1st day than the other two days (ANOVA p>0.05). In contrast, as shown in Figure 4, skin temperature was the same at rest throughout heat exposure and during recovery during all 3 days. As shown in Figure 6, the numbers of calories consumed at each time interval measurement was the same for day 2 and 3 and was significantly lower than day 1 in the younger group of subjects.
Figure 4.
Illustrated here is the blood flow (in flux) in the younger group of subjects measured at rest and at 30, 60, 90, 120, 180 seconds after application of heat and at 5, 7.5, 10, 15, and 20 minutes after application of heat and well as at 30 sec, 60 sec, 2 min, 3 min and 5 min post removal of the heat. Data is shown for the 1st day (diamonds), the 2nd day of heat exposure (squares), and the 3rd day of heat exposure (triangles). Each point illustrates the mean of 10 subjects ± the respective standard deviations.
Figure 6.
Illustrated here is the thermal heat transfer (in calories) in the younger group of subjects measured at rest and at 30, 60, 90, 120, 180 seconds after application of heat and at 5, 7.5, 10, 15, and 20 minutes after application of heat and well as at 30 sec, 60 sec, 2 min, 3 min and 5 min post removal of the heat. Data is shown for the 1st day (diamonds), the 2nd day of heat exposure (squares), and the 3rd day of heat exposure (triangles). Each point illustrates the mean of 10 subjects ± the respective standard deviations.
Repeat heat response in older subjects
The effect of repeat heat exposure on blood flow, skin temperature, and calories consumed by the skin is shown in Figures 7–9 for the older groups of subjects. As shown in these figures, there was no significant difference in the blood flow response between day 1, 2, and 3 (Figure 7), the skin temperature (Figure 8), or in the calories consumed by the skin to warm the temperature (Figure 9) (p>0.05).
Figure 7.
Illustrated here is the blood flow (in flux) in the older group of subjects measured at rest and at 30, 60, 90, 120, 180 seconds after application of heat and at 5, 7.5, 10, 15, and 20 minutes after application of heat and well as at 30 sec, 60 sec, 2 min, 3 min and 5 min post removal of the heat. Data is shown for the 1st day (diamonds), the 2nd day of heat exposure (squares), and the 3rd day of heat exposure (triangles). Each point illustrates the mean of 10 subjects ± the respective standard deviations.
Figure 9.
Illustrated here is the thermal heat transfer (in calories) in the older group of subjects measured at rest and at 30, 60, 90, 120, 180 seconds after application of heat and at 5, 7.5, 10, 15, and 20 minutes after application of heat and well as at 30 sec, 60 sec, 2 min, 3 min and 5 min post removal of the heat. Data is shown for the 1st day (diamonds), the 2nd day of heat exposure (squares), and the 3rd day of heat exposure (triangles). Each point illustrates the mean of 10 subjects ± the respective standard deviations.
Figure 8.
Illustrated here is the skin temperature (in °C) in the older group of subjects measured at rest and at 30, 60, 90, 120, 180 seconds after application of heat and at 5, 7.5, 10, 15, and 20 minutes after application of heat and well as at 30 sec, 60 sec, 2 min, 3 min and 5 min post removal of the heat. Data is shown for the 1st day (diamonds), the 2nd day of heat exposure (squares), and the 3rd day of heat exposure (triangles). Each point illustrates the mean of 10 subjects ± the respective standard deviations.
Discussion
Numerous studies have been conducted examining the whole body response to global heat and how the body acclimatizes depending to environmental temperatures and exercise [31]. Studies on the response to local heat generally examine a single heat exposure and the response to sustained heat. In one recent study, twelve healthy young subjects were studied to assess the effect of repeated heat at a maximum temperature of 41°C [32]. The duration of the heat was 30 minutes. The results showed desensitization in the nitric oxide mediated vasodilatation at 2 hours but not 4 hours after local heat exposure. This nitric oxide desensitization has been shown in other tissues [33,34].
In the present investigation, this study was expanded in several ways. First, both younger and older subjects were examined. Second, a warmer temperature was used which elicits a duel response to heat mediated at first by neuropeptides and later by nitric oxide. In addition, the calories consumed by skin, blood flow and skin temperature were examined for 3 successive days of repeated heat exposure.
For younger individuals, there was an adaptation to repeated use of local heat from the 1st to the 2nd day. On the 1st day, there was a rapid increase in blood flow which was diminished on the 2nd and 3rd day. However, by 20 minutes exposure to heat, the blood flow response was the same each day. The greater number of calories consumed in the first few minutes of heat exposure in the 1st day might well be accounted for in terms of the higher blood flow response seen in the skin. When Pennes first modeled the response of the thermal transfer characteristics of the skin to heat exposure [22], he emphasized the major role of circulation in carrying heat from the skin. Certainly, the fact that circulation rose much quicker and to a greater extent in the 1st compared to the 2nd and 3rd day would account for the greater number of calories necessary to increase skin temperature. The high blood flows during the 1st day would remove heat from the thermode and make it more difficult to raise skin temperature.
The mechanism for this faster increase in skin blood flow in the initial response but not sustained response to heat response in the 1st day compared to the other 2 days may be linked to the fact that the skin blood flow increase to local heat is a two part mechanism. During the first 1–3 minutes, the increase in circulation following local warm heat exposure to a 44°C heat source, is mediated by TRPV1 voltage gated vanilloid receptors on tactile neurons [11]. These voltage gated calcium channels are not on endothelial cells but on sensory neurons. When they increase calcium flux in to the sensory neurons in response to warm skin temperature, they release the vasodilators substance P and calcitonin gene related peptide other vasoactive agents [11]. The initial response to heat is believed to be related to a change in membrane potential across these receptors [11]. These receptors adapt to heat and their membrane potential, due to potassium efflux from the neuron, subsides after a few minutes [11]. During these first few minutes, the neuropeptides diffuse to the blood vessels and mediate vasodilatation.
After this initial response, the increase in circulation is mediated by another type of voltage gated calcium channel, the TRPV4 channels. This channel is in the vascular endothelial cell and is also temperature sensitive. Its response is slower than the sensory TRPV1 channels and, in endothelial cells, the increase in cellular calcium, activates endothelial nitric oxide synthetase, allows for vasodilator release[1]. In younger individuals, 2 vasodilators are released due to a temperature increase, prostacyclin and nitric oxide [1,3]. Thus, looking at the data in Figures 1–3, it would appear that the enhanced response from the 1st day is most likely mediated by the TRPV1 channels. An enhanced response in these channels would account for the greater blood flow in the 1st day in the first minute after heat was applied whereas the fact that blood flows were similar in day 1, 2, and 3 toward the end of the heat exposure would seem to indicate that the other vasodilator pathway mediated by TRPV4 vanilloid channels remains unaffected by daily heat exposure. Thus, some type of adaptive mechanism is probably found in the TRPV1 channels that alters the response after the 1st day of heat exposure. A recent study has provided evidence that oxidized linoleic acid metabolites increase the temperature sensitivity of TRPV1 channels [35]. Perhaps this pathway adapts to repeated exposure with impaired linoleic acid production due to free radical oxidation and reduces thermal sensitivity. The results then are in agreement with the study by Ciplak et al. [32]. The sustained response (nitric oxide mediated) was not altered with 24 hours between exposure to heat as found in this study after 4 hours of repeated heat exposure.
However, in older individuals, the blood flow response was slower and of less amplitude than seen in the younger individuals for all 3 days. Although there was no statistical difference in the skin temperature response to heating in older individuals in days 1, 2, and 3, the blood flow response was of less amplitude and sluggish compared to the younger individuals. This would seem to indicate that part of the ageing impairment in increasing skin circulation to heat would be a defect in the TRPV1 channels since the initial response to heat was slower in older vs. younger people and unaltered by repeated daily heat exposure. There is evidence that sensory neurons loose sensitivity with the ageing process [36]. TRPV1 loss of sensory function to thermal stimuli with age may be related to age related loss in the ability to oxidize linoleic acid in other parts of the nervous system [37–39]. Since the thermo-sensitive properties of TRPV1 channels have been linked to oxidation of linoleic acid [35], a loss of this function with age would disrupt thermal sensitivity.
For the sustained heat response of the skin circulation, there has been ample documentation that the TRPV4 temperature channels on vascular endothelial cells and the nitric oxide pathway in particular show senescence with ageing [40]. Prostacyclin, a major dilator in younger individuals is largely replaced by nitric oxide for dilation to local heat in older individuals. This may explain the lower absolute amplitude in circulation in response to heat after 15 or 20 minutes as shown in the older compared to the younger individuals. However, the sluggishness with which the circulation increases, would certainly show an ageing effect on the TRPV1 channels which has not been reported as of yet. The low circulation and the similarity of the circulation of days 1, 2, and 3 would therefore, using the Pennes model, explain why the number of calories transferred to the skin is the same after day 1, 2, and 3 for older individuals. However, the smaller increase in circulation and absolute increase in circulation throughout the heat exposure would also then explain why for younger individuals; many more calories were transferred into the skin since the thermal coefficient of the skin with a lower circulation would allow the skin to heat up with fewer calories than for younger individuals.
Thus, it is of no surprise then, that older individuals are much more susceptible to thermal injury given the fact that the circulatory response is sluggish in terms of its ability to protect the skin from a caloric heat load. Sustained temperatures even at a moderately low level (e.g. 42–45°C) would heat the heat skin up very readily. In the present experiment, a thermode was used where the temperature was clamped by the water in the water jacket of the thermode. In a real life situation, this would not be the case. With a higher temperature applied to the skin such as a warm heat pack, where temperature is substantially higher 43–44°C, skin temperature would climb rapidly with very few calories and lead to burns much more readily in younger individuals. Questions unanswered are the duration of this adaptive response; would 2 days between exposures return the skin response to normal. Is the response greater if heat is applied 4 hours apart? These must be answered in future studies.
Conclusions
The rate of rise in skin blood flow immediately after the application of local heat is reduced with ageing.
Skin blood flow during a 20 minute heat exposure is reduced with ageing.
In younger individuals, there is an adaptive mechanism in the skin blood flow response to repeated heat exposure not seen in older individuals; the initial rapid response to heat is lost when heat is applied 24 hours apart.
Figure 2.
Illustrated here is the skin temperature (in °C) measured at rest and at 30, 60, 90, 120, 180 seconds after exposure to heat and at 5, 7.5, 10, 15, and 20 minutes after exposure to heat and for 5 minutes after the heat was removed with measurements at 30 sec, 60 sec, 2 min, 3 min and 5 min as the average of 10 younger (diamonds) and 10 older (squares). Each point is the mean ± the respective standard deviation.
Figure 5.
Illustrated here is the skin temperature (in °C) in the younger group of subjects measured at rest and at 30, 60, 90, 120, 180 seconds after application of heat and at 5, 7.5, 10, 15, and 20 minutes after application of heat and well as at 30 sec, 60 sec, 2 min, 3 min and 5 min post removal of the heat. Data is shown for the 1st day (diamonds), the 2nd day of heat exposure (squares), and the 3rd day of heat exposure (triangles). Each point illustrates the mean of 10 subjects ± the respective standard deviations.
Footnotes
Source of support: Departmental sources
References
- 1.Kellogg DL, Jr, et al. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol. 1999;86(4):1185–90. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- 2.Kellogg DL, Jr, Zhao JL, Wu Y. Roles of nitric oxide synthase isoforms in cutaneous vasodilation induced by local warming of the skin and whole body heat stress in humans. J Appl Physiol. 2009;107(5):1438–44. doi: 10.1152/japplphysiol.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91(4):1619–26. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 4.Namer B, et al. Chemically and electrically induced sweating and flare reaction. Auton Neurosci. 2004;114(1–2):72–82. doi: 10.1016/j.autneu.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Petrofsky JS, Al-Malty A-M, Prowse M. Relationship between multiple stimuli and skin blood flow. Med Sci Monit. 2008;14(8):CR399–405. [PubMed] [Google Scholar]
- 6.Sauerstein K, et al. Electrically evoked neuropeptide release and neurogenic inflammation differ between rat and human skin. J Physiol. 2000;529(Pt 3):803–10. doi: 10.1111/j.1469-7793.2000.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54(1):75–159. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- 8.Rowell LB. Reflex control of the cutaneous vasculature. J Invest Dermatol. 1977;69(1):154–66. doi: 10.1111/1523-1747.ep12497938. [DOI] [PubMed] [Google Scholar]
- 9.Almalty AM, et al. An effective method for skin blood flow measurement using local heat combined with electrical stimulation. J Med Eng Technol. 2009;33(8):663–69. doi: 10.3109/03091900903271646. [DOI] [PubMed] [Google Scholar]
- 10.Petrofsky JS, et al. The effect of the moisture content of a local heat source on the blood flow response of the skin. Arch Dermatol Res. 2009;301(8):581–85. doi: 10.1007/s00403-009-0957-3. [DOI] [PubMed] [Google Scholar]
- 11.Malmberg AB, Bley KR, editors. Turning up the heat on pain: TRPV1 receptors in pain and inflammation. Vol. 2. Springer Science; 2005. p. 49. [Google Scholar]
- 12.Minson CT, et al. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol. 2002;93(5):1644–49. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- 13.Evans E, et al. Thermally-induced cutaneous vasodilatation in aging. J Gerontol. 1993;48(2):M53–57. doi: 10.1093/geronj/48.2.m53. [DOI] [PubMed] [Google Scholar]
- 14.Holowatz LA, et al. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol. 2003;284(5):H1662–67. doi: 10.1152/ajpheart.00871.2002. [DOI] [PubMed] [Google Scholar]
- 15.Martin HL, Loomis JL, Kenney WL. Maximal skin vascular conductance in subjects aged 5–85 yr. J Appl Physiol. 1995;79(1):297–301. doi: 10.1152/jappl.1995.79.1.297. [DOI] [PubMed] [Google Scholar]
- 16.Petrofsky JS, et al. The influence of ageing on the ability of the skin to dissipate heat. Med Sci Monit. 2009;15(6):CR261–68. [PubMed] [Google Scholar]
- 17.Richardson D. Effects of age on cutaneous circulatory response to direct heat on the forearm. J Gerontol. 1989;44(6):M189–94. doi: 10.1093/geronj/44.6.m189. [DOI] [PubMed] [Google Scholar]
- 18.Rooke GA, Savage MV, Brengelmann GL. Maximal skin blood flow is decreased in elderly men. J Appl Physiol. 1994;77(1):11–14. doi: 10.1152/jappl.1994.77.1.11. [DOI] [PubMed] [Google Scholar]
- 19.Weiss M, et al. Analysis of the diminished skin perfusion in elderly people by laser Doppler flowmetry. Age Ageing. 1992;21(4):237–41. doi: 10.1093/ageing/21.4.237. [DOI] [PubMed] [Google Scholar]
- 20.Schellen L, et al. Differences between young adults and elderly in thermal comfort, productivity, and thermal physiology in response to a moderate temperature drift and a steady-state condition. Indoor Air. 2010;20(4):273–83. doi: 10.1111/j.1600-0668.2010.00657.x. [DOI] [PubMed] [Google Scholar]
- 21.Taylor NA, Allsopp NK, Parkes DG. Preferred room temperature of young vs aged males: the influence of thermal sensation, thermal comfort, and affect. J Gerontol A Biol Sci Med Sci. 1995;50(4):M216–21. doi: 10.1093/gerona/50a.4.m216. [DOI] [PubMed] [Google Scholar]
- 22.Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol. 1948;1(2):93–122. doi: 10.1152/jappl.1948.1.2.93. [DOI] [PubMed] [Google Scholar]
- 23.Petrofsky J, et al. Does skin moisture influence the blood flow response to local heat? A re-evaluation of the Pennes model. Journal of Medical Engineering & Technology. 2009;33(7):532–37. doi: 10.1080/03091900902952683. [DOI] [PubMed] [Google Scholar]
- 24.Brink H, Werner J. Efficiency function: improvement of classical bio-heat approach. J Appl Physiol. 1994;77(4):1617–22. doi: 10.1152/jappl.1994.77.4.1617. [DOI] [PubMed] [Google Scholar]
- 25.Petrofsky JS, McLellan K. Galvanic skin resistance – a marker for endothelial damage in diabetes. Diabetes Technol Ther. 2009;11(7):461–67. doi: 10.1089/dia.2008.0096. [DOI] [PubMed] [Google Scholar]
- 26.Farage M, Miller K, Maibach H, editors. Text Book of Ageing Skin. Springer; Berlin Heidelberg: 2010. Influence of Race, Gender, Age and Diabetes on the Skin Circluation. [Google Scholar]
- 27.Petrofsky JS, Laymon M. Heat transfer to deep tissue: the effect of body fat and heating modality. J Med Eng Technol. 2009;33(5):337–48. doi: 10.1080/03091900802069547. [DOI] [PubMed] [Google Scholar]
- 28.Parker BA, et al. Evidence for reduced sympatholysis in leg resistance vasculature of healthy older women. Am J Physiol Heart Circ Physiol. 2007;292(2):H1148–56. doi: 10.1152/ajpheart.00729.2006. [DOI] [PubMed] [Google Scholar]
- 29.Koch DW, Newcomer SC, Proctor DN. Blood flow to exercising limbs varies with age, gender, and training status. Can J Appl Physiol. 2005;30(5):554–75. doi: 10.1139/h05-141. [DOI] [PubMed] [Google Scholar]
- 30.Holowatz LA, Thompson-Torgerson CS, Kenney WL. Altered mechanisms of vasodilation in aged human skin. Exerc Sport Sci Rev. 2007;35(3):119–25. doi: 10.1097/jes.0b013e3180a02f85. [DOI] [PubMed] [Google Scholar]
- 31.Mundel T. Exercise heat stress and metabolism. Med Sport Sci. 2008;53:121–29. doi: 10.1159/000151554. [DOI] [PubMed] [Google Scholar]
- 32.Ciplak M, et al. The vasodilatory response of skin microcirculation to local heating is subject to desensitization. Microcirculation. 2009;16(3):265–75. doi: 10.1080/10739680802595880. [DOI] [PubMed] [Google Scholar]
- 33.Halvey EJ, et al. Mechanisms of activity-dependent plasticity in cellular nitric oxide-cGMP signaling. J Biol Chem. 2009;284(38):25630–41. doi: 10.1074/jbc.M109.030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ring A, et al. Exogenous Nitric Oxide Donation Causes Desensitization of Arteriolar Relaxing Activity In Vivo: An Intravital Analysis in Mice. J Surg Res. 2010;164(1):169–74. doi: 10.1016/j.jss.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 35.Patwardhan AM, et al. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J Clin Invest. 2010;120(5):1617–26. doi: 10.1172/JCI41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang S, Albers KM. Behavioral and cellular level changes in the aging somatosensory system. Ann NY Acad Sci. 2009;1170:745–49. doi: 10.1111/j.1749-6632.2009.04011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bourre JM. Roles of unsaturated fatty acids (especially omega-3 fatty acids) in the brain at various ages and during ageing. J Nutr Health Aging. 2004;8(3):163–74. [PubMed] [Google Scholar]
- 38.Freemantle E, et al. Omega-3 fatty acids, energy substrates, and brain function during aging. Prostaglandins Leukot Essent Fatty Acids. 2006;75(3):213–20. doi: 10.1016/j.plefa.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 39.McKay DL, et al. Chronic and acute effects of walnuts on antioxidant capacity and nutritional status in humans: a randomized, cross-over pilot study. Nutr J. 2010;9:21. doi: 10.1186/1475-2891-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rush JW. Exercising an option to prevent age related decline of vascular BH4 and uncoupling of eNOS. J Physiol. 2009;587(Pt 15):3755. doi: 10.1113/jphysiol.2009.177261. [DOI] [PMC free article] [PubMed] [Google Scholar]