Summary
Background
Chronic alcohol intake exerts myocardial damage en route to the development of alcoholic cardiomyopathy (ACM), although the precise pathogenesis of ACM is unknown. Carnitine is known to participate in the regulation of metabolism in a number of heart diseases. This study was designed to examine the interplay between myocardial metabolism and carnitine in the development of ACM.
Material/Methods
Experimental animals were divided into 3 groups: (i) group A: alcohol-fed. (ii) group B: alcohol/carnitine: (200mg/kg/d, p.o. by mixing carnitine in rat chow). (iii) group C: control. Blood levels of free fatty acid (FFA), total carnitine (TC) and free carnitine (FC) were monitored in rats receiving alcohol with or without carnitine. Mitochondrial adenine nucleotide translocator-1 (ANT1) activity, ATPase activity, high energy phosphate concentration, peroxisome proliferator-activated receptor-α (PPARα), carnitine-palmitoyl transferase I (CPT-I), medium-chain acyl-coenzyme A dehydrogenase (MCAD), ANT1 and ATPase mRNA and protein expression were also monitored in myocardial tissue.
Results
Experimental animals received alcohol with or without carnitine for six6 months. Our results indicated that FFA increased abruptly. TC and FC were significantly decreased in groups receiving alcohol at 4 months. The concentration of ATP, ADP and AMP in the myocardium decreased following 2 months of alcohol administration. mRNA and protein expression of PPARα, CPT-I, MCAD, ANT1 and ATPase expressions were gradually altered in groups following alcohol feeding.
Conclusions
These observations suggest that abnormal metabolism is present in the myocardium during the development of ACM. Carnitine may improve myocardial metabolism by elevating the content of PPARα, CPT-I and MCAD.
Keywords: carnitine, alcoholic cardiomyopathy, metabolism, structural remodeling, mechanism
Background
Chronic alcohol consumption usually leads to the onset and progression of a nonischemic, dilated cardiomyopathy known as alcoholic cardiomyopathy (ACM) [1]. ACM is mainly manifested as ventricular dysfunction, which may ultimately result in congestive heart failure and arrhythmia. The occurrence of ACM is associated with a prolonged high daily intake of alcohol. Nonetheless, the prevalence of ACM is variable and fortunately does not appear in all heavy drinkers. ACM represents about 3.8% of all cardiomyopathy cases [2]. Similar to other forms of dilated cardiomyopathies, ACM is characterized by an increase in left ventricular mass, dilation of ventricles, wall thinning and ventricular dysfunction. More importantly, these functional and geometric changes may develop in the absence of coronary artery disease and/or nutritional deficiencies [3]. To the best of our knowledge, little information is available with regards to specific pharmacotherapeutics in patients with ACM. Based on current guidelines from the Agency for Health Care Policy and Research and Heart Failure Consensus [4,5], patients with ACM should be treated if they present heart failure with systolic dysfunction (EF 40%).
To-date, the precise nature of the pathogenesis of ACM remains obscure, although altered cell energy metabolism has been speculated to play a role [6]. Carnitine, an essential factor that transports long-chain fatty acids into mitochondrial matrix for β-oxidation, plays an important role in the cellular energy metabolism [7]. Carnitine is a quaternary ammonium compound biosynthesized from the amino acids lysine and methionine [8]. In living cells, carnitine is required for the transport of fatty acids from cytosol into mitochondria during conversion of lipids (or fats) into metabolic energy. Moreover, carnitine is essential to the sustainability of human life through tightly regulated metabolic pathways [9]. Carnitine transports long-chain acyl groups from fatty acids into the mitochondrial matrix where they are broken down through β-oxidation to acetate to obtain usable energy via the citric acid cycle. Towards the end of the 20th century, much research was directed towards investigating the effects of L-carnitine supplementation on exercise performance, the main premise being that increasing carnitine availability would increase fat oxidation during prolonged exercise, spare glycogen stores and, thus, delay the onset of fatigue [10]. Carnitine plays an important role in cellular energy metabolism. The heart muscle uses fat for energy by carnitine. It is also known that carnitine is important for sustaining human life; people who suffer a heart attack appear to have a reduced risk of dying if they supplement with carnitine, as a consequence of tightly regulated metabolic pathways, and alleviate heart failure [7,11]. However, whether carnitine-mediated cell energy metabolism participates in the development of ACM remains elusive. The objective of this study was to investigate whether carnitine affects abnormal metabolism and structural remodeling in ACM. We examined the process of myocardial metabolism in a rat model of ACM and our findings provide the first evidence for a role of carnitine in the development of ACM.
Material and Methods
Animal models of ACM
All animal procedures have been approved by our institutional animal care and use committee. In brief, adult male Wistar rats weighing 300±50 g were purchased from the laboratory animal center of the First Affiliated Hospital of Harbin Medical University. Ninety rats were randomly assigned into the following 3 groups: (i) group A: alcohol-fed (n=30; 10% alcohol ad libitum in drinking water and 60% alcohol, 5ml/kg daily by intragastric administration in the first week; 20% alcohol ad libitum in drinking water and 60% alcohol, 10ml/kg twice per day by intragastric administration during the second week; and 30% alcohol ad libitum in drinking water and 60% alcohol, 15ml/kg by intragastric administration 3 times per day for 2 months). (ii) group B: alcohol/carnitine: (n=30; animals received alcohol plus carnitine 200mg/kg/d, p.o. by mixing carnitine in rat chow). (iii) group C: control (n=30; animals received water intragastrically in lieu of alcohol). All animals were maintained under similar environments for 2, 4 and 6 months. To ensure similarities of physical activity and nutritional intake between the alcohol-treated control animals, all animals were weighed twice weekly. Venous blood samples were collected at the onset of the study and at intervals of 1 month thereafter for nutritional status assessment. Random blood samples were obtained from the experimental animals at various times of the day to estimate the average level of alcohol in the blood.
Measurement of LV functional performance
Studies Before Alcohol. Before initiation of alcohol administration in all animals, baseline steady state, left ventricular dimension and left ventricular function data were acquired, and blood was collected for biochemical assay from an LA catheter at rest.
Studies After Alcohol. The protocol as outlined above was repeated early morning the next day, 1 to 2 hours before alcohol intake, twice per week after alcohol initiation, and throughout the development of alcoholism over 6 months. LV end-diastolic dimension (LVEDD), LV end-systolic dimension (LVESD), ejection fraction (EF), fractional shortening (FS) and interventricular septal thickness in diastole (IVSD) were analyzed.
Histopathological and electron microscopic examination
Rats were scarified following 6 months of alcohol intake. The hearts were removed, rinsed in saline and fixed in 4% paraformaldehyde prior to storage at 4°C. Fixed heart tissues were embedded in paraffin, sliced perpendicular to the long axis and stained with haematoxylin-eosin. The extent of inflammatory lesions in the myocardium was visualized using a light microscope (OLYMPUS BX 60). To assess the alcohol-induced ultrastructural changes through conventional electron microscopy, hearts were fixed in situ with Karnovsky fixative (2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate buffer, pH 7.4). Ultra thin sections (50~100 nm) were stained by uranyl acetate and lead citrate before examination with electron microscopy (JEM21200EX).
Measurement of free fatty acid and carnitine concentrations
Levels of free fatty acid (FFA), total carnitine (TC) and free carnitine (FC) were determined in the blood. Serum FFA concentration was determined using the modified absorbance spectrum method of Nixon [12]. The concentration of TC and FC was measured as described [13].
Investigation of mitochondrial ANT1 activity and ATPase activity
Myocardial mitochondria were isolated from the left ventricles including septum using a previously described method [14]. Mitochondrial adenine nucleotide translocator-1 (ANT1) activity was detected by the atractyloside (ATR)-inhibitor stop assay [15–17]. Mitochondrial ATPase activity was assayed according to the published method of Monk and colleagues [18].
High energy phosphate concentration in myocardial tissue
For measurement of high-energy phosphates, myocardial tissue was rapidly frozen in liquid nitrogen between 2 pre-cooled aluminum blocks following 2, 4 and 6 months of alcohol intake. ATP was measured using the reaction of bioluminescence with purified firefly luciferase, which has the advantage of producing an almost constant light emission proportional to the ATP concentration. ADP and AMP were measured after enzymatic conversion to ATP. Phosphocreatine (Pcr) was measured using a bioluminescent technique modified according to Ellis and Gardner [16,17].
Expression of Peroxisome Proliferator-Activated Receptor-α (PPARα), Carnitine-Palmitoyl Transferase I (CPT-I), Medium-Chain Acyl-Coenzyme A Dehydrogenase (MCAD), Adenine Nucleotide Translocator (ANT1) and ATPase Protein in Myocardial Tissue
Proteins were separated on 5–8% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk for 2h at room temperature. The membranes were incubated overnight at 4°C with anti-PPARα, CPT-I, MCAD, ANT1 and ATPase antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at a 1:1000 dilutions and then incubated with peroxidase-conjugated goat anti-rabbit IgG for 1 h. Proteins were detected by enhanced chemiluminescence (ECL). Bands were visualized by autoradiography and quantified using commercially available software. Results were normalized to the optical density of a standard sample. Experiments were repeated in triplicate for all conditions.
PPARα, CPT-I, MCAD, ANT1 and ATPase mRNA Expression in Myocardial Tissue
Total RNA was extracted from the myocardial tissue using TRIZOL reagent according to the manufacturer’s protocol. The primers for PPARα, CPT-I, MCAD, ANT1 and ATPase (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) were chosen to bind to separated exons to avoid genomic DNA amplification. Forward and reverse primer sequence for each gene and its corresponding amplicon size are provided in Table 1.
Table 1.
Dogs LVW/BW (mg/g).
| Group | Month | Weight (g) | LVW (mg) | LVW/BW(mg/g) |
|---|---|---|---|---|
| A | Baseline | 320±17 | 824±27 | 2.58±0.11 |
| 6 | 320±19 | 856±25 | 2.68±0.15 | |
| B | Baseline | 314±15 | 816±21 | 2.5±0.09 |
| 6 | 338±18 | 847±14 | 2.51±0.08 | |
| C | Baseline | 315±17 | 819±17 | 2.44±0.13 |
| 6 | 321±20 | 837±13 | 2.4±0.12 |
LV – left ventricle; LVW – left ventricular weight; BW – body weight; ARB – angiotensin receptor blocker. Group A – alcohol-feeding; group B – alcohol/carnitine.
Quantitative RT-PCR was carried out on a Light Cycler II instrument (Roche Diagnostics) and a standard Light Cycler amplification cycle protocol was established for each gene. The specificity of RT-PCR was verified by checking that the PCR products were of the expected size by gel electrophoresis.
Statistical analysis
All values are presented as mean ±SD. Statistical analyses were performed using SPSS software (version 15.0). Summary statistics were compared by paired or unpaired Student’s t-test where appropriate, or a Wilcoxon signed-rank test in case of non-normal distributions. A P value less than 0.05 was considered to be statistically significant.
Results
Rats’ left ventricular weight/body weight
Body weights (BW) of rats were measured at baseline and after 6 months of alcohol feeding.. Body weights of rats from group A slowly increased between baseline and 6 months, although the LV weight increased quickly. The left ventricular weight (LVW)/BW ratios at baseline and 6 months were 2.44±0.13 and 2.4±0.12 mg/g in group C, 2.58±0.11 and 2.68±0.15 mg/g in group A (p<0.01 vs. group C), and 2.50±0.09 and 2.51±0.08 mg/g in group B (Table 1).
LV Function with chronic alcohol consumption: Effects of carnitine
LV function and hemodynamic alterations measured for the 3 groups of animals are summarized in Table 2. Compared with controls, 6 months after alcohol intake, LV end-diastolic dimension and LV end-systolic dimension (alcohol-fed 40.32±1.29 mm and 31.75±2.23 mm versus control 30.55±3.93 mm and 21.16±3.34 mm) were significantly increased, accompanied by reduced LV ejection fraction and fractional shortening (EF 29.57±1.50% vs 62.75±5.94%, FS 19.35±3.03% vs 30.85±2.63%). In contrast, LV function and general hemodynamics were similar between the control and alcohol/carnitine groups. Concomitant carnitine with alcohol prevented alcohol-induced decreased LV contractility (Table 2).
Table 2.
LV Function and general hemodynamic variables with chronic alcohol consumption: Effects of cartine.
| Alcohol (n=30) | Alcohol/carnitine (n=30) | Control (n=30) | ||||
|---|---|---|---|---|---|---|
| Baseline A1 | 6 months A2 | Baseline B1 | 6 months B2 | Baseline C1 | 6 months C2 | |
| Hear rate, bpm | 106±6 | 110±6 | 109±8 | 106±4 | 106±4 | 109±7 |
| LV end-diastolic dimesion, mm | 31.15±1.1 | 40.32±1.29*,** | 31.0±1.9 | 34.96±1.76 | 30.1±1.86 | 30.55±1.93 |
| LV end-systolic dimension, mm | 21.36±1.21 | 31.75±2.23 | 21.63±2.17 | 23.18±1.12 | 21.20±2.36 | 21.16±3.34 |
| LV end-diastolic volume, mL | 45.1±7.4 | 50.6±6.4*,** | 44.5±8.0 | 45.4±9.1 | 43.8±6.3 | 44.2±7.2 |
| LV end-sstolic volume, mL | 29.5±8.8 | 42.2±7.0*,** | 28.9±7.6 | 30.2±11.9 | 28.2±6.1 | 28.2±6.3 |
| Ejection fraction | 64.3±1.78 | 29.57±1.5*,** | 63.7±1.72 | 57.71±4.78 | 62.75±5.94 | 63±1.83 |
| Fractional shortening | 33.27±2.09 | 19.35±3.03*,** | 34.12±1.98 | 29.72±1.65 | 33.46±1.57 | 30.85±2.63 |
| Interventricularseptal thickness | 5.79±0.72 | 7.21±1.07*,** | 5.87±0.67 | 6.06±0.60 | 5.81±0.47 | 5.94±0.47 |
n – indicates number of rats;
P<0.05, A2 vs C1 and A2 vs B2.
P<0.05, A1 vs A2.
H&E and electron microscopy in myocardium
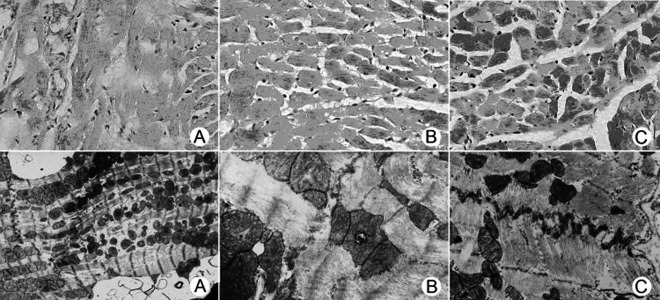
Following 6 months of chronic alcohol intake, myocardial morphology was examined using the H&E staining and electron microscopic techniques. Our data revealed that myocardium from group A developed overt fibrosis and fragmentation as observed by the H&E staining. Electron microscopic examination depicted focal fragmentation, mitochondrial flocculation and appearance of phagolysosomes in myofilament, breakage of gap junctions and disappearance of macula densa in the macula adherens conjunction in tissue specimens from group A. These observations were consistent with a previously established model of ACM (Figure 1).
Figure 1.
Myocardial H&E staining and electron microscopic assessment. H&E staining: (A) Myofilament alignment disorder manifested by scattered fibrosis and fragmentation. (200×); (B) Normal myocardial fiber structure with focal myocardial fiber swelling. (200×); (C) Normal myocardial fibers (200×). Microscopic images: (D) Localized myofilament fragmentation, mitochondrial flocculation and appearance of phagolysosomes in myofilament. Discontinuation of gap junction and disappearance of macula densa in the macula adherens conjunction (10000×); (E) Chromoplasm condensation (lighter in the cellular nucleus) in otherwise normal structured cardiomyocytes. Cellular organelles were abundant (10000×); (F) Ultrastructure of normal cardiac muscle fibers (10000×). Panel A, B and C are from groups D, E and F, respectively.
Measurement of carnitine concentration
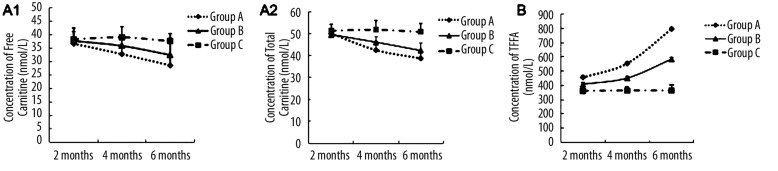
Carnitine concentration in serum was measured following 2, 4 and 6 months of alcohol feeding. As shown in Figure 2A, concentrations of free carnitine (FC) and total carnitine (TC) were significantly decreased in serum from group A during the feeding period (P<0.05 vs. groups B and C at 4 and 6 months). TC and FC concentrations in serum were significantly decreased in group A and B from 4 months. There were substantial differences in TC/FC concentrations among groups A, B and C following 6 months of alcohol administration. FC concentrations in groups A and B were 76.6% and 86.4%, respectively, of that from group C, while the TC concentrations were 74.8% and 81.7%, respectively, when normalized to group C. Response to medical prevention, mean values of TC, and FC concentration from group B were higher than group A.
Figure 2.
Panel A1 and A2: Concentrations of free carnitine and total carnitine in serum measured in each animal group at 2, 4 and 6 months following initiation of alcohol feeding. Levels of carnitine were significantly reduced in sera from group A and B during the feeding period (2–6 months) compared with serum from group C. Values are expressed as means ±SEM, n=10 per group. Panel B: Concentration of free fatty acid in serum measured from each animal group at 2, 4 and 6 months following initiation of alcohol feeding. Concentration of free fatty acid was significantly elevated in serum from group A and B during the feeding period (2–6 months) compared with that of group C. Values are expressed as means ±SEM, n=10 per group.
Measurement of free fatty acid concentration
Concentration of free fatty acid (FFA) in serum of groups A, B and C was measured following 2, 4 and 6 months of alcohol intake. Figure 2B shows that the contents of FFA in serum of group A increased rapidly (457.8±13.1nmol/L, 556.1±12.3nmol/L and 798.2±10.7nmol/L, respectively) and became 2.18-fold higher than that in group C at 6 months of alcohol feeding (P<0.05). Response to medical prevention and the increase of concentration of FFA in group B was not as fast as that of group A. FFA concentrations from serum of groups A and B were also significantly different following 2, 4 and 6 months of alcohol intake. No significant difference was found in FFA concentration between groups B and C after 2 months of alcohol feeding. Therefore, a longer period of alcohol intake was required to attain an effect on FFA concentration in serum.
High energy phosphate concentration in myocardial tissue
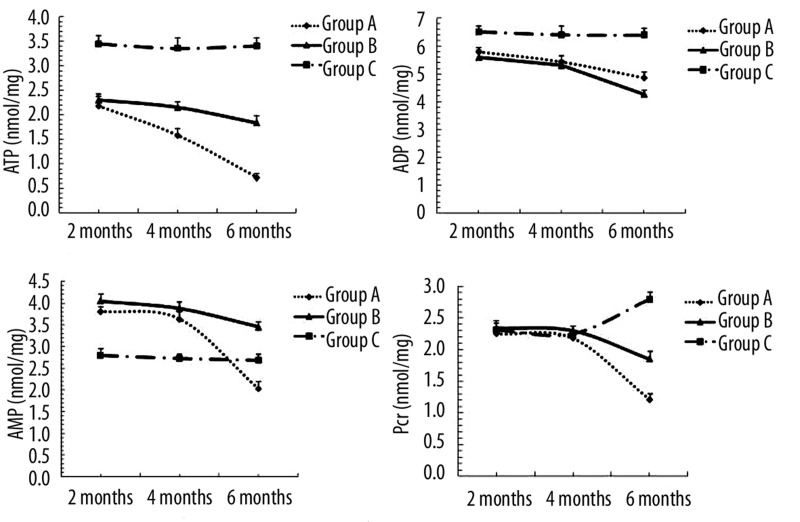
Concentrations of ATP, ADP, AMP and Pcr in myocardial tissue from groups A, B and C were measured at 2, 4 and 6 months (Figure 3). The content of ATP, ADP and AMP in myocardial tissue decreased dramatically in groups A and B compared to group C at 2 months (P<0.05 vs. group C). There was no significant difference in content of Pcr among the 3 groups until 6 months after alcohol feeding (group A/B vs. group C, P<0.05; group A vs. group B, P<0.05). Metabolic disturbance of fatty acid did not elicit any significant effect on the concentration of energy substrates at early stages of alcohol intake.
Figure 3.
Concentrations of ATP, ADP, AMP and Pcr measured at 2, 4 and 6 months in myocardial tissue from groups A, B and C. Values are expressed as means ±SEM, n=10 per group. The content of ATP, ADP and AMP in myocardial tissue at 2 months (P<0.05 or P<0.01 groups A and B vs. group C). The content of Pcr until 6 months (group A/B vs. group C, P<0.01; group A vs. group B, P<0.05).
ATPase activity and mitochondrial ANT1 activity analysis
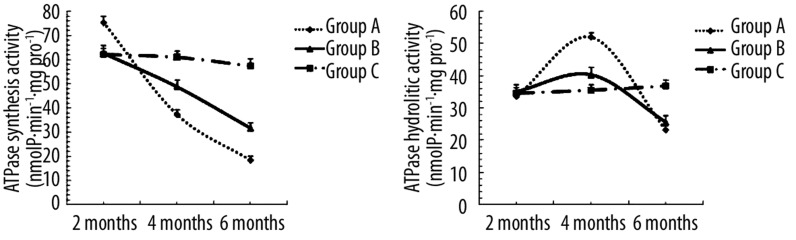
Mitochondrial ATPase activity from myocardial tissue is shown in Figure 4. Following 2 months of alcohol intake, the synthetic activities of ATPase rose steadily in rats from group A. However, the synthetic activity was reduced in groups A and B following 4 months of alcohol feeding, while the hydrolytic activity was increased. At time points of up to 6 months of alcohol feeding, both synthetic and hydrolytic activity of ATPase declined (P<0.05). These data suggest that the synthesis activity of ATPase was facilitated to supply energy at early stages of alcohol challenge.
Figure 4.
Mitochondrial ATPase activity measured in myocardial tissue from groups A, B and C. Values are expressed as means ±SEM, n=10 per group. The synthetic activities of ATPase was raised at 2 months (P<0.05, group A vs. group C). The synthetic activity was reduced following 4 months (P<0.05 or P<0.01, groupA/B vs. group C). The hydrolytic activity was increased at 4months (P<0.05 or P<0.01, groupA/B vs. group C). While up to 6 months, both synthetic and hydrolytic activity of ATPase declined (P<0.05 or P<0.01, groupA/B vs. group C).
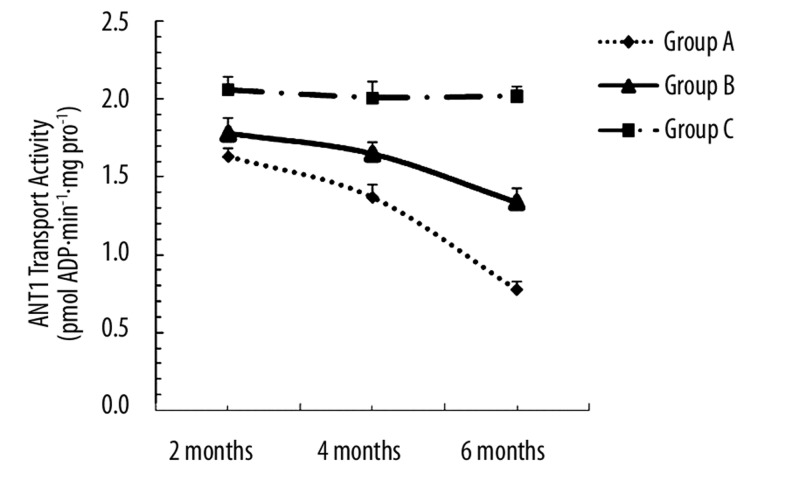
Our data further revealed that alcohol reduced ANT1 transport activity, the effect of which was prevented by carnitine. Analysis of ANT1 transport activity (Figure 5.) shows that the ANT1 transport activity of groups A and B gradually decreased from the second month, and was only 38% and 66% compared with group C by 6 months of alcohol intake (P<0.05).
Figure 5.
Mitochondrial ANT1 transport activity measured in myocardial tissue from groups A, B and C. Values are expressed as means ±SEM, n=10 per group. ANT1 transport activity of groups A and B gradually decreased from the second month and was only 38% and 66% compared with group C by 6 months of alcohol intake (P<0.05 or P<0.01).
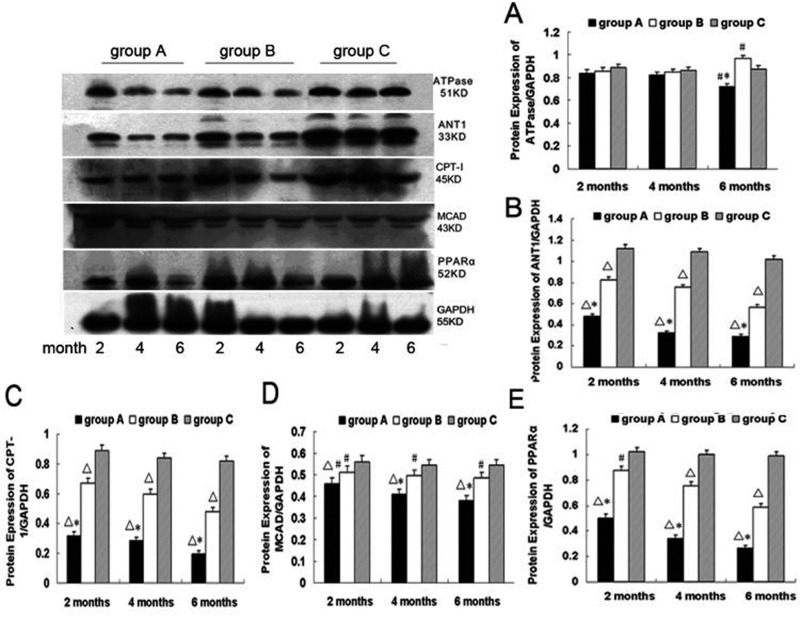
mRNA and protein expression of PPARα, CPT-I, MCAD, ANT1 and ATPase in the myocardium
Figures 6 and 7 depict mRNA and protein expression of PPARα, CPT-I, MCAD, ANT1 and ATPase. No significant difference was found in the mRNA expression of ATPase among all 3 groups at 2 and 4 months. The mRNA expression of ATPase from group A was significantly lower than group C at 6 months (ATPase mRNA/β-actin, 0.51±0.034 vs. 0.59±0.075; P<0.05). The mRNA expression of ATPase was significantly greater in group B than group C at 6 months (ATPase mRNA/β-actin, 0.67±0.036 vs 0.59±0.075; P<0.05). mRNA expression of ANT1, CPT-I, MCAD and PPARα were gradually reduced in both group A and group B compared with group C from 2 months to 6 months of alcohol feeding, respectively (P<0.05 group A/B vs. group C).
Figure 6.
The mRNA level of ATPase, ANT1, CPT-I, MCAD and PPARα in myocardium from groups A, B and C at 2, 4 and 6 months. Values are expressed as means ±SEM, n=10 per group. □P<0.01 vs. group C; #P<0.05 vs. group C; *P<0.05 vs. group B.
Figure 7.
ATPase, ANT1, CPT-I, MCAD and PPARα protein expression in myocardial tissue from group A, B and C at 2, 4 and 6 months. Values are expressed as means ±SEM, n=10 per group. □P<0.01 vs. group C; #P<0.05 vs. group C; *P<0.01 vs. group B.
No significant difference was found in the protein expression of ATPase among the 3 groups following 2 and 4 months of alcohol intake. The protein expression of ATPase from group A was significantly lower than group C at 6 months (0.72±0.033 vs. 0.86±0.031; P<0.05). The protein expression of ATPase was significantly greater in group B than group C at 6 months (0.97±0.025 vs 0.86±0.031; P<0.05). Protein expression of ANT1, CPT-I, MCAD and PPARα gradually declined in group A and group B compared with group C from 2 months to 6 months of alcohol feeding, respectively (P<0.05 group A/B vs. group C).
Discussion
Alcohol intoxication is known to trigger myocardial dysfunction, and aberrant metabolism and function [19,20]. In normal metabolic process of myocardial cells, energy is generated from fatty acids by oxidative degradation through β-oxidation. Fatty acids are transported across the outer mitochondrial membrane by CPT-I and then couriered across the inner mitochondrial membrane by carnitine [8,21]. Alcohol may promote carnitine escape from myocardial cells either directly or indirectly, leading to fatty acid metabolic disorder. In consequence, fatty acid accumulates in mitochondria to accelerate reversible myocardial damage.
In our studies, previous investigations with dogs administered alcohol for 6 months or longer demonstrated a clear impairment of LV relaxation and diastolic filling and an abnormal response to angiotensin infusion, which is consistent with our findings. However, these earlier investigations failed to show chronic alcohol-induced depression in LV contractility, LV dilatation, or clinical congestive heart failure. We found that chronic alcohol ingestion produces direct inhibition of LV contractility and causes progressive LV systolic and diastolic functional impairment and LV structural remodeling. These findings are similar to the clinical syndrome of ACM. The chronic alcohol-induced LV dysfunction and cardiac failure were prevented by administration of carnitine during ingestion of alcohol.
Moreover, we evaluated concentration of carnitine and free fatty acid in serum. Concentrations of carnitine decreased in groups A and B following 4 months of alcohol intake, while the concentration of free fatty acid rose from 2 months of feeding and beyond. In addition, the serum concentrations of free fatty acid in groups A and B following 6 months of alcohol intake were 1.75- and 1.43-fold, respectively, after 2 months of alcohol feeding. The content of ATP, ADP and AMP in myocardial tissue displayed a robust decrease in groups A and B compared to group C following 2 months of alcohol intake. There was little difference in PCr content among the 3 groups after up to 6 months of alcohol feeding. These data indicated the possible existence of a compensatory mechanism in the development of ACM. It is plausible to speculate that metabolic disturbance elicited by alcohol intake could not alter the concentration of carnitine and energy substrate during early stages of alcohol intake. However, following chronic exposure to alcohol, myocardial tissue cannot compensate for the damage caused by metabolic disorder of fatty acids. In consequence, acyl-carnitine was accumulated in mitochondria in conjunction with reduced free carnitine level. These changes usually result in a slowed velocity of fatty acid β-oxidation and destroyed myocardial/mitochondrial function by fatty acid ethanol ester, a process referred to as metabolic remodeling.
Numerous studies have demonstrated down-regulation of certain fatty acid oxidation regulatory genes in myocardium following remodeling in response to pressure overload, myocardial infarction or rapid pacing [22,23]. Among these genes, PPARα plays an essential role in the regulation of cellular differentiation, development and metabolism (carbohydrate, lipid, and protein) of higher organisms [24–27]. CPT-I and MCAD may contribute to the progression of cardiac hypertrophy and transition into heart failure, independent of any other notable metabolic changes [27]. However, little evidence is available supporting the notion that PPARα contributes to these metabolic changes or cardiac remodeling associated with ACM. In this study, for the first time, we confirmed down-regulated expression of CPT-I and MCAD occurred following alcohol intake, and was associated with altered expression of regulatory enzyme PPARα involved in fatty acid metabolism.
In our present study, we evaluated the PPARα protein levels following 2, 4 and 6 months of alcohol intake. Our results demonstrated a gradual decline in the expression of the metabolic gene PPARα during ACM development. The mRNA and protein expression of PPARα in group A were only 43% and 28%, respectively, of group C following 6 months of alcohol intake. With the carnitine treatment in group B, the alcohol-induced down-regulation of PPARα was partially reversed. Our result further indicate that the expression of CPT-I and MCAD is regulated by concentration of the medium-chain-length fatty acid in blood. Down-regulated expression of CPT-I and MCAD has a negative feedback regulation on expression of PPARα. Long-chain Acyl-CoA is conjugated to carnitine by carnitine acyltransferase I (palmitoyltransferase), located on the outer mitochondrial membrane [28]. Carnitine can prevent Acyl-CoA from accumulating in mitochondria to induce mitochondrial damage. In addition, carnitine may provide a protective effect against lipid peroxidation of phospholipid membranes and oxidative stress at myocardial and endothelial cell levels [29]. Carnitine reserves high-energy phosphates in myocardial tissues and compromised fatty acid metabolism. In consequence, carnitine has an indirect effect on up-regulated expression of PPARα. Nonetheless, further study is waranted to elucidate the direct effect of carnitine on PPARα, and the role of inflammatory cytokines in such processes.
The etiology of high energy phosphate metabolic disturbance in myocardial tissue is unclear. Numerous studies have suggested that ANT1 and ATPase are important proteins in the mitochondrial inner membrane, while ATPase may serve as a key enzyme for link-coupling of oxidation and phosphorylation, which may affect the oxidative phosphorylation status. ANT facilitates the exchange of ADP/ATP by an antiport mechanism and is thus essential for the utilization of ATP produced by oxidative phosphorylation. In the present study, we confirmed that the synthetic activities of ATPase increased following 2 months of alcohol feeding, although the enzymatic activity decreased following 4 months of feeding when the hydrolytic activity was enhanced. By the sixth month, both synthetic and hydrolytic activity of ATPase decreased. Down-regulated expression of ATPase mRNA and protein was noted following 4 months of alcohol feeding, demonstrating a nice correlation between protein expression and the steady-state mRNA level for ATPase in the current experimental setting. This result indicates that regulation of these proteins depends, in part, upon changes in transcriptional rates and/or in stability of transcriptional products. Our results further illustrate that synthetic activity of ATPase is promoted to supply myocardial energy required during early stages of ACM. However, synthesis of ATPase cannot compensate for extra energy consumption following chronic alcohol intake. Down-regulated expression of ATPase mRNA and protein, corresponding to transcription and post-transcriptional regulation, elicits alteration of enzymatic structure and loss of enzyme content [30–32]. These changes in structure and content of enzyme may lead to cellular energy deficiency.
ANT1 is expressed in heart and skeletal muscle, whereas rats express only 2 isoforms of ANT (ANT1 and ANT2). We found that ANT1 transport activity plays a role in the process of oxidized respiration. The decrease of ANT1 transport activity relates to limited ATP synthetic capacity and high energy phosphate kinetic abnormalities, and thus cannot transport ATP out of the mitochondria. Thus, these data support the notion that ANT deficit is linked to the high energy phosphate abnormalities and the limited respiratory capacity observed in ACM hearts. The effect of chronic alcohol consumption on myocardial energy metabolism reveals that alterations in cardiac function are likely accompanied by changes in the levels of the high-energy metabolites, ATP and creatine phosphate. These observations further confirm that energy transport obstacles may be essential to the development of ACM.
The results of our study suggest that carnitine can increases the content of adenyl acid and promotes the activity of ATPase and ANT1 to improve cardiac energy metabolism. Further study is warranted to elucidate the mechanism of carnitine-offered cardiac protection. Alcoholic cardiomyopathy in humans has long been recognized. The direct effect of alcohol on the heart is difficult to determine because of multiple confounding factors such as the role of alterations in the immune system and changes in the metabolism of organ systems. Several studies have shown that a single high dose of alcohol, mimicking binge drinking in humans, produced liver oxidative stress and injury [33–35]. The heart also suffers the same oxidative damage as the liver from a single high dose alcohol administration. This damage would reflect the direct detrimental action of alcohol and/or its metabolites in the heart [36]. Several scenarios have been postulated for the pathogenesis of alcoholic cardiomyopathy, including direct and indirect toxicity from ethanol and its metabolite acetaldehyde on mitochondria and sarcoplasmic reticula, disturbance in intracellular calcium homeostasis [37,38], modifications of lipoprotein and apolipoprotein particles, as well as accumulation of reactive oxygen species and fatty acid ethyl esters [7,9]. Others have suggested that ethanol-induced changes occur in the presence of oxidative stress and ethanol metabolism into more reactive molecules [39]. Although these theories may offer some explanations toward a better understanding of alcohol-induced tissue damage, specific pathogenic molecular mechanisms remain unknown. Further study is warranted to discover the mechanisms of ACM.
Conclusions
Data from the present study demonstrates that carnitine plays an essential role in myocardial metabolism and remodeling. Carnitine may improve myocardial metabolism by elevating the content of PPARα, CPT-I and MCAD. However, the effect of metabolic products of medicine on heart requires further research. In addition, further study is needed to evaluate the impact of metabolism in the liver, adipose tissue and skeletal muscle on myocardial metabolism.
Abbreviations
- ACM
alcoholic cardiomyopathy
- FFA
free fatty acid
- TC
total carnitine
- FC
free carnitine
- ANT1
Mitochondrial adenine nucleotide translocator-1
- PPARα
peroxisome proliferator-activated receptor-α
- CPT-I
carnitine-palmitoyl transferase I
- MCAD
medium-chain acyl-coenzyme A dehydrogenase
- LVEDD
LV end-diastolic dimension
- LVESD
LV end-systolic dimension
- EF
ejection fraction
- FS
fractional shortening
- IVSD
interventricular septal thickness in diastole
Footnotes
Source of support: This work was supported by the Youth Foundation of Heilongjiang Province, China (QC07C89)
References
- 1.Piano MR. Alcoholic cardiomyopathy: incidence, clinical characteristics, and pathophysiology. Chest. 2002;121:1638–50. doi: 10.1378/chest.121.5.1638. [DOI] [PubMed] [Google Scholar]
- 2.Van Camp G. Toxic cardiopathies. Acta Clinica Belgica. 2005;60:293–300. doi: 10.1179/acb.2005.047. [DOI] [PubMed] [Google Scholar]
- 3.De Keulenaer GW, Brutsaert DL. Dilated cardiomyopathies: changing pathophysiological concepts and mechanisms of dysfunction. J Card Surg. 1999;14:64–74. doi: 10.1111/j.1540-8191.1999.tb00953.x. [DOI] [PubMed] [Google Scholar]
- 4.Konstam MA, Dracup K, Baker DW, et al. Heart failure: evaluation and care of patients with left ventricular systolic dysfunction. J Card Fail. 1995:183–87. doi: 10.1016/1071-9164(95)90021-7. [DOI] [PubMed] [Google Scholar]
- 5.Packer M, Cohn JN, Abraham WT. Consensus recommendations for the management of chronic heart failure. Am J Cardiol. 1999;83:1A–38A. [PubMed] [Google Scholar]
- 6.Li SY, Ren J. Cardiac overexpression of alcohol dehydrogenase exacerbates chronic ethanol ingestion-induced myocardial dysfunction and hypertrophy: role of insulin signaling and ER stress. J Mol Cell Cardiol. 2008;44:992–1001. doi: 10.1016/j.yjmcc.2008.02.276. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Takahashi R, Asai T, Murakami H, et al. Pressure overload – Induced cardiomyopathy in heterozygous carrier mice of carnitine transporter gene mutation. Hypertension. 2007;50:497–502. doi: 10.1161/HYPERTENSIONAHA.107.088609. [DOI] [PubMed] [Google Scholar]
- 8.Steiber A, Kerner J, Hoppel CL. Carnitine: a nutritional, biosynthetic, and functional perspective. Mol Aspects Med. 2004;25:455–73. doi: 10.1016/j.mam.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Hothi DK, Geary DF, Fisher L, et al. Short-term effects of nocturnal haemodialysis on carnitine metabolism. Nephrol Dial Transplant. 2006;21:2637–41. doi: 10.1093/ndt/gfl312. [DOI] [PubMed] [Google Scholar]
- 10.Stephens FB, Constantin-Teodosiu D, Greenhaff PL. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J Physiol. 2007;581:431–44. doi: 10.1113/jphysiol.2006.125799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontanive P, Saponati G, Iurato A, et al. L-Arginine in Heart Failure Study Group. Effects of L-arginine on the Minnesota Living with Heart Failure Questionnaire quality-of-life score in patients with chronic systolic heart failure. Med Sci Monit. 2009;15(12):CR606–11. [PubMed] [Google Scholar]
- 12.Nixon M. A simple and sensitive colorimetric method for the determination of long-chain free fatty acids in subcelluar or ganelles. Anal Biochem. 1979;97:403–9. doi: 10.1016/0003-2697(79)90093-9. [DOI] [PubMed] [Google Scholar]
- 13.Wan L, Hubbard RW. Determination of free and total carnitine with a random access chemistry analyzer. Clin Chem. 1998;44:8102–6. [PubMed] [Google Scholar]
- 14.Zydowo MM, Swierczynski J, Nagel G. The respiration and calcium content of heart mitochondria from rats with vitamin D-induced cardionecrosis. Biochem J. 1985;226:155–61. doi: 10.1042/bj2260155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Florian V, Schonfeld P. Alteration of the ADP/ATP translocase isoform pattern improves ATP expenditure in developing rat liver mitochondria. FEBS Lett. 1998;433:261–64. doi: 10.1016/s0014-5793(98)00925-9. [DOI] [PubMed] [Google Scholar]
- 16.Schonfeld P, Bohnensack R. Developmental changes of the adenine nucleotide translocation in rat brain. Biochim Biophys Acta. 1995;1232:75–80. doi: 10.1016/0005-2728(95)00114-9. [DOI] [PubMed] [Google Scholar]
- 17.Schonfeld P, Schild L, Bohnensack R. Expression of the ADP/ATP carrier and expansion of the mitochondrial (ATP+ADP) pool contribute to postnatal maturation of the rat heart. Eur J Biochem. 1996;241:895–900. doi: 10.1111/j.1432-1033.1996.00895.x. [DOI] [PubMed] [Google Scholar]
- 18.Monk BC, Kellerman GM. A rapid method for the assay of mitochondrial ATPase activity. Analytical Biochemistry. 1976;73:187–91. doi: 10.1016/0003-2697(76)90153-6. [DOI] [PubMed] [Google Scholar]
- 19.Kozlovski IV. Early and progressing alcohol cardiomyopathy. Klin Med. 2007;85:51–54. [PubMed] [Google Scholar]
- 20.Skotzko CE, Vrinceanu A, Krueger L, et al. Alcohol use and congestive heart failure: incidence, importance, and approaches to improved history taking. Heart Failure Reviews. 2007;22:43–46. doi: 10.1007/s10741-007-9048-8. [DOI] [PubMed] [Google Scholar]
- 21.De Vivo DC, Bohan TP, Coulter DL, et al. L-Carnitine supplementation in childhood epilepsy: current perspectives. Epilepsia. 1998;39:1216–25. doi: 10.1111/j.1528-1157.1998.tb01315.x. [DOI] [PubMed] [Google Scholar]
- 22.Rosenblatt-Velin N, Montessuit C, Papageorgiou I, et al. Postinfarction heart failure in rats is associated with upregulation of GLUT-1 and downregulation of genes of fatty acid metabolism. Cardiovasc Res. 2001;52:407–16. doi: 10.1016/s0008-6363(01)00393-5. [DOI] [PubMed] [Google Scholar]
- 23.Zhai P, Gao S, Holle E, et al. Glycogen synthase kinase-3 reduces cardiac growth and pressure overload induced cardiac hypertrophy by inhibition of extracellular signal-regulated kinases. J Biol Chem. 2007;45:33181–91. doi: 10.1074/jbc.M705133200. [DOI] [PubMed] [Google Scholar]
- 24.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–35. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 25.Feige JN, Gelman L, Michalik L, et al. From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog Lipid Res. 2006;45:120–59. doi: 10.1016/j.plipres.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Barger PM, Brandt JM, Leone TC, et al. Deactivation of peroxisome proliferator-activated receptor-alpha during cardiac hypertrophic growth. J Clin Invest. 2000;105:1723–30. doi: 10.1172/JCI9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finck BN, Han X, Courtois M, et al. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci USA. 2003;100:1226–31. doi: 10.1073/pnas.0336724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olpin SE. Fatty acid oxidation defects as a cause of neuromyopathic disease in infants and adults. Clin Lab. 2005;51:289–306. [PubMed] [Google Scholar]
- 29.Calabrese V, Giuffrida Stella AM, Calvani M, et al. Acetylcarnitine and cellular stress response: roles in nutritional redox homeostasis and regulation of longevity genes. J Nutri Biochem. 2006;17:73–88. doi: 10.1016/j.jnutbio.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 30.Baniene R, Nauciene Z, Maslauskaite S, et al. Contribution of ATP synthase to stimulation of respiration by Ca2+ in heart mitochondria. Syst Biol(Stevenage) 2006;153:350–53. doi: 10.1049/ip-syb:20060009. [DOI] [PubMed] [Google Scholar]
- 31.Chen C, Saxena AK, Simcoke WN, et al. Mitochondrial ATP synthase. crystal structure of the catalytic F1 unit in a vanadate-induced transition-like state and implications for mechanism. J Biol Chem. 2006;281:13777–83. doi: 10.1074/jbc.M513369200. [DOI] [PubMed] [Google Scholar]
- 32.Zharova TV, Vinogradov AD. Requirement of medium ADP for the steady-state hydrolysis of ATP by the proton-translocating Paracoccus denitrificans Fo.F1-ATP synthase. Biochim Biophys Acta. 2006;1757:304–10. doi: 10.1016/j.bbabio.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Z, Sun X, Kang YJ. Ethanol-induced apoptosis in mouse liver: Fas-and cytochrome c-mediated caspase-3 activation pathway. Am J Pathol. 2001;159:329–38. doi: 10.1016/S0002-9440(10)61699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Z, Sun X, James Kang Y. Metallothionein protection against alcoholic liver injury through inhibition of oxidative Stress. Exp Biol Med. 2002;227:214–22. doi: 10.1177/153537020222700310. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Z, Wang L, Song Z, et al. A critical involvement of oxidative stress in acute alcohol-induced hepatic TNF-α production. Am J Pathol. 2003;163:1137–46. doi: 10.1016/s0002-9440(10)63473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kannan M, Wang L, Kang YJ. Myocardial oxidative stress and toxicity induced by acute ethanol exposure in mice. Exp Biol Med. 2004;229:553–59. doi: 10.1177/153537020422900614. [DOI] [PubMed] [Google Scholar]
- 37.Doser TA, Turdi S, Thomas DP, et al. Transgenic overexpression of aldehyde dehydrogenase-2 rescues chronic alcohol intake-induced myocardial hypertrophy and contractile dysfunction. Circulation. 2009;119:1941–49. doi: 10.1161/CIRCULATIONAHA.108.823799. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Li SY, Li Q, Shen JJ, Dong F, et al. Attenuation of acetaldehyde-induced cell injury by overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene in human cardiac myocytes: role of MAP kinase signaling. J Mol Cell Cardiol. 2006;40:283–94. doi: 10.1016/j.yjmcc.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Li SY, Brown RA, Ren J. Ethanol and acetaldehyde in alcoholic cardiomyopathy: from bad to ugly en route to oxidative stress. Alcohol. 2004;437:175–86. doi: 10.1016/j.alcohol.2004.01.005. [DOI] [PubMed] [Google Scholar]