Summary
Background
The aim of this study was to assess the effect of diet supplementation with L-ascorbic acid (500 mg/L), tocopherol (3 mg/kg b.w.), and/or a water soluble analog of tocopherol (Trolox) (48 mg/L) on ion transport in the colon of rats subjected to a chronic exposure (9 months) to 0.1% lead acetate in drinking water.
Material/Methods
The electrophysiological parameters of the colon wall were measured with Ussing methods. Lead content in the whole blood was analyzed by graphite furnace atomic absorption spectrometry (GFAAS) using Zeeman correction. L-ascorbic acid and tocopherol in plasma was measured by high performance liquid chromatography. Immunohistochemical reaction was carried out for visualization of occludin, the intracellular tight junction protein.
Results
We showed a strong inhibitory effect of lead on the electrophysiological parameters, changes in intestinal permeability, disappearance of junctional occludin, decreased amount of mucus covering the colon surface, and the accumulation of PAS-positive substance in the apical region of the cytoplasm in the absorptive cells.
Conclusions
Supplementation with tocopherol or Trolox did not exert a beneficial influence on the studied parameters. L-ascorbic acid positively influenced the examined electrophysiological parameters, as it cancelled the inhibitory influence of lead on ion transport in the rat colon. L-ascorbic acid also protected against tight junction disruption of epithelial cells in the colon of the lead-treated rats. A similar effect was observed in the group of rats receiving lead and supplemented with L-ascorbic acid plus Trolox.
Keywords: rat colon, ion transport, electrophysiological parameters, lead (Pb), tight junction, L-ascorbic acid, tocopherol supplementation
Background
Factors associated with environmental pollution are among the suggested causes of inflammatory and proliferative diseases of the large intestine. The most common and dangerous pollutant is lead (Pb), which, regardless of more restrictive laws, is still present in the environment, as it is practically non-biodegradable (CDC, 2004). Acute lead poisoning is rarely seen today, but chronic exposure to Pb in low concentration (hitherto considered safe for humans) may disrupt cell morphology and metabolism (Marchlewicz et al., 2009, Baranowska-Bosiacka et al., 2009).
The transporting epithelia of the small and large intestines are characterized by layers of anatomically and biochemically polarized cells that are connected to each other by tight junctions (TJ), and which rest on basement membranes. The cell membranes are anatomically polarized into an apical (luminal, mucosal) region, which faces the intestinal lumen, and a basolateral region. The tight junction forms the borders between the apical and basolateral membranes. Biochemical polarity is reflected in distinct enzymes and transport proteins in the apical (such as Na+-coupled glucose, phosphate and amino-acid carriers, H+-ATP-ase and amiloride-sensitive Na+ channels) and basolateral membranes (such as Na+, K+-ATPase, Ca2+-ATP-ase, Na+/K+-exchanger, Cl−/HCO3− exchanger, sodium independent glucose and amino-acid carriers and barium-sensitive K+ channels) (Diamond 2000). The tight junction is a region of particularly close association of the plasma membranes of adjacent epithelial cells. The junction serves as a permeability barrier between adjacent cells. The permeability properties of tight junctions vary considerably. Epithelia that exhibit low tight-junction permeability to ions tend to exhibit relatively high electrical resistance (low conductance) and relatively high transepithelial electrical potential differences (PD). Such epithelia are classified as “tight epithelia”, and examples include the mammalian renal distal tubule and colon. By contrast, “leaky epithelia” tend to exhibit high tight junction permeability to ions, relatively low electrical resistance and low PD (Dragsten et al., 1981). The mammalian renal proximal tubule and small intestine fall into this category. In leaky epithelia, a large fraction of solute transport occurs between adjacent epithelial cells across the tight junctions, referred to as the paracellular pathway. By contrast, solute transport in tight epithelia is predominantly across the cell, or transcellular. Because the lipid bilayer of plasma membranes constitutes a formidable barrier to diffusion of charged species, this requires specialized transport proteins in the apical and basolateral cell membranes to facilitate transcellular transport of ions (Diamond 2000).
Cellular membranes are among the main targets of Pb toxicity. Disorders of cellular metabolism can, to a great extent, be associated with the resultant imbalance between pro- and anti-oxidants (Slobozhanina et al., 2005). Although Pb does not directly participate in ROS reactions of oxidation and reduction, they are capable of changing the structure of cellular membranes, limiting the mobility of membrane phospholipids and facilitating free radical reactions (Gurer and Ercal, 2000; Slobozhanina et al., 2005). It has also been demonstrated that Pb intoxication may impair blood-brain barrier integrity by the reduction of occludin expression, which is an intracellular tight junction protein (Wang et al., 2007). Lead transport in the small intestine has been studied in the in situ perfused mouse intestine and in vitro isolated segments of rat intestine (for review see Diamond 2000). Lead crosses the apical membrane of the small intestine, possibly through Ca2+ channels or by endocytosis of a lead-binding protein (PbBP). In the epithelial cell, Pb may displace Ca2+ from calcium-binding proteins (CaBP) such as the calbindins, or bind to other proteins, including metallothionein (Pb-MT). Lead may exit the cell across the basolateral membrane by displacing Ca2+ from the Ca2+-ATPase or the Ca2+/Na+-exchanger (for review see Diamond 2000).
However, we have not found any reports in the available literature on the effect of Pb on intestinal permeability and ion transport. Until now, it has not been known if exposure to Pb affects the transport of ions in the epithelium of the large intestine, increasing pro-inflammatory activity of Pb and the risk of large intestine cancer among individuals chronically exposed to lead.
Ion transport in the colon occurs via channels and ionic pumps in cell membranes, being responsible for the distribution of ions on both sides of the epithelial tissue, and the transepithelial electrical potential difference, which is a parameter determining ion transport (Kosik-Bogacka et al. 2000, 2002, 2003; Tyrakowski et al. 1998, 1998b). Ion transport, and, more exactly, the state between the process of absorption and secretion of Na+ and Cl− ions, influences the quantity and the composition of the mucus layer, and indirectly affects the degree of hydration and the physical state of the mucus covering the gastrointestinal tract (Snyder and Walker, 1987).
Mucin glycoproteins play an important role in the protection of the intestine against chemical or physical injury. This protective function depends on the quantity of mucin released and the viscosity of the mucus blanket. It is also a barrier in which immunological, allergic and inflammable reactions begin, caused by infection or intoxication, for example with lead (Field and Semrad, 1993).
Protective functions against the cytotoxicity of Pb can be exerted by intracellular components such as L-ascorbic acid and tocopherol (Marchlewicz et al., 2006). L-ascorbic acid can act inside a cell, but primarily it is an antioxidant of extracellular liquids. In certain conditions, however, it shows pro-oxidative properties (Halliwell and Gutteridge, 1995). Tocopherol, as a component of cell membranes and lipoproteins, is the main antioxidant in the hydrophobic structures of the cell (Halliwell and Gutteridge, 1995; Ziaei et al., 2009). Thus far, little has been known about the role of L-ascorbic acid and tocopherol in the transport of Pb into cells (Simon and Hudes, 1999), and the influence they can exert on the transport of other ions in the presence of Pb (Clarkson, 1993).
The aim of our research was to determine if chronic exposure to Pb influences electrophysiological parameters and tight junction permeability in the colon of rats, and whether supplementation with L-ascorbic acid, tocopherol and a water-soluble analog of tocopherol -Trolox can exert a positive influence on the parameters examined in this study.
Material and Methods
Animals and treatments
The research was carried out on mature (3-month-old) male Wistar rats (300–350 g initial weight), randomly divided into 10 groups for the experiment. The 9-month experiment was conducted according to the following scheme:
K group (n=9): control rats, drank distilled water;
KE group (n=9): received tocopherol in feed (Vitaminum E, Medana, Poland; 3 mg/kg b.w/24h per os);
KTrx group (n=9): drank distilled water containing 48 mg/L Trolox, water-soluble analog of tocopherol (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid, Sigma-Aldrich, Poland), the dose adequate to the 3 mg/kg b.w/24h per os of tocopherol;
KC group (n=9): drank distilled water containing 500 mg/L L-ascorbic acid (Ascorgem, PPF Gemi, Poland);
KCTrx group (n=9): drank distilled water containing 500 mg/L L-ascorbic acid, plus 48 mg/L Trolox;
Pb group (n=9): drank distilled water containing 0.1% lead acetate Pb(CH3COO)2×3H2O; (PbAc); (ultra-pure grade, Merck, Poland);
PbE group (n=9): drank distilled water containing 0.1% PbAc and tocopherol in feed (3 mg/kg b.w/24 h per os);
PbTrx group (n=9): drank distilled water containing 0.1%
PbAc and 48 mg/L Trolox;
PbC group (n=9): drank distilled water containing 0.1% PbAc and 500 mg/L L-ascorbic acid;
PbCTrx group (n=9): drank distilled water containing 0.1% PbAc, 500 mg/L L-ascorbic acid, plus 48 mg/L Trolox.
In all groups, the animals drank from 30 to 50 ml of water or PbAc solution every 24 hours, and the amount of liquid consumed did not differ between the groups.
After the experiment, rats were sacrificed by Tiopental anaesthesia (Biochemie GmbH, Austria), administered at 120 mg/kg body weight (b.w.) intraperitoneally (i.p.). Blood was collected from the heart, and EDTA or heparin was added as anticoagulant. The proximal colon (Lindström et al., 1979) was collected from each rat. All animals were treated humanely and with regard for alleviation of suffering, and all animal procedures complied with National Institute of Environmental Health Sciences (NIEHS)/NIH animal care guidelines (NIH, 1999). Our experiments were approved by the local ethics committee.
Chemicals
The following chemicals were used in the study: for the determination of Pb in whole blood: gradient grade ammonium pyrrolidine dithiocarbamate (APDC), Triton X-100 and isobutyl methyl ketone (IBMK) were obtained from Sigma-Aldrich, Poland. Nitric acid (suprapur) was purchased from Merck, Poland. Water was filtered through a Milli-Q (Millipore) purification system. For determination of L-ascorbic acid: metaphosphoric acid and EDTA (ethylenediaminetetraacetic acid disodium salt dihydrate) were obtained from Sigma-Aldrich, Poland. For determination of tocopherol: trans-β-apo-8′-carotenal, di-tert-butyl-4-methylphenol, hexane, ethanol, methanol, and acetonitrile were purchased from Merck, Poland. Electrophysiological research used Ringer’s solution (mmol/l): Na+ (142.2); K+ (4.0); Ca2+ (4.4); Cl− (155.6); HEPES, N-[2-hydroxyethyl]-piperazine-N′-[2-ethanesulphonic acid] (10.0) (Sigma-Aldrich, Poland). For immunohistochemical reaction: primary rabbit polyclonal anti-occludin antibody (H-279, cat. no. sc-5562) from Santa Cruz Biotechnology Inc., USA; LSAB+DAB Visualization System DAKO (HRP; Rabbit/Mouse/Goat (DAB+) cat. no. K0679; from Dako Cytomation), citrate buffer (pH 6.0), PBS (Sigma-Aldrich, Poland); hematoxylin (PPH POCH SA, Poland). For mucin staining: Periodic Schiff Acid (Parafuchsin – hydrochlorid Standard C19H18N3Cl; Fluka, Poland), HCl and Na2S2O5 (Sigma-Aldrich, Poland).
Determination of lead in whole blood and colon
Lead content in whole blood was analyzed by atomic absorption spectrometry (Model -Solar 969, Hewlett Packard, USA) according to the National Institute for Occupational Safety and Health method (NIOSH, 1994), as described in our previous study (Baranowska-Bosiacka at al., 2009). The whole blood (5 mL) was treated with 1 mL 3% APDC in 5% Triton X-100 and extracted into 5 mL IBMK. The concentration of lead was analyzed by atomic absorption spectrometry (AAS-Solar 969) in an air-acetylene flame at 217 nm, because of the favorable signal-to-noise ratio, compared to 238.3 nm. The detection limit was 0.2 μg/dL.
Lead content in the colon was analyzed by Zeeman Effect graphite furnace atomic absorption spectrometry (GFAAS). The 4100 ZL, Perkin Elmer polarized Zeeman atomic absorption spectrometer, equipped with graphite furnace system, was used. The samples were placed into a closed container, cleaned earlier with HNO3 hot steam. Next, 1 mL of 65% HNO3 was added, and the samples were mineralized in 120°C, 16 h, thermo block. After cooling, samples were treated with 1 mL 30% H2O2 and mineralized for 24 h in the same condition. The obtained solution was diluted with deionized water to 10 mL volume. The concentration of lead was analyzed by GFAAS. The detection limit was 3 μg/g. Internal quality control used 3 standards, prepared by the Heavy Metal Toxicology Central Laboratory of the Work Medicine Institute in Lodz, Poland. Control assays were carried out every 10 samples.
Separation of L-ascorbic acid and tocopherol in plasma by High Performance Liquid Chromatography (HPLC)
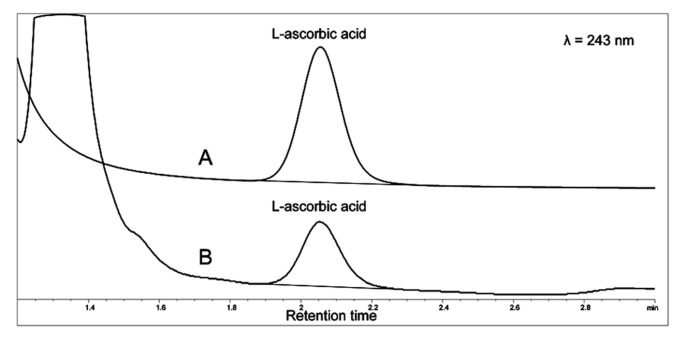
In order to determine L-ascorbic acid in plasma, the blood was heparinized and immediately centrifuged (5000 G for 5 min at +4°C). Plasma was mixed with an equal amount of 10% metaphosphoric acid containing 1 mM EDTA at 0°C, and samples were protected from light. The samples were centrifuged (10000 G for 10 min at +4°C) and the supernatant was analyzed with an isocratic HPLC method (Agilent 1050/1100 chromatograph) at +21°C on a LiChrospher 100 RP-18 125×4 mm column (Merck, Germany), using 0.1 M phosphate buffer (pH=3) as mobile phase (Muto et al., 1997). Detection of the L-ascorbic acid was conducted at 243 nm, and quantification was based on peak areas (Figure 1)
Figure 1.
Chromatograms of L-ascorbic acid in a standard (A) and rat plasma sample (B). The detection of the ascorbic acid was conducted at 243 nm and the quantification was based on peak areas.
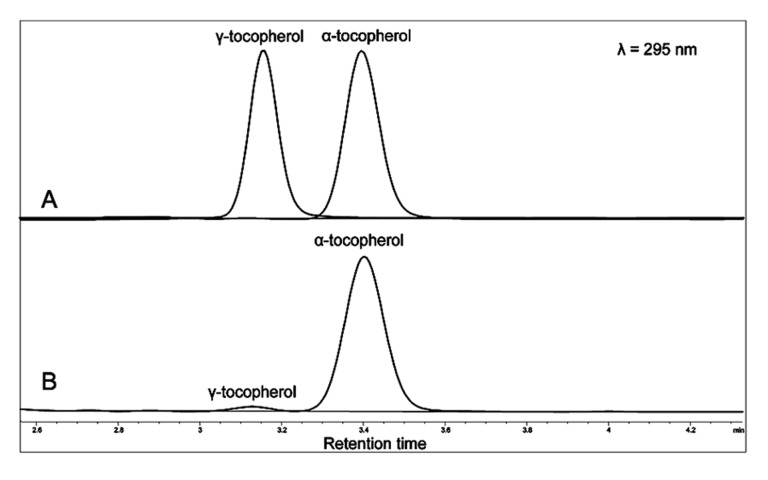
Concentrations of α- and γ-tocopherol were measured using a slightly modified version of the previously described HPLC method (Stachowska et al., 2005). Blood was anticoagulated with EDTA and centrifuged (10000 G for 10 min at +4°C). Plasma was stored at −80°C in the dark in plastic epp. tubes until assayed. Volumes of 150 μL of plasma were mixed with 150 μL of ethanol containing 0.5 mg/L trans-β-apo-8′-carotenal (internal standard) and 100 mg/L di-tert-butyl-4-methylphenol (antioxidant), and vortexed for 30 s. Next, 600 μL of hexane was added, and the mixture was vortexed for 2 min (Epler et al., 1993). Following centrifugation (10000 G at 0°C for 5 min), 480 μl of the upper layer was transferred to a glass tube and evaporated under N2 until dry. The sediment was dissolved in 100 μl ethanol with intense vortexing for 30 s. The temperature at all stages was 0°C, and samples were protected from light. Reversed phase HPLC was performed with an Agilent 1200 chromatograph (Agilent, PaloAlto, CA, USA) equipped with a diode array detector (DAD), thermostatted column compartment and thermostatted autosampler. A 201TP54 (250 × 4.6 mm) column and a 201GK54 precolumn (Vydac, Warsaw, Poland) were used. Tocopherols were separated isocratically at 21°C. The mobile phase was methanol–tetrahydrofurane–acetonitrile (88.5: 7.5: 5.0 v/v), flowing at 1.5 mL/min. The detection of tocopherols was at 295 nm, whereas the internal standard was detected at 450 nm. Quantification was based on peak areas Figure 2). The sample volume was 20 μL, and the time between runs was 10 min. Calibration was performed with an ethanolic solution of added α-tocopherol (Sigma-Aldrich, Poland), instead of ethanol.
Figure 2.
Chromatograms of tocopherols in a standard (A) and rat plasma sample (B). The detection of tocopherols was at 295 nm, whereas the internal standard was detected at 450 nm. Quantification was based on peak areas.
Electrophysiology
The electrophysiological parameters of the colon wall, measured with Ussing methods (Koefoeld-Johnsen and Ussing, 1958), were: the transepithelial electrical potential difference (PD), changes in the transepithelial electrical potential difference during mechanical stimulation (dPD), and transepithelial electrical resistance (R). PD was established when the compensation current intensity of the external battery was 0 mA. The transepithelial electrical resistance was determined using an electrical stimuli I=±10 mA, followed by respective voltage change measurements, then R was calculated from Ohm’s law. R was determined both before and after mechanical stimulation (MS). Since the values of this parameter before and after MS were the same, mechanical stimulation has been given.
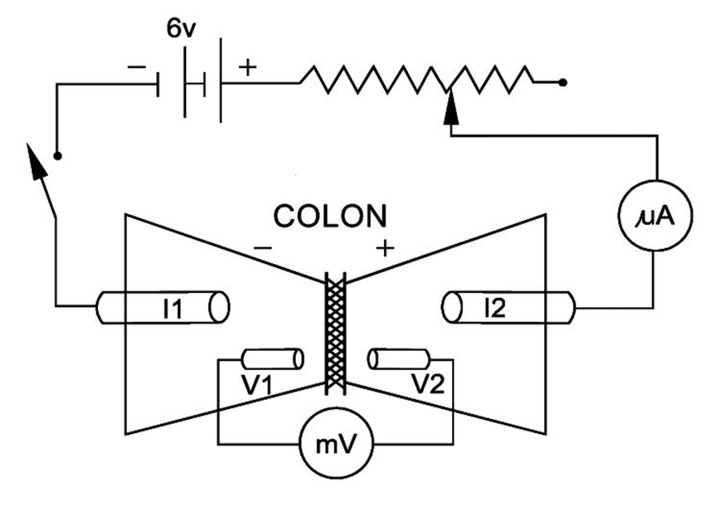
A modified Ussing system was used in the experiments. The modification of a conventional Ussing chamber consisted of placing the tissue horizontally (Figure 3). A nozzle was mounted on one half of the chamber and connected to a peristaltic pump. A gentle MS was applied to the mucosal surface of the tissue by a jet of stimulation fluid from the nozzle.
Figure 3.
Diagram of electrical circuit for evaluation of electrical parameters describing isolated epithelial organs according to Ussing’s method. Abbreviations use are: V1 and V2, Ag/AgCl electrodes for measuring transepithelial electrical potential, connecting voltmeter with measuring chamber through electrolyte agar bridges; I1 and I2, Ag/AgCl electrodes for measuring electrical current, connecting the voltage compensation device with measuring chamber through electrolyte agar bridges; mA, ammeter; mV, voltmeter.
The halves of the chambers were connected by means of 2 pairs of agar bridges, mounted on both sides of the tissue to silver/silver-chloride electrodes. The electrodes were then linked to a voltage/current clamp apparatus, EVC4000 (WPI, USA) and BD 111 recorder (Kipp & Zonen, The Netherlands). One pair of electrodes was used for measuring the transepithelial electrical potential difference, and a second was used for electrical impulses.
The colon walls were gently excised, rinsed of chyme, cut longitudinally and divided into pieces of about 2.5 cm2. After 1-hour incubation in oxygenated Ringer solution, each specimen was mounted in Ussing chambers filled with bathing fluid (Kosik-Bogacka et al. 2000, 2002a and b, Wolska et al. 2005). After 15 min, the mucosal surface of the tissues was stimulated with fluid from a 1.5-mm diameter nozzle, set 12 mm from the intestinal surface. The standard stimulus lasted 30 s, and consisted of 14–16 fluid discharges, with a total volume of 3.6 ml.
Each measurement was followed by a control test experiment with a synthetic cellophane membrane. The current-clamp measuring mode was applied for this test, with current set at ±80 μA, which produced a PD of ±2 mV on the cellophane membrane. Next, the cellophane was stimulated in the same manner as all stimulating fluids used in the experiment.
Histological and immunohistochemical staining
Paraffin-embedded rat colon sections (3–5 μm) were used for immunostaining and for mucins staining with PAS (Periodic Acid Schiff) method according to McManus (Totty, 2002). Immunohistochemical (IHC) reaction was carried out for visualization of occludin, the intracellular tight junction protein, using primary rabbit polyclonal anti-occludin antibody. Next, the deparaffinized sections were microwave irradiated (twice for 4 and 3 min. at high power 700 W) in citrate buffer (pH 6.0) for epitope retrieval. After slow cooling to room temperature, slides were washed in PBS twice for 5 minutes, and then incubated for 30 minutes with primary antibody. After that, sections were stained with an avidin-biotin-peroxidase system, with diaminobenzidine as the chromogen (HRP; Rabbit/Mouse/Goat (DAB+)), in conformity with staining procedure instructions included with the Dako LSAB+ System-HRP. Sections were washed in distilled water and counterstained with hematoxylin. For negative controls, specimens were processed in the absence of the primary antibody. Positive staining was defined microscopically by visual identification of brown pigmentation.
To estimate the ratio of absorptive cells to goblet cells’ number within epithelium of rats’ colons, the cell nuclei of 10 random, longitudinal sectioned crypts of control (n=3) and Pb-treated (n=3) animals were counted. In addition, goblet cells (PAS-positive) within analyzed crypts were also counted. Enumerated, percentage contents of absorptive and goblet cells within colon crypts of control rats were compared with scores achieved with Pb-treated rats.
Statistical analysis
The obtained results were analyzed statistically using Statistica 6.1 software. Arithmetical mean and standard deviation (±SD) were calculated for each of the studied parameters. As most of the distributions deviated from the normal distribution (Shapiro-Wilk test), non-parametric tests were used for the analyses. Correlations between the parameters were examined with Spearman rank correlation coefficient (Rs). To assess the differences between the parameters studied, Mann-Whitney tests were used. The level of significance was p<0.05.
Results
Lead concentration in whole blood and colon
Administration of 0.1% lead acetate to rats in drinking water, or administration of Pb and supplemental L-ascorbic acid (PbC) or tocopherol (PbE) for 9 months, caused a significantly higher whole blood Pb concentration and higher colon Pb concentration in all the examined groups compared to their respective control groups (Table 1). The concentration of Pb in the blood was strongly positively correlated with the concentrations of Pb in the colon (Rs=+0.80, p<0.0001).
Table 1.
The concentration of lead in whole blood and in the colon of control, Pb-treated and vitamins supplemented rats.
| Group | E | Trx | C | CTrx | |
|---|---|---|---|---|---|
| Blood (μg/dL) | |||||
| K | 0.34±0.23 | 0.33±0.20 | 0.33±0.15 | 0.32±0.15 | 0.33±0.20 |
| Pb | 7.21±1.27** | 6.89±0.16** | 6.52±1.07** | 6.63±0.62** | 6.69±0.50** |
| Colon (μg/g d.m.) | |||||
| K | 1.19±0.06 | 1.11±0.12 | 1.14±0.18 | 1.19±0.12 | 1.22±0.20 |
| Pb | 16.35±2.64** | 15.79±0.46** | 15.52±0.30** | 17.11±0.63** | 16.73±0.78** |
Values are mean ±SD. Abbreviations use are: group E – rats received 3 mg/kg body wt. vitamin E in feed; group Trx – rats drank distilled water containing 48 mg/L Trolox; group C – rats drank distilled water containing 500 mg/L vitamin C; group CTrx – rats drank distilled water containing 500 mg/L vitamin C plus 48 mg/L Trolox; group K – control rats; group Pb – rats drank distilled water containing 0.1% lead acetate.
difference statistically significant (p<0.01) in comparison with respective control group (Mann-Whitney test).
No statistically significant differences were found between the control (K) and control supplemented groups (KE, KTrx, KC, KCTr) (Mann-Whitney test)
No statistically significant differences were found between the Pb-treated (Pb) and Pb-treated and vitamins supplemented groups (PbE, PbTrx, PbC, PbCTr) (Mann-Whitney test).
L-ascorbic acid concentration in plasma
Rats from the experimental (PbC, PbCTrx) and control (KC, KCTrx) groups had a significantly higher concentration of L-ascorbic acid in plasma compared to the K group (Table 2). The concentration of L-ascorbic acid in rat plasma in all the Pb-exposed groups was significantly lower in comparison to the respective control groups (Table 2). L-ascorbic acid concentration in plasma negatively correlated with the Pb concentration in the blood (Rs=−0.44, p<0.0001) and the colon (Rs=−0.34, p<0.0001).
Table 2.
The concentration of ascorbic acid (mg/L) and sum of tocopherols a and (mg/L) in plasma of control, Pb-treated and vitamins supplemented rats.
| Group | E | Trx | C | CTrx | |
|---|---|---|---|---|---|
| Ascorbic acid (mg/L) | |||||
| K | 0.32±0.15 | 0.23±0.09 | 0.33±0.09 | 0.93±0.26# | 1.078±0.23# |
| Pb | 0.21±0.02* | 0.14±0.03** | 0.17±0.02** | 0.54±0.23*,a | 0.52**±0.03a |
| Tocopherol (mg/L) | |||||
| K | 9.27±0.80 | 11.89±2.6# | 10.23±0.58# | 7.67±1.78 | 10.32±1.08# |
| Pb | 7.16±1.65* | 11.16±3.46a | 9.73±1.87a | 7.31±1.56 | 9.97±0.94a |
Explanations, see Table 1. Values are mean ±SD.
(p<0.01);
(p<0.05) difference statistically significant in comparison with respective control group (Mann-Whitney test);
(p<0.05) difference statistically significant in comparison with K group (Mann-Whitney test);
(p<0.05) difference statistically significant in comparison with Pb group (Mann-Whitney test).
Tocopherol concentration in plasma
Tocopherol supplementation, both in the experimental (PbTrx, PbCTrx, PbE) and control rats (KTrx, KCTrx, KE), resulted in a significantly higher tocopherol concentration in plasma compared to the respective control (Table 2). Concentration of tocopherol in rat plasma was significantly lower than in the respective control group only in the Pb group (Table 2). Tocopherol concentration in plasma was negatively correlated with the Pb concentration in the blood (Rs=−0.25, p=0.023) and the colon (Rs=−0.36, p<0.0001).
Transepithelial electrical potential difference (PD)
In rats exposed to lead (Pb), and in groups receiving lead supplemented with tocopherol (PbE), we observed a statistically significantly lower value of PD in the colon compared to the respective control group (Table 3). However, in the control group of rats receiving tocopherol (KE), the PD value in the colon was significantly lower than in the K group (Table 3). In the PbC and PbCTrx groups, the PD value was significantly higher than in the Pb group (Table 3). The PD value in the colon was positively correlated with blood Pb concentration (Rs=+0.41, p<0.0001), negatively correlated with the concentration of L-ascorbic acid (Rs=−0.47, p=0.0003), and weakly positively correlated with tocopherol concentration (Rs=+0.28, p=0.037) in rat plasma.
Table 3.
The influence of lead on electrophysiological parameters in the colon of control, treated with lead and vitamins supplemented rats.
| Group | K | KE | KTrx | KC | KCTrx | Pb | PbE | PbTrx | PbC | PbCTrx |
|---|---|---|---|---|---|---|---|---|---|---|
| PD (mV) | ||||||||||
|
| ||||||||||
| (n) | (9) | (3) | (5) | (3) | (3) | (4) | (3) | (4) | (3) | (3) |
| Mean | −1.5 | −0.7a | −1.2 | −1.2 | −1.0 | −0.3a | −0.2a | 0.0a | −1.6b | −1.0b |
| ±SD | 0.3 | 0.2 | 0.4 | 0.3 | 0.2 | 0.1 | 0.1 | 0.0 | 0.2 | 0.0 |
|
| ||||||||||
| R (Ω× cm2) | ||||||||||
|
| ||||||||||
| Mean | 207.2 | 170.0a | 148.6a | 172.0a | 166.0a | 168.8a | 131.0a,b | 150.0a | 150.0a | 167.0a |
| ±SD | 7.1 | 4.0 | 13.4 | 1.5 | 9.3 | 12.0 | 0.0 | 0.0 | 0.0 | 4.4 |
|
| ||||||||||
| MS dPD (mV) | ||||||||||
|
| ||||||||||
| Mean | −0.7 | −0.2a | −0.2a | −0.3a | −0.2a | −0.0a | −0.3a | 0.0a | −0.3a | −0.4a,b |
| ±SD | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.0 | 0.1 | 0.0 |
Mean value ±SE of mean are given; n number of the studied fragments; PD transepithelial electrical potential; dPD difference between maximal value of PD and control value; difference statistically significant in comparison with a –control group (K), b – lead treated group (Pb) group (Student’s t-test p<0.05).
Differences between the maximum stimulated value and the control value of PD (dPD)
In rats exposed to Pb and in groups PbTrx, the value of dPD in the colon was significantly lower than in the respective control group (Table 3). However, dPD in control groups supplemented with vitamins was also significantly lower than in the control group (Table 3; Figure 4). In rats of the PbCTrx group, dPD value was significantly higher than in the Pb group. The dPD value in the rat colon was strongly positively correlated to Pb concentration in the blood (Rs=+0.51, p<0.0001), moderately positively correlated with Pb concentration in the colon (Rs=0.35, p=0.007), and strongly negatively correlated with L-ascorbic acid concentration (Rs=−0.54, p=0.0003) in rat plasma.
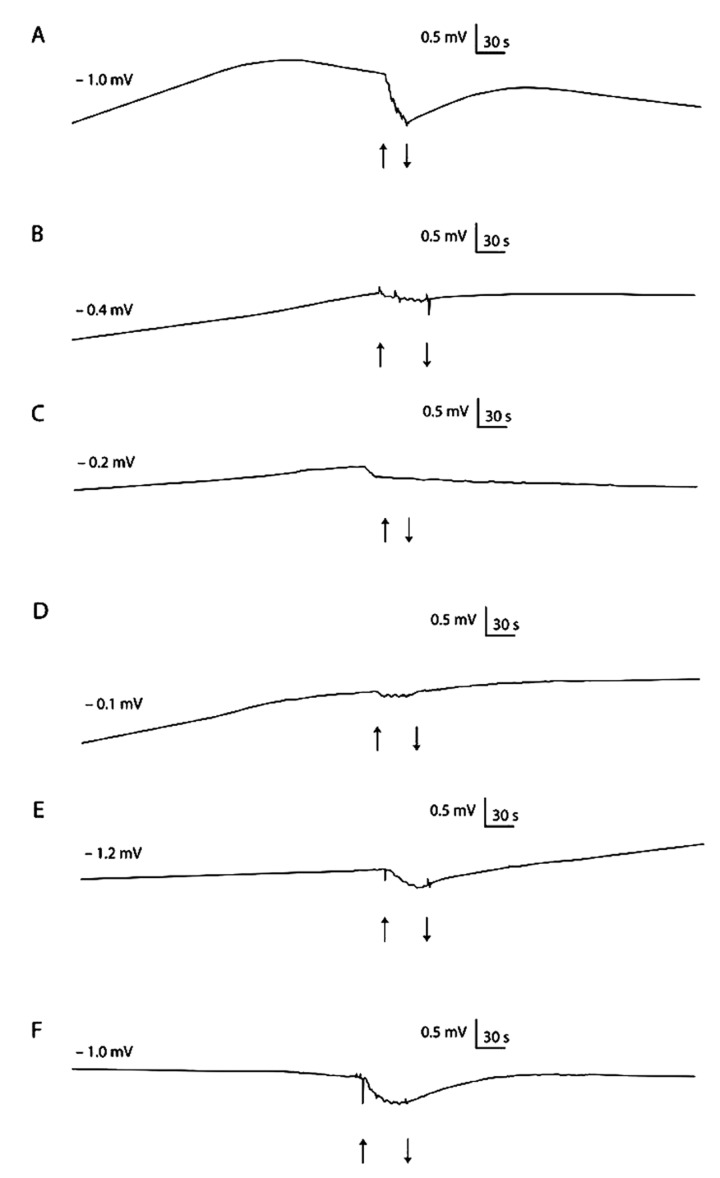
Figure 4.
Hyperpolarisation reaction (dPD) at the time of mechanical stimulation of isolated colon wall in: control condition (A), Pb-treated rats (B) Pb-treated rats and vit. E supplemented (C), Pb-treated rats and Trolox supplemented (D), Pb-treated rats and vit. C supplemented (E) Pb-treated rats and vit. C and Trolox supplemented (F). Stimulation was 30s of jet rising. The start and finish of stimulation are denoted by the pair of arrows. Single experiments are shown.
Transepithelial electrical resistance (R)
In rats exposed to Pb, the value of R in the colon was significantly lower than in the K group (Table 3). Simultaneously, in all the control groups of rats supplemented with vitamins, we observed R in the colon at a significantly lower level than in the K group (Table 3). The R value in group PbTrx, PbC, PbCTrx was not statistically different than in the Pb group, whereas in the PbE group we observed significantly lower R value than in the colon of the Pb group. The value of R in the colon was strongly negatively correlated with Pb concentration in the blood (Rs=−0.73, p<0.0001) and with Pb concentration in the colon (Rs=−0.59, p<0.0001). A moderately positive correlation was observed between the value of R and the concentration of L-ascorbic acid (Rs=−0.30, p=0.002) in rat plasma.
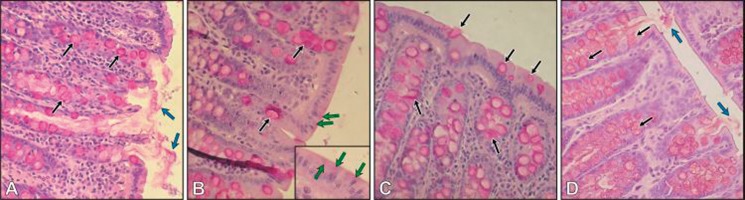
PAS reaction, mucin staining
In control group (K) slides stained with PAS method, a thick layer of mucin was presented on the surface of the colon epithelium (Figure 5A; blue arrows). The frequently presented goblet cells within crypts were filled with mucin (Figure 5A; black arrows). We did not observe any differences among control groups (data not shown).
Figure 5.
Colon of control (A), Pb-intoxicated (B) received lead acetate with vitamin C (C) and received lead acetate with vitamin C plus Trolox (D) rats. PAS-staining. Objective magnification A, B, C, D ×40; insert ×100.
In rats treated with Pb, a notable decrease of mucus covering epithelium of the colon was visible (Figure 5B). In addition to being present in goblet cells, mucin was also present in the apical region of some enterocytes (Figure 5B, insert; green arrows). We did not observe statistically significant differences in the number of goblet cells in comparison to the K group. The goblet cells within the control group constitute 52% of crypts’ cells, and 51% within the Pb-treated group (Figure 5A, B, black arrows).
Results of histological examination of the colon of rats receiving Pb supplemented with tocopherol (PbE) or Trolox (PbTrx) were not different from rats receiving only Pb (data not shown).
In the colon PbC rats, the number of goblet cells (Figure 5C; black arrows) was similar to the control (K), but mucus covering the epithelium and PAS-positive enterocytes were not observed. In the colon of PbCTrx rats, the number of goblet cells (Figure 5D, black arrows) was similar to the K group and similar to the KC group. PAS-positive enterocytes were not present, and mucus covering the epithelium (Figure 5D, blue arrows) appeared, but less abundantly than in the K group.
Occludin immunoexpression
In control rats (K), occludin was localized in both levels: within epithelial cells covering the lumen (Figure 6A, B; back arrows), and also lining the crypts (Figure 6A, green arrows) of the colon. The superficial enterocytes were immunopositive for occludin in the upper part of cells at the cell-cell contact site corresponding to the tight junctions (Figure 6B; black arrow), whereas, within goblet cells, occludin was localized, mostly in the basal region (Figure 6A; green arrows). The immunoexpression of occludin at a different intensity was also visible within cells of the lamina propria of the mucosa (Figure 6A, B; red arrows). We did not observe any differences among control groups (data not shown).
Figure 6.
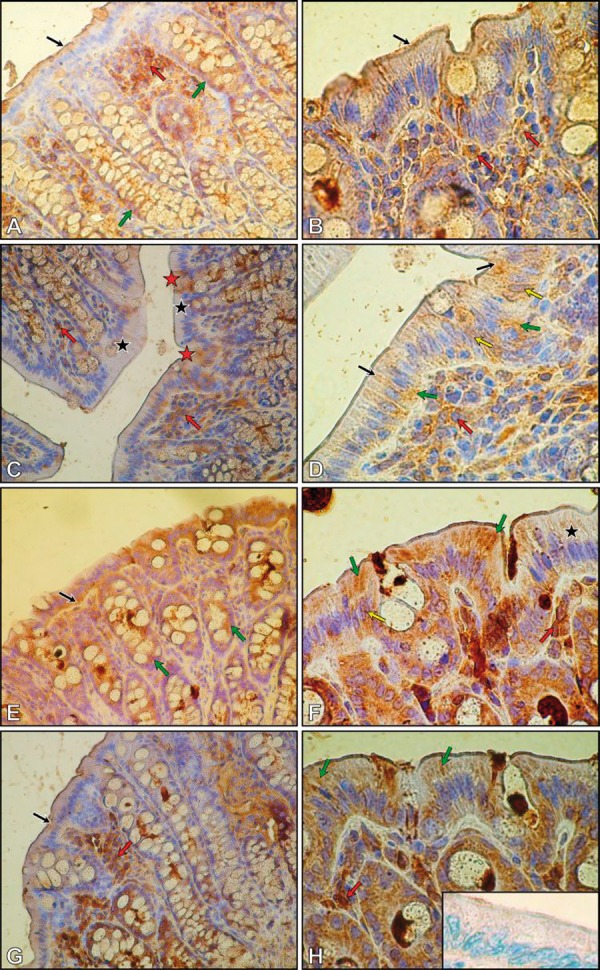
Immunohistochemical reaction for occludin in colon of control (A, B), Pb intoxicated (C, D), received lead acetate with vitamin C (E, F) and received lead acetate with vit. C plus Trolox (G, H) rats. Negative control (H, insert). Objective magnification: A, C, E, G ×40; B, D, F, H ×100.
In the colon of Pb-treated rats, the immunoexpression for occludin was considerably lower (Figure 6C, D) than in control rats. The brown pigmentation, indicating a positive score of IHC, had a mosaic pattern (Figure 6C, D). Some enterocytes were immunonegative (Figure 6C; black asterisks), and others were immunopositive (Figure 6C, red asterisks) and showed diffuse speckled, cytoplasmic reaction for occludin (Figure 6D, black arrows). Moreover, occludin was also observed at the basal portion of several colonic enterocytes (Figure 6D; green arrows), and within nuclei of some of them (Figure 6D; yellow arrows). Similar to the control group, numerous occludin-positive cells were localized in the lamina propia of colon mucosa of Pb-treated rats (Figure 6C, D; red arrows). The number of these cells was nearly the same as in the control group.
The immunohistochemical examination of the colon of rats of the PbE group and PbTrx group did not show any differences in occludin expression in comparison to Pb-treated rats (data not shown).
In the colon of the PbC group of rats (Figure 6E, F), the intensity of occludin immunoexpresion was higher than in Pb-treated rats, and similar to the K group. The diffuse reaction was localized mainly in the basal portion (Figure 6E; black arrow), and also in the apical region of enterocytes (Figure 6F; green arrows). Additionally, both immunonegative epithelial cells (Figure 6F; black asterisk), and cells with occludin-positive nuclei, were observed (Figure 6F; yellow arrow). Within mucosa, the cells that expressed occludin were widely spread (Figure 6F; red arrow). In the goblet cells, occludin was localized at the basal portion, both in the control and the Pb-treated groups (Figure 6E, green arrows).
In the colon of the PbCTxr group of rats (Figure 6G, H), the intensity of occludin immunoexpresion was higher than in Pb-treated rats, and similar to the control group, and occludin immunoexpresion was visible in the upper part of enterocytes (Figure 6G; black arrow). In comparison to the PbC group, the diffuse immunoexpression of occludin was visible primarily in the apical region of enterocytes (Figure 6H; green arrows). The cells of the lamina propria of mucosa also were occludin-positive (Figure 6G, H; red arrows).
Negative control of immunohistochemical reaction in the step using primary anti-occludin antibody have been omitted shows insert (Figure 6H).
Discussion
Although the determined whole blood Pb concentration in the rats was within norms hitherto considered safe for humans (below 10 μg/dL) (CDC, 1991), this study showed a strong inhibitory effect of Pb on electrophysiological parameters, observed in decreased PD, dPD and R in the rat colon.
The secretory function of the epithelium of the gastrointestinal tract is regulated by the enteric nervous system (ENS), a part of the autonomous nervous system (Maggi, 1995). Due to the fact that the investigated fragments of the colon contained intact nervous elements, the possibility of an interaction between ENS and the epithelium on the nervous and neurotransmitter levels exists (Cooke, 1986). There are sensory endings of C fibers in the epithelium of the mammalian colon, which, in addition to generating afferent signals, react to stimulation by secreting neuropeptides of the nonadrenergic noncholinergic systems – NANC (e.g., neurokinin A – NKA, substance P – SP and calcitonin gene – related polypeptide – CGRP), influencing the function of smooth muscles, the production of mucus by intestinal glands, and ion transport by the epithelium (Maggi, 1995; Miller, 1985). In this study, the mechanical stimulation was applied as a gentle washing of the mucosal surface of the colon with an incubation solution. This stimulation caused changes in the transepithelial ion transport, expressed in the reaction of hyperpolarization (Figure 4A). In agreement with findings in the literature, we ascertained that the movement of the solution on the surface of the colon caused the stimulation of sensory receptors, which caused the secretion of NANC neuropeptides from C fibers, changing the transepithelial ion transport (Bevan and Geppetti, 1994; Kosik-Bogacka et al., 2000; 2002, 2002a, 2002b; 2003; Tyrakowski et al., 1998, 1998b). Because transepithelial electrical resistance depends on the permeability of tight junctions, we can infer that the changes in PD during the mechanical stimulation of the isolated colon of the rats resulted from changes in the active transport of ions at the stable level of the extracellular neutral flow of ions. Similar reactions to mechanical stimulation were described previously in an isolated cecum (Kosik-Bogacka et al., 2000, 2002,2002a, 2002b, 2003) and trachea (Tyrakowski et al., 1998, 1998b) of a rabbit, and in the skin of a frog (Kosik-Bogacka et al. 2000, 2002).
In this study, chronic intoxication with lead caused a decrease, almost to zero, in the value of PD, and inhibited the dPD in the colon of rats. In our opinion, this may indicate a Pb-induced increase in the permeability of blood-vessels and the epithelium. Moreover, the hyperstimulation of C fibers, as a result of the destruction of epithelial cells, causes an antidromic stimulation of all branches, which additionally stimulates the release of NANC neuropeptides (axis reflex) (Bevan and Geppetti, 1994; Pendry et al., 1993). Consequently, there occurs a contraction of smooth muscles, stimulation or inhibition of the ionic transport, and changes in the mucus layer of the colon.
Our reasoning concerning the increase in the permeability of epithelium in chronic intoxication with lead is also confirmed by a decrease in the electrical resistance of the colon, which could be due to an increase in the permeability of the epithelial tight junction. Loosening, opening TJ, and an increase in the permeability of the epithelium, was observed by Bohme et al. (1992) in research on the influence of mercury on the colon of rats. Our histological study confirmed that changes in intestinal permeability accompanied the reorganization of tight junction structure. We have shown, for the first time, that lead treatment caused disappearance of junctional occludin from localization at cell-cell contacts, together with appearance within the basal portion of cytoplasm and within nuclei of enterocytes. Similar changes were observed by Fries et al. (2008) during experimental colitis in mice. The authors suggest that the cytoplasmic localization of occludin may be the result of the removal of TJ-associated proteins through the caveolae-mediated endocytosis. Moreover, Turner (2006) suggests that the nuclear expression of occludin may correspond to repair processes. The disruption of TJ integrity was possibly caused by the direct influence of lead on junctional protein structures (by binding to the -SH groups) and their function. This influence might also be indirect, due to induced actin filament depolarization, which could induce tight junction dysfunction (Shen et al., 2005).
Our study also showed a decrease in the amount of mucus present on enterocytes in rats receiving Pb, which could significantly impair colon protection. Our previous research (Marchlewicz et al., 2006), and the results of several other studies, indicate that Pb toxicity seems to be a result of metabolic disorders, which are connected with a disruption in the balance between pro- and anti-oxidants and the propagation of radical oxygen species reactions (Behrend et al., 2003; Roessner et al., 2008). In cells of the large intestine, hydrogen peroxide, which belongs to the reactive oxygen species (ROS), is produced physiologically in metabolic processes (Roessner et al., 2008). However, it has been shown that hydrogen peroxide plays a role in the formation of inflammatory diseases of the large intestine, and especially in the creation of the hydroxyl radical, which is produced from hydrogen peroxide in the presence of Fe2+ and is chemically very reactive. In the lumen of the large intestine, 90–95% of consumed iron compounds are subjected to condensation, increasing the production of ROS (especially the hydroxyl radical) (Lund et al., 2001; Parkin, 2006; Pravda, 2005; Roessner et al., 2008). Through the propagation of lipid peroxidation and oxygenation of DNA bases, the hydroxyl radical increases the risk of gene mutation and uncontrolled cellular proliferation in the large intestine (Scibior et al., 2008; Wiseman and Halliwell, 1996). L-ascorbic acid, in large concentrations, shows antioxidative properties, but in small concentrations it may initiate the peroxidation of lipids in the presence of Fe3+ (eg, bound by the ferritin of the mucous membrane in the intestine) (Halliwell and Gutteridge, 1995). Fe2+ ions can reduce oxygen, thus producing the anion superoxide radical, whose disintegration produces hydrogen peroxide. Pb appears to exacerbate lipid peroxidation induced by hydrogen peroxide and iron (Villeda-Hernandez et al., 2001).
It has been observed that L-ascorbic acid has a regulatory function in ion transport in the cornea of amphibians, and, more exactly, that in a physiological concentration it stimulates the active transport of Cl− (Scott and Cooperstein, 1975). Fischer et al. (2004) also ascertained that L-ascorbic acid was a biological regulator of epithelial CFTR Cl− channel in the human respiratory tract. However, there are no reports concerning the biological role of L-ascorbic acid in the transport of water or salt in the gastrointestinal tract of mammals. In our study of rats treated with lead and supplemented with L-ascorbic acid or L-ascorbic acid plus Trolox, we observed a complete cancellation of the inhibitory influence of lead on ion transport in the colon of rats, which can be explained by the stimulation of Cl− secretion by L-ascorbic acid.
The decline of reaction during mechanical stimulation (dPD), observed by us in rats supplemented by vitamins only, might be caused by previous excessive stimulation of Cl- secretion by vitamins, and depletion of NANC neuropeptides secreted from C fibers endings during stimulation. This issue requires further study. However, in our histological examination, we noticed that the mucin blanket in the colon of Pb-treated and L-ascorbic acid supplemented rats was reduced, but that the number of goblet cells within the colon was comparable to the control group. Such a positive effect on the electrophysiological parameters examined in this study was not confirmed for supplementation with tocopherol or Trolox in Pb-treated rats. We may also suppose that the observed positive effect of supplementation with L-ascorbic acid plus Trolox could have been due more to the activity of L-ascorbic acid than Trolox, as we did not observe the positive effect of supplementation with Trolox on the parameters examined in this study; only the PD value in rat colon was weakly positively correlated with tocopherol concentration in plasma. The lack of inhibitory effect of tocopherol on the ion transport in the rat colon is surprising, and requires further study. The available literature provides no data on this subject.
We can assume that L-ascorbic acid supplementation does not retract the disadvantages of lead influence on the mucin blanket protector of intestinal epithelial cells, but it is possible that the above mentioned improvement of ion transport could be the result of undisrupted tight junctions. As we noticed, the changes of immunoexpression of occludin in the colon of rats receiving Pb and L-ascorbic acid, in comparison to the Pb group, were less, which could suggest that L-ascorbic acid supplementation to a greater or lesser degree protects the intestinal barrier. Because L-ascorbic acid is endogenously produced in rats, we should be careful in stating its protective activity in the human large intestine. In our study, supplementation with L-ascorbic acid and L-ascorbic acid plus Trolox had no effect on Pb levels in the blood and colon of rats. Although opposing reports exist on this subject, L-ascorbic acid in the diet may decrease lead absorption (Simon and Hudes, 1999). In our study, an increased lead concentration in the blood and colon had a negative effect on L-ascorbic acid concentration in plasma in the rats. It is possible that Pb may affect L-ascorbic acid absorption in the small intestine, or lead to its increased excretion, but further detailed studies are needed to address this possibility.
Conclusions
Chronic lead intoxication of rats (i) inhibited the ion transport in the epithelium of the colon; (ii) increased tight junction permeability; and (iii) consequently disrupted ion transport and decreased the amount of mucus that constitutes a protective layer covering the epithelium of the colon.
Supplementation with (i) tocopherol or Trolox did not exert a positive influence on studied parameters; and (ii) L-ascorbic acid exerted a significant positive influence on electrophysiological parameters of the rat colon, exhibiting a protective action on the mucous membrane, where it cancelled the inhibitory influence of Pb on ion transport in the colon, and exhibited a protective influence on intracellular tight junctions.
Acknowledgements
The authors thank Ms Tatiana Małolepsza for her help with animal care.
Abbreviations
- dPD
changes in transepithelial electrical potential difference during mechanical stimulation (mV)
- MS
mechanical stimulation
- NANC
non-adrenergic non-cholinergic
- Pb
lead
- PD
transepithelial electrical potential difference (mV)
- R
transepithelial electrical resistance (Ω×cm2)
- TJ
tight junction
Footnotes
Source of support: none
References
- 1.CDC. Centers for Disease Control. United States Department of Health and Human Services; Atlanta: 2004. [Google Scholar]
- 2.Marchlewicz M, Baranowska-Bosiacka I, Kolasa A, et al. Disturbances of energetic metabolism in rat epididymal epithelial cells as a consequence of chronic lead intoxication. BioMetals. 2009;22:877–87. doi: 10.1007/s10534-009-9238-z. [DOI] [PubMed] [Google Scholar]
- 3.Baranowska-Bosiacka I, Dziedziejko V, Safranow K, et al. Inhibition of erythrocyte phosphoribosyltransferases (APRT and HPRT) by Pb2+: A potential mechanism of lead toxicity. Toxicology. 2009;259:77–83. doi: 10.1016/j.tox.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Diamond GL. Transport of metals in the gastrointestinal system and kidneys. In: Zalups RK, Koropatrick J, editors. Molecular Biology and Toxicology of Metals. Taylor and Francis; 2000. [Google Scholar]
- 5.Dragsten PR, Blumenthal R, Handler JS. Membrane asymmetry in epithelia: Is the tight junction a barrier to diffusion in the plasma membrane? Nature. 1981;294:718–22. doi: 10.1038/294718a0. [DOI] [PubMed] [Google Scholar]
- 6.Slobozhanina EI, Kozlova NM, Lukyanenko LM, et al. Lead-induced changes in human erythrocytes and lymphocytes. J Appl Toxicol. 2005;25:109–14. doi: 10.1002/jat.1043. [DOI] [PubMed] [Google Scholar]
- 7.Gurer H, Ercal N. Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic Biol Med. 2000;29:927–45. doi: 10.1016/s0891-5849(00)00413-5. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, Luo W, Zheng W, et al. Iron supplement prevents lead-induced disruption of the blood-brain barrier during rat development. Toxicol Appl Pharmacol. 2007;219:33–41. doi: 10.1016/j.taap.2006.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosik-Bogacka DI, Banach B, Tyrakowski T, Bilicka B. Pharmacological modification of ionic currents elicited in epithelia by sensory neuropeptides. Med Sci Monit. 2000;6(5):887–91. [PubMed] [Google Scholar]
- 10.Kosik-Bogacka DI, Banach B, Tyrakowski T, Wojciechowska I. Effect of capsaicin and dimethyl sulphoxide on ion transport in selected experimental models. Pol J Pharmacol. 2002;54:267–74. [PubMed] [Google Scholar]
- 11.Kosik-Bogacka DI, Banach B, Tyrakowski T, et al. Effects of amiloride and bumetanide on ion transport in the caecum of rabbit. Pol J Pharmacol. 2003;55:213–19. [PubMed] [Google Scholar]
- 12.Tyrakowski T, Greczko I, Sedlaczek A, et al. Electrophysiological investigation of the effects of ambroxol on the transepithelial Na+ ion transport pathway in airways. Pol J Pharmacol. 1998;50:31–38. [PubMed] [Google Scholar]
- 13.Tyrakowski T, Banach B, Mościbroda A, et al. Reappraisal of amiloride action on transepithelial electrical potential difference of isolated tracheal wall. Arch Immunol Therap Exp (Warsaw) 1998b;46:45–50. [PubMed] [Google Scholar]
- 14.Snyder JD, Walker WA. Structure and function of intestinal mucin: developmental aspects. Int Arch Allergy Appl Immunol. 1987;82:351–56. doi: 10.1159/000234225. [DOI] [PubMed] [Google Scholar]
- 15.Field M, Semrad CE. Toxigenic diarrheas, congenital diarrheas, and cystis fibrosis: disorders of intestinal ion transport. Annu Rev Physiol. 1993;55:631–55. doi: 10.1146/annurev.ph.55.030193.003215. [DOI] [PubMed] [Google Scholar]
- 16.Marchlewicz M, Wiszniewska B, Gonet B, et al. Increased lipid peroxidation and ascorbic acid utilization in testis and epididymis of rats chronically exposed to lead. Biometals. 2006;20:13–19. doi: 10.1007/s10534-006-9009-z. [DOI] [PubMed] [Google Scholar]
- 17.Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. Oxford, New York: Oxford University Press; 1995. [Google Scholar]
- 18.Ziaei S, Kazemnejad A, Sedighi A. The effect of vitamin E on the treatment of menstrual migraine. Med Sci Monit. 2009;15(1):CR16–19. [PubMed] [Google Scholar]
- 19.Simon JA, Hudes ES. Relationship of ascorbic acid to blood lead levels. JAMA. 1999;281:2289–93. doi: 10.1001/jama.281.24.2289. [DOI] [PubMed] [Google Scholar]
- 20.Clarkson TW. Molecular and ionic mimicry of toxic metals. Annu Rev Pharmacol Toxicol. 1993;33:545–71. doi: 10.1146/annurev.pa.33.040193.002553. [DOI] [PubMed] [Google Scholar]
- 21.Lindström CG, Rosengren JE, Fork FT. Colon of the rat. An anatomic, histological and radiographic investigation. Acta Radiol. 1979;20:523–36. doi: 10.1177/028418517902000314. [DOI] [PubMed] [Google Scholar]
- 22.NIH. DES Research Update. Bethesda, MD: National Institute of Health; 1999. [Google Scholar]
- 23.NIOSH (National Institute for Occupational Safety Health) Lead in blood and urine 8003. Manual of Analytical Methods, NIOSH. (4th ed) 1994;2:1–4. [Google Scholar]
- 24.Muto N, Ohta T, Suzuki T, et al. Evidence for the involvement of a muscarinic receptor in ascorbic acid secretion in the rat stomach. Biochem Pharmacol. 1997;53:553–59. doi: 10.1016/s0006-2952(96)00792-7. [DOI] [PubMed] [Google Scholar]
- 25.Stachowska E, Wesołowska T, Olszewska M, et al. Elements of Mediterranean diet improve oxidative status in blood of kidney graft recipients. Br J Nutr. 2005;93:345–52. doi: 10.1079/bjn20051374. [DOI] [PubMed] [Google Scholar]
- 26.Epler KS, Ziegler RG, Craft NE. Liquid chromatographic method for the determination of carotenoids, retinoids and tocopherols in human serum and in food. J Chromatogr. 1993;619:37–48. doi: 10.1016/0378-4347(93)80444-9. [DOI] [PubMed] [Google Scholar]
- 27.Koefoeld-Johnsen V, Ussing HH. The nature of the frog skin potential. Acta Physiol Scand. 1958;42:289–308. doi: 10.1111/j.1748-1716.1958.tb01563.x. [DOI] [PubMed] [Google Scholar]
- 28.Kosik-Bogacka D, Tyrakowski T. Model study of toluene diisocyanate effect on transepithelial ion transport. Med Sci Monit. 2002;8(5):BR187–92. [PubMed] [Google Scholar]
- 29.Kosik-Bogacka D, Tyrakowski T. Effect of ambroxol on ion transport – a model study. Med Sci Monit. 2002;8(6):BR236–41. [PubMed] [Google Scholar]
- 30.Wolska E, Danielewicz NM, Kaczorowski P, et al. Postmortem examination of transepithelial ion currents in rabbit colon and trachea in relation to temperature of storage and its importance for interlethal reactions. Forensic Sci Int. 2005;154:85–91. doi: 10.1016/j.forsciint.2004.09.110. [DOI] [PubMed] [Google Scholar]
- 31.Totty BA. Mucins. In: Bancroft JD, Gamble M, editors. Theory and practice of histological techniques. London: Churchill Livingstone; 2002. pp. 163–200. [Google Scholar]
- 32.CDC. Centres for Disease Control. United States Department of Health and Human Services; Atlanta: 1991. [Google Scholar]
- 33.Maggi CA. Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Prog Neurobiol. 1995;45:1–98. doi: 10.1016/0301-0082(94)e0017-b. [DOI] [PubMed] [Google Scholar]
- 34.Cooke HJ. Neurobiology of the intestinal mucosa. Gastroenterology. 1986;90:1057–81. doi: 10.1016/0016-5085(86)90889-9. [DOI] [PubMed] [Google Scholar]
- 35.Miller RJ. Control of epithelial ion transport by neuropeptides. Regul Pept Suppl. 1985;4:203–8. [PubMed] [Google Scholar]
- 36.Bevan S, Geppetti P. Protons: small stimulants of capsaicin-sensitive sensory nerves. Trends Neurosci. 1994;17:509–12. doi: 10.1016/0166-2236(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 37.Pendry YD. Neuronal control of airways smooth muscle. Pharmacol Ther. 1993;57:171–202. doi: 10.1016/0163-7258(93)90055-i. [DOI] [PubMed] [Google Scholar]
- 38.Bohme M, Diener M, Mestres P, Rummel W. Direct and indirect actions of HgCl2 and methyl mercury chloride on permeability and chloride secretion across the rat colonic mucosa. Toxicol Appl Pharmacol. 1992;114:285–94. doi: 10.1016/0041-008x(92)90079-8. [DOI] [PubMed] [Google Scholar]
- 39.Fries W, Muja C, Crisafulli C, et al. Dynamics of enterocyte tight junctions: effect of experimental colitis and two different anti-TNF strategies. Am J Physiol Gastrointest Liver Physiol. 2008;294:938–47. doi: 10.1152/ajpgi.00469.2007. [DOI] [PubMed] [Google Scholar]
- 40.Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–9. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen L, Turner JR. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol Biol Cell. 2005;16:3919–36. doi: 10.1091/mbc.E04-12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Behrend L, Henderson G, Zwacka RM. Reactive oxygen species in oncogenic transformation. Biochem Soc Trans. 2003;31:1441–44. doi: 10.1042/bst0311441. [DOI] [PubMed] [Google Scholar]
- 43.Roessner A, Kuester D, Malfertheiner P, Schneider-Stock R. Oxidative stress in ulcerative colitis-associated carcinogenesis. Patrol Res Pract. 2008;204:511–24. doi: 10.1016/j.prp.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Lund EK, Fairweather-Tait SJ, Wharf SG, Johnson IT. Chronic exposure to high levels of dietary iron fortification increases lipid peroxidation in the mucosa of the rat large intestine. J Nutr. 2001;131:2928–31. doi: 10.1093/jn/131.11.2928. [DOI] [PubMed] [Google Scholar]
- 45.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–44. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 46.Pravda J. Radical induction theory of ulcerative colitis. World J Gastroenterol. 2005;11:2371–84. doi: 10.3748/wjg.v11.i16.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scibior D, Skrzycki M, Podsiad M, Czeczot H. Glutathione level and glutathione-dependent enzyme activities in blood serum of patients with gastrointestinal tract tumors. Clin Biochem. 2008;41:852–58. doi: 10.1016/j.clinbiochem.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Villeda-Hernandez J, Barroso-Moguel R, Mendez-Armenta M, et al. Enhanced brain regional lipid peroxidation in developing rats exposed to low level lead acetate. Brain Res Bull. 2001;55:247–51. doi: 10.1016/s0361-9230(01)00512-3. [DOI] [PubMed] [Google Scholar]
- 50.Scott WN, Cooperstein DF. Ascorbic acid stimulates chloride transport in the amphibian cornea. Invest Ophthalmol. 1975;14:763–66. [PubMed] [Google Scholar]
- 51.Fischer H, Schwarzer C, Illek B. Vitamin C controls the cystic fibrosis transmembrane conductance regulator chloride channel. Proc Natl Acad Sci USA. 2004;101:3691–96. doi: 10.1073/pnas.0308393100. [DOI] [PMC free article] [PubMed] [Google Scholar]