Summary
Background
Osteogenic growth peptide (OGP) is a 14-mer peptide found in relevant concentration in blood, and its carboxy-terminal fragment [OGP(10-14)] represents the active portion of the full-length peptide. In addition to stimulating bone formation, OGP(10-14) shows hematological activity. In fact, it highly enhances hematopoiesis-affecting stem progenitors. Moreover, OGP(10-14) reduces the growth and induces the differentiation of the hematological tumour cell line trombophoietin(TPO)-primed M07-e by interfering with RhoA and Src kinase pathways. In the present report, we went deeper into this mechanism and evaluated the possible interference of the OGP(10-14) signal pathway with TGFβ1 and TPO receptor Mpl.
Material/Methods
In OGP(10-14)-treated M07-e cells cultured with or without RhoA and Src kinases inhibitors (C3 and PP2), expression of TGFβ1, Mpl, and Src kinases was analyzed by immunoperoxidase technique. Activated RhoA expression was studied using the G-LISA™ quantitative test.
Results
In M07-e cells, both OGP(10-14) and PP2 activate RhoA, inhibit Src kinases, reduce Mpl expression and increase TGFβ1 expression. OGP(10-14) and PP2 show the same behavior, causing an additive effect when associated.
Conclusions
OGP(10-14) induces TPO-primed M07-e cells differentiation through RhoA/TGFβ1/SFKs signalling pathway. In particular OGP(10-14) acts as a Src inhibitor, showing the same effects of PP2.
Keywords: OGP(10-14), RhoA, Src kinases, M07-e, Human thrombopoietin (TPO), TGFβ1
Background
The carboxy-terminal fragment of osteogenic growth peptide [OGP(10-14)] represents the bioactive form of the bone anabolic and hematopoietic stimulator agent OGP, retaining its effects [1]. The pentapeptide OGP(10-14) induces proliferation in fibroblast and osteoblast cell lines and enhances hematopoiesis both in vitro and in vivo [2–7]. Previous studies demonstrated that OGP(10-14) reduces proliferation and induces differentiation of myeloblastic HL60 and megakaryoblastic TPO-primed M07-e cells (4,8,9). The small G-protein RhoA and the tyrosine kinase Fyn belonging to the Src family (SFKs) are involved in these effects [8,9]. It has been demonstrated that, in megakaryocytic cells, TPO activates Src kinases by its receptor Mpl [10]. Mpl display can be modulated by TPO binding and receptor internalization [11]. It has also been demonstrated that TGFβ1, a member of the large superfamily of growth factors that control cell growth, proliferation, differentiation, and apoptosis in multiple lineages, is involved in megakaryocytic differentiation [12,13]. Taking into account our previous study showing that OGP(10-14) reduces proliferation and induces M07-e differentiation through RhoA activation and Src inhibition [9], in the present study we have gone deeper into this signal transduction pathway and investigated the possible involvement of TGFβ1 and Mpl.
Material and Methods
Cell culture and culture conditions
Megakaryoblastic M07-e TPO responsive cell line (DSMZ: German Collection of Micro-organisms and Cell Cultures) was maintained in RPMI 1640 medium (Sigma, St. Louis, MO, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS-Gibco, Gaithersburg, MD, USA), 2 mM L-Glutamine (Sigma), 100 U/L penicillin-100 g/L streptomycin (Sigma), and 50 ng/mL rh TPO (Pepro-Tech EC Ltd, London, UK) at 37°C in a humidified atmosphere containing 5% CO2. For all experiments, cells were pre-cultured for 24 hours in IST (Sigma)-RPMI medium and then treated with OGP(10-14) (linear osteogenic growth peptide 10-14; Abiogen Pharma SpA, Pisa, Italy), PP2 SFK inhibitor (Calbiochem, La Jolla, CA, USA) and C3 Rho inhibitor (Upstate Biotechnology, Lake Placid, NY, USA) at 10−11 M, 1 μM and 2 μg/ml, respectively, according to the conditions already reported in our previous paper [9]. PP2 SFK inhibitor was dissolved in DMSO and added to cell cultures 40 minutes before adding OGP(10-14). DMSO was used at a concentration that does not interfere with M07-e cell proliferation [9]. Experiments were performed at 1, 72 and 144 hours on the basis of the previous results, showing in M07-e cells RhoA activation and signs of differentiation at 1h and 144h, respectively [9].
All experiments were repeated at least 3 times. The significance of differences between experimental variables was determined using a parametric t-test.
Quantitative expression of activated RhoA
To measure activated RhoA, we used serum-free conditions to avoid artifacts from serum protein that activate RhoA [14]. According to RhoA G-LISA™ test instructions (Cytoskeleton, Denver, CO, USA), cells treated with OGP(10-14) and/or PP2 for 1 hour were lysed and snap frozen in liquid nitrogen. Then, cell lysates were thawed and the protein concentrations normalized to total protein amount. Cell lysates were added to the microplate containing the Rho-GTP-binding protein linked to the wells. After 30 minutes samples were washed twice and incubated with the antigen presenting buffer for 2 minutes and with the anti-RhoA primary antibody for 45 minutes. After 2 washes, samples were incubated with the secondary horse radish peroxidase conjugate (HRP) antibody for 45 minutes, and finally with HRP detection reagent for 15 minutes. The absorbance values were recorded at 490 nm using a Bio-Rad microplate reader (Hercules, CA, USA). For all experiments, positive (CTRL+) and negative (CTRL−) controls were used. In particular, CTRL+ and CTRL− consisted of constitutively active RhoA (RhoA-GTP) and lysis buffer, respectively.
Immunocytochemical analysis
Cells were plated on glass slides by centrifugation at 400 rpm for 5 minutes, air-dried and fixed with 1% formalin for 10 minutes at 4°C. For antigen retrieval, cytospins were exposed to 0.2% Triton X-100 solution (Sigma) for 10 minutes (for Mpl detection) or to microwaves for 3×5 minutes at 600 W in 10 mM sodium citrate (for TGFβ1 and Src activated detection). Slides were then treated with 0.6% H2O2/cold methanol for 15 minutes, with normal swine serum (1:20; Sigma) for 20 minutes and with anti TGFβ1 (sc-146; Santa Cruz Biotechnology; Santa Cruz, CA, USA), activated Src (Tyr 416; Santa Cruz Biotechnology) and c-Mpl (sc-15403, Santa Cruz Biotechnology) rabbit polyclonal antibodies diluted in 0.1% BSA/PBS overnight at 4°C. The detection protocol was carried out using biotinylated link antibodies and streptavidin-peroxidase complex (LSAB kit, Dakopatts, Glostrup, Denmark), and diaminobenzidine (DAB, Sigma) or 10% nickel-DAB enhanced (for c-Mpl detection) as chromogens for 5 minutes in the dark. After counterstaining by hematoxylin or nuclear fast red, samples were dehydrated, mounted with DPX mountant (Fluka, Buchs, Switzerland) and observed with a DMRB Leica microscope. Negative controls were obtained by incubating the specimens with 0.1% BSA-PBS, omitting the primary antibody. Results were evaluated either by optical observation on 50 cells minimum per sample, or by image analysis.
Image analysis system
A “Quantimet +” image analysis system was used to evaluate the reacting surfaces per cell and the degree of reactivity. Two series of 10 microscopic fields, the former from immunostained slides, the latter from negative controls, were frozen and captured. The gray levels of immunostained samples were compared with those of negative controls and the image analysis data were obtained according to conditions already reported by our group [15]. The final rate of immunoreactivity/cell was calculated by the product of the extension (percentage of positive cell surface) and the intensity (mean gray level of the reaction), on 50 cells per sample.
Results
Expression of Mpl
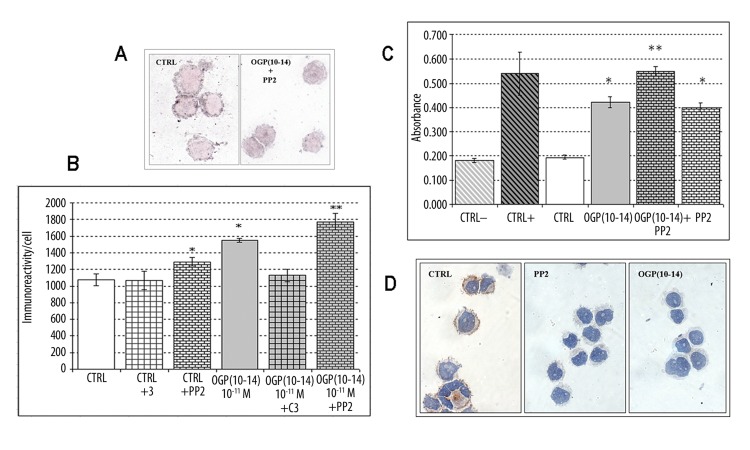
Immunocytochemical evaluation of Mpl expression was performed on the samples treated with OGP(10-14) and/or PP2 for 1, 72 and 144 hours. Mpl receptor was expressed in all samples as demonstrated by immunoreactivity localized at the level of every cell, especially along the cell membrane. A decreased Mpl expression was detected only in the cells treated with PP2/OGP for 144 hours as demonstrated by the lower degree of positivity (+) shown by these cells compared to that (++) shown by the other samples (Figure 1A).
Figure 1.
(A) Mpl expression in M07-e cells cultured for 144 hours. Immunoreaction (silver) is higher on control cells (CTRL) than in OGP(10-14)+PP2 treated cells. Original magnification ×1000. (B) Image analysis of TGFβ1 expression in M07-e cells cultured for 144 hours. *P<0.01 vs control; ** P<0.01 vs OGP(10-14). Error bars represent the SD of the means (n=6). (C) Quantitative activated RhoA expression in M07-e cells cultured for 1 hour. CTRL−: lysis buffer, CTRL+: RhoA-GTP. *P<0.01 vs control; ** P<0.01 vs OGP(10-14). Error bars represent the SD of the means (n=6). (D) Activated Src expression in M07-e cells cultured for 1 hour. Immunoreaction (brown) is present only in control cells (CTRL) Original magnification ×1000.
Expression of TGFβ1
Intracellular TGFβ1 immunocytochemical expression was performed on samples treated with OGP (10-14) for 72 and 144 hours, with or without PP2 or C3 inhibitors. TGFβ1 factor was expressed on all the samples at the level of every cell, variously distributed in the cytoplasm. Image analysis performed on the 144-hour treated samples showed a higher immunoreactivity compared to 72-hour-treated samples. Image analysis data, summarised in Figure 1B, showed that OGP(10-14) induced an increase of TGFβ1 as demonstrated by the degree of immunoreactivity/cell that was 1551.17±23.07 on OGP-treated cells and 1076.30±71.51 on control cells (P=1.9E-040). Moreover, RhoA inhibition by C3 prevented OGP-mediated TGFβ1 increases. In fact, the immunoreactivity/cell of OGP/C3 treated samples was comparable to that of controls (1126.57±74.98 on OGP/C3 treated cells vs 1076.30±71.51 on control cells; P=0.223). On the contrary, the SFK inhibition by PP2 induced a TGFβ1 increase not only in OGP(10-14)-treated cells (1551.17±23.07 on OGP-treated cells vs 1774.16±97.97 on OGP/PP2-treated cells; P=0.009) but also in control cells (1076.30±71.51 on control cells vs 1289.68±50.63 on PP2/control cells; P=0.006).
Expression of activated RhoA
The results showed that in 1-hour OGP(10-14)-treated cells RhoA was activated as demonstrated by absorbance value that was approximately double that of control cells (CTRL) incubated only with TPO (0.422±0.023 nm in OGP-treated cells vs 0.195±0.009 nm in CTRL; P=1.3E-04). As expected, CTRL did not display activated RhoA, showing absorbance value comparable to that of negative control samples (0.182±0.008 nm in CTRL− vs 0.195±0.009 nm in CTRL; P=0.147). Moreover, PP2 addition induced a further significant increase of activated RhoA (0.550±0.019 nm in PP2/OGP-treated cells vs 0.422±0.023 nm in OGP-treated cells; P=4.2E-04), and PP2 alone activated an amount of RhoA similar to OGP(10-14) (0.401±0.018 nm in PP2-treated cells vs 0.422±0.023 nm in OGP-treated cells; P=0.306 and 0.401±0.018 nm in PP2-treated cells vs 0.195±0.009 nm in CTRL; P=3.5E-05) (Figure 1C).
Expression of activated Src
Expression of activated Src was evaluated by immunocytochemical analysis with an antibody that detects endogenous levels of Src only when they are phosphorylated at Tyr416. As we expected, control samples, treated with TPO, showed Src activated at both 1 and 72 hours, while PP2 and OGP(10-14) prevented this activation. The immunoreaction was present in the control cells only (Figure 1D).
Discussion
OGP(10-14) promotes bone formation and hemopoiesis, while it reduces Mk-CFU growth of bone marrow-derived cells from patients affected by idiopathic myelofibrosis, and it reduces the proliferation of megakaryoblastic M07-e cells promoting their differentiation [4,9]. Our group has previously demonstrated that RhoA and Src kinases are involved in the OGP(10-14)-induced differentiation of the megakaryoblastic M07-e cell line. In particular, we showed that PP2 reduces proliferation and induces differentiation as OGP(10-14), demonstrating a co-operative action of these agents [9]. Here, we have demonstrated by quantitative data that both OGP(10-14) and PP2 activate RhoA, and that their association activates a larger amount of RhoA compared to those activated by the 2 molecules separately. Moreover, we showed that OGP(10-14) prevents SFK activation, as does PP2. Taking into account that Mpl-SFK interaction is necessary for the activation of Src proteins by TPO [16], we checked if OGP(10-14) could interfere with Mpl expression. The results obtained by enhanced immunocytochemistry showed that OGP(10-14) can modulate Mpl expression in the M07-e cells, as demonstrated by its decrease in the cells treated also with PP2. However, since the modulation is evident only after the association of OGP(10-14) with PP2, a more sensitive test should be used. In any case, the involvement of the Mpl TPO receptor in OGP(10-14)-induced M07-e differentiation could explain our previous results showing that the cell proliferation was inhibited by OGP(10-14) only in TPO-dependent, but not in GM-CSF-dependent M07-e cells [9]. TGFβ1 could be the key molecule that links RhoA to Mpl; in fact, TGFβ1 is able to down-modulate the Mpl receptor on hematopoietic stem/progenitors [17] and to antagonize the proliferative effect of TPO on the M07-e cell line [13]. Here, we have found that in these cells OGP(10-14) modulates TGFβ1 expression, inducing an increase through RhoA, since it is prevented by the C3 treatment. Also, PP2 enhances TGFβ1 expression, showing again the same behavior of OGP(10-14) and causing an additive effect when associated with it. PP2 could induce a TGFβ1 increase, preventing the inactivation of RhoA due to SFK activation.
Conclusions
In a previous study we have demonstrated that OGP(10-14) and PP2 reduce proliferation and induce differentiation of TPO-primed M07-e cells through RhoA/SFK signalling pathways. In the present study we found again that PP2 and OGP(10-14) show the same effects, increasing TGFβ1 expression and RhoA activation, and inhibiting Src activation. Thus, on the basis of previous [9] and present results, we suggest an OGP(10-14) differentiation signaling pathway model (Figure 2), showing that OGP(10-14) induces M07-e cell differentiation preventing SFK activation caused by activated RhoA. In this pathway activated RhoA induces a TGFβ1 increase that down-modulates Mpl [17], preventing SFK activation.
Figure 2.
Proposed model of OGP(10-14) differentiation signalling. (A) Control cells: TPO induces M07-e cells proliferation by SFK activation and RhoA inhibition. (B) OGP(10-14)-treated cells: the pentapeptide induces cell differentiation by activating RhoA and preventing SFKs activation through a down-modulation of Mpl induced by an increase of TGFβ1.
Footnotes
Source of support: Partially supported by RRMR-CUCCS (Rete Regionale Toscana Medicina Rigenerativa – Centro per l’Uso Clinico di Cellule Staminali) and AIL (Associazione Italiana per la lotta alle Leucemie, linfomi e mieloma)
References
- 1.Gabarin N, Gavish H, Muhlrad A, et al. Mitogenic Gi protein-MAP kinase signaling cascade in MC3T3-E1 osteogenic cells: activation by c-terminal pentapeptide of osteogenic growth peptide (OGP 10-14) and attenuation of activation by camp. J Cell Biochem. 2001;81:594–602. doi: 10.1002/jcb.1083. [DOI] [PubMed] [Google Scholar]
- 2.Fazzi R, Testi R, Trasciatti S, et al. Bone and bone-marrow interactions: haematological activity of osteoblastic growth peptide (OGP)-derived carboxy-terminal pentapeptide. Mobilizing properties on white blood cells and peripheral blood stem cells in mice. Leuk Res. 2002;26:19–27. doi: 10.1016/s0145-2126(01)00091-1. [DOI] [PubMed] [Google Scholar]
- 3.Fazzi R, Galimberti S, Testi R, et al. Bone and bone marrow interactions: hematological activity of osteoblastic growth peptide (OGP)-derived carboxy-terminal pentapeptide. II. Action on human hematopoietic stem cells. Leuk Res. 2002;26:839–48. doi: 10.1016/s0145-2126(02)00008-5. [DOI] [PubMed] [Google Scholar]
- 4.Fazzi R, Pacini S, Testi R, et al. Carboxy-terminal fragment of osteogenic growth peptide in vitro increases bone marrow cell density in idiopathic myelofibrosis. Br J of Haematol. 2003;121:76–85. doi: 10.1046/j.1365-2141.2003.04250.x. [DOI] [PubMed] [Google Scholar]
- 5.Spreafico A, Frediani B, Capperucci C. Osteogenic growth peptide effects on primary human osteoblast cultures: potential relevance for the treatment of glucocorticoid-induced osteoporosis. J Cell Biochem. 2006;98:1007–20. doi: 10.1002/jcb.20836. [DOI] [PubMed] [Google Scholar]
- 6.Chen ZX, Chang M, Peng YL, et al. Osteogenic growth peptide C-terminal pentapeptide [OGP(10-14)] acts on rat bone marrow mesenchymal stem cells to promote differentiation to osteoblasts and to inhibit differentiation to adipocytes. Regul Pept. 2007;142:16–23. doi: 10.1016/j.regpep.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z, Chang M, Peng Y, et al. Regulation of endochondral ossification by osteogenic growth peptide C-terminal pentapeptide [OGP(10-14)] Protein Pept Lett. 2009;16:1074–80. doi: 10.2174/092986609789055395. [DOI] [PubMed] [Google Scholar]
- 8.Mattii L, Fazzi R, Moscato S, et al. Carboxy-terminal fragment of osteogenic growth peptide regulates myeloid differentiation through RhoA. J Cell Biochem. 2004;93:1231–41. doi: 10.1002/jcb.20248. [DOI] [PubMed] [Google Scholar]
- 9.Mattii L, Battolla B, Moscato S, et al. The small peptide OGP(10-14) acts through Src kinases and RhoA pathways in Mo-7e cells: morphologic and immunologic evaluation. Med Sci Monit. 2008;14:103–8. [PubMed] [Google Scholar]
- 10.Lannutti BJ, Shim MH, Blake N, et al. Identification and activation of Src family kinases in primary megakaryocytes. Exp Hematol. 2003;31:1268–74. doi: 10.1016/j.exphem.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Kaushansky K. The molecular mechanisms that control thrombopoiesis. J Clin Invest. 2005;115:3339–47. doi: 10.1172/JCI26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soderberg SS, Karlsson G, Karlsson S. Complex and Context Dependent Regulation of Hematopoiesis by TGF-β Superfamily Signaling. Ann N Y Acad Sci. 2009;1176:55–69. doi: 10.1111/j.1749-6632.2009.04569.x. [DOI] [PubMed] [Google Scholar]
- 13.Kalina U, Koschmieder S, Holfmann WK, et al. Transforming growth factor-beta 1 interferes with thrombopoietin-induced signal transduction in megakaryoblastic and erythroleukemic cells. Exp Hematol. 2001;29:602–8. doi: 10.1016/s0301-472x(01)00628-2. [DOI] [PubMed] [Google Scholar]
- 14.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–99. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 15.Mattii L, Bianchi F, Da Prato I, et al. Renal cell cultures for the study of growth factor interactions underlying kidney organogenesis. In Vitro Cell Dev Biol Anim. 2001;37:251–58. doi: 10.1007/BF02577538. [DOI] [PubMed] [Google Scholar]
- 16.Lannutti BJ, Drachman JG. Lyn tyrosine kinase regulates thrombopoietin-induced proliferation of hematopoietic cell lines and primary megakaryocytic progenitors. Blood. 2004;103:3736–43. doi: 10.1182/blood-2003-10-3566. [DOI] [PubMed] [Google Scholar]
- 17.Fortunel NO, Hatzfeld JA, Monier MN, Hatzfeld A. Control of hematopoietic stem/progenitor cell fate by transforming growth factor-beta. Oncol Res. 2003;13:445–53. doi: 10.3727/096504003108748483. [DOI] [PubMed] [Google Scholar]