Summary
Background
Obese patients with prostate cancer may have lower preoperative PSA concentration due to hemodilution. Lower PSA concentration may falsely affect assessing the risk of progression after radical prostatectomy (RP). The aim of this study was to determine preoperative PSA mass as the absolute amount of PSA protein secreted into circulation, and evaluation of its usefulness in prediction of biochemical recurrence after RP.
Material/Methods
177 patients after RP due to prostate cancer were included in the study. On the basis of formulas, PSA mass was calculated {PSA mass [μg] = (weight [kg])0.425 × (height [cm])0.72 × 0.007184 × 1.670 × PSA concentration [ng/ml]}. Patients were divided into 3 groups according to increasing values of PSA mass. The following features were assessed and compared between these groups (χ-square test): pathologic stage T3, nodal metastases, positive surgical margins, biochemical and local recurrence and the rate of death. Cancer-specific survival was assessed depending on PSA mass (Kaplan-Meier curves with log rank test). The usefulness of PSA mass in prediction of biochemical recurrence was compared with PSA concentration (logistic regression with ROC curves).
Results
Pathologic stage T3, nodal metastases, positive surgical margins and progression were more common in patients with higher levels of PSA mass (p<0.01). Cancer-specific survival was significantly shorter in patients with elevated values of PSA mass (p=0.02). Preoperative PSA mass was a more sensitive predictor of biochemical recurrence than was PSA concentration (p=0.04).
Conclusions
The preoperative PSA mass is a better predictor of biochemical recurrence after RP than PSA concentration.
Keywords: hemodilution, obesity, prostate cancer, PSA mass, radical prostatectomy
Background
Progression is diagnosed in 1 out of 4 patients after 3 years of radical treatment of prostate cancer [1]. Progression includes biochemical recurrence, local recurrence, distant metastases and death. Elevated preoperative PSA concentration, among other factors, is a strong predictor of progression after radical prostatectomy (RP) due to prostate cancer [2]. However, this marker has some limitations. Undoubtedly, a negative feature of PSA concentration is the fact that it is subject to hemodilution. Some authors claim that in overweight and obese patients PSA concentration is lower, which is, in the first place, caused by the aforementioned phenomenon. This phenomenon is supposed to consist of the dissolution of PSA mass in a large amount of plasma, finally resulting in lower PSA concentration [3,4]. Lower PSA concentration may falsely affect assessing the risk of progression after radical prostatectomy.
PSA is a protease whose physiological function is to liquefy semen. Every adult male is characterized by a quite invariable amount (mass) of this, secreted into the blood protein, depending on age, the size of prostate, the presence of cancer or other prostate diseases. However, standard PSA determination means that PSA mass is dissolved in plasma volume, which is mainly dependant on the degree of obesity.
PSA mass, as the absolute amount of PSA protein secreted into circulation, may be quite easily computed on the basis of physiological formulas for estimated body surface (EBS) and plasma volume (PV), and therefore is independent of the hemodilution phenomenon (Table 1).
Table 1.
The formulas to estimate plasma volume and PSA mass.
| Estimated Body Surface (EBS) | Plasma volume [liters] (PV) | PSA mass [μg] |
|---|---|---|
| (weight) 0.425 × (height) 0.72 × 0.007184 | EBS × 1.670 | PV × PSA concentration |
In order to eliminate hemodilution, it was decided to evaluate the PSA mass in patients with prostate cancer and compare its usefulness with PSA concentration in prediction of cancer progression after RP.
Material and Methods
Ethics
The study was approved by the appropriate ethics committee and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All patients gave their informed consent prior to inclusion in the study.
Patients
From 1994 until the end of 2007, 206 radical retropubic prostatectomies in Caucasian men suffering from prostate cancer were carried out in the Department of Urology in Zabrze, Medical University of Silesia in Katowice. The patients who underwent preoperative anti-androgen therapy, chemotherapy or radiotherapy were excluded from the study (29 patients), and 177 patients were qualified for inclusion.
All patients were divided according to:
BMI – into 3 groups: I – 45 patients with normal weight (BMI <25), II – 95 overweight patients (BMI – 25–29.9), III – 37 obese patients (BMI ≥30).
Preoperative PSA concentration – into 3 groups: I – 79 patients with PSA <10 ng/ml, II – 66 patients with PSA 10–19.9 ng/ml, III – 32 patients with PSA ≥20 ng/ml.
Preoperative PSA mass – into 3 groups: I – 71 patients with PSA <40 μg, II – 78 patients with PSA 40–69.9 μg and III – 28 patients with PSA ≥70 μg.
The characteristics of the study group and subgroups are shown in Tables 2–4.
Table 2.
Characteristics of the study group (177 patients).
| Mean | Standard deviation | Range | |
|---|---|---|---|
| Age (years) | 62.3 | 6.1 | 48–76 |
| BMI (kg/m2) | 27.5 | 3.4 | 17.9–40.3 |
| PSA concentration (ng/ml) | 13.8 | 10.4 | 1.83–61.7 |
| Gleason score (median) | 6.0 | 1.9 | 2–10 |
| PSA mass (μg) | 45.1 | 33.7 | 6.5–196.6 |
| Plasma volume (liters) | 3.2 | 0.2 | 2.7–4.1 |
Table 4.
Characteristics of patients in BMI, PSA concentration and PSA mass subgroups.
| BMI (kg/m2) | PSA concentration (ng/ml) | PSA mass (μg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | I | II | III | I | II | III | ||
| pT3 | Yes | 12 (18.1%) | 33 (50%) | 21 (31.9%) | 14 (21.2%) | 32 (48.5%) | 20 (30.3%) | 13 (19.6%) | 34 (51.5%) | 19 (28.7%) |
| No | 33 (29.7%) | 62 (55.8%) | 16 (14.5%) | 65 (58.5%) | 34 (30.6%) | 12 (10.9%) | 58 (52.2%) | 44 (39.6%) | 9 (8.1%) | |
| p value (χ-square test) | 0.01 | <0.001 | <0.001 | |||||||
| Positive lymph nodes | Yes | 2 (20%) | 4 (40%) | 4 (40%) | 1 (10%) | 5 (50%) | 4 (40%) | 1 (10%) | 3 (30%) | 6 (60%) |
| No | 43 (25.7%) | 91 (54.5%) | 33 (19.8%) | 78 (46.7%) | 61 (36.5%) | 28 (16.7%) | 70 (41.9%) | 75 (44.9%) | 22 (13.1%) | |
| p value (χ-square test) | 0.31 | 0.04 | <0.001 | |||||||
| Positive surgical margin | Yes | 13 (26%) | 28 (56%) | 9 (18%) | 9 (18%) | 27 (54%) | 14 (28%) | 7 (14%) | 29 (58%) | 14 (28%) |
| No | 32 (25.1%) | 67 (52.7%) | 28 (22%) | 70 (55.1%) | 39 (30.7%) | 18 (14.1%) | 64 (50.3%) | 49 (38.5%) | 14 (11%) | |
| p value (χ-square test) | 0.83 | <0.001 | <0.001 | |||||||
| Biochemical recurrence | Yes | 13 (20%) | 34 (52.3%) | 18 (27.6%) | 15 (23%) | 30 (46.1%) | 20 (30.7%) | 14 (21.5%) | 33 (50.7%) | 18 (27.6%) |
| No | 32 (28.5%) | 61 (54.4%) | 19 (16.9%) | 64 (57.1%) | 36 (32.1%) | 12 (10.7%) | 57 (50.8%) | 45 (41.1%) | 10 (8.9%) | |
| p value (χ-square test) | 0.17 | <0.001 | <0.001 | |||||||
| Local recurrence | Yes | 4 (20%) | 10 (50%) | 6 (30%) | 5 (25%) | 7 (35%) | 8 (40%) | 5 (25%) | 7 (35%) | 8 (40%) |
| No | 41 (26.1%) | 85 (54.1%) | 31 (19.7%) | 66 (42%) | 71 (45.2%) | 20 (12.7%) | 66 (42%) | 71 (45.2%) | 20 (12.7%) | |
| p value (χ-square test) | 0.54 | <0.01 | <0.01 | |||||||
| Death | Yes | 1 (6.6%) | 6 (40%) | 8 (53.3%) | 1 (6.6%) | 10 (66.6%) | 4 (26.6%) | 0 (0%) | 9 (60%) | 6 (40%) |
| No | 44 (27.1%) | 89 (54.9%) | 29 (17.9%) | 78 (48.1%) | 56 (34.5%) | 28 (17.2%) | 71 (43.8%) | 69 (42.5%) | 22 (13.5%) | |
| p value (χ-square test) | 0.01 | <0.01 | <0.01 | |||||||
Evaluations
Two types of data were subjects of analysis. Preoperative data, such as age, height, weight, BMI, PSA concentration (immunoenzymatic Elecsys test; Cobas 6000 Hitachi), and postoperative data such as the extent of histopathologic differentiation of prostate tissue in Gleason score, extracapsular extension (pT3), the presence of lymph nodes metastases and the presence of positive surgical margins. Patients were under constant control in the Hospital Outpatient Clinic, thanks to which data concerning progression (biochemical recurrence, local recurrence, death) were also collected, and the cancer-specific survival time was determined. The total volume of plasma and the PSA mass were calculated on the basis of the formulas (Table 1) [5,6].
Statistics
All constant variables’ distributions were analyzed with regard to normality by means of Kolmogorov-Smirnov and Lilliefors tests. By means of descriptive statistics the following characteristics were determined: mean or median, standard deviation and maximal and minimal values.
In order to determine differences between the groups, where variables were of categorical character, χ-square test was used. In order to determine differences between a number of independent groups, where continuous variables have distribution other than normal, Kruskal-Wallis test was used.
A multiple regression model was created to assess the correlation between BMI and PSA concentration. Covariates in the model included age, the extent of prostate cancer differentiation in Gleason score, extracapsular extension (pT3) and positive surgical margins.
In order to evaluate and compare the odds ratio of biochemical recurrence together with the elevated PSA concentration or mass, 2 models of logistic regression were used. Covariates in the models included age, BMI, the extent of prostate cancer differentiation in Gleason score, nodal metastases, extracapsular extension (pT3) and positive surgical margins. As both the concentration and the PSA mass did not show normal distribution, logarithmic (decimal) transformation of data was performed. With the assistance of receiver operating characteristic (ROC), curves from 2 models of logistic regression were compared.
Cancer-specific survival of patients was evaluated by means of Kaplan-Meier analysis, while the significance of differences between them was evaluated by means of log-rank test.
For all statistical tests, the critical level of significance was adopted at p<0.05. The statistical analysis was calculated by means of StatSoft Statistica v. 8.0.
Results
Predictive value of PSA mass and PSA concentration
Increasing values of PSA mass and PSA concentration had a statistically significant influence on the following features: extracapsular extension, presence of metastases in the surrounding lymph nodes, frequency of positive surgical margins, presence of biochemical and local recurrence and the rate of death (Table 4).
Relationship between PSA concentration and BMI
The research demonstrated that PSA concentration and PSA mass do not differ in BMI groups (Table 3). Differences in preoperative PSA concentration between the 3 groups of patients are statistically insignificant (p=0.28). The total plasma volume is higher in obese patients (p<0.001). The model of multiple regression proved the lack of statistically significant correlation between preoperative PSA concentration and BMI (p=0.99). In patients with increasing BMI, pathologic stage T3 was more frequently observed (Table 4). After excluding from the analysis patients with stage T3, we observed a statistically significant negative correlation between BMI and PSA concentration (p=0.04) in multiple regression analysis.
Table 3.
Characteristics of patients in BMI, PSA concentration and PSA mass subgroups.
| BMI (kg/m2) | PSA (ng/ml) | PSA mass (μg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | I | II | III | I | II | III | ||
| Age (years) | Mean | 62.8 | 62.2 | 62.1 | 63 | 61.4 | 62.6 | 62.8 | 61.8 | 62.7 |
| SD | 6.7 | 5.9 | 6 | 5.7 | 6.9 | 5.5 | 5.8 | 6.5 | 6.0 | |
| Range | 50–76 | 48–74 | 49–71 | 49–74 | 48–76 | 52–72 | 49–74 | 48–76 | 52–72 | |
| p value (Kruskal-Wallis test) | 0.85 | 0.47 | 0.71 | |||||||
| BMI (kg/m2) | Mean | 23.4 | 27.4 | 32.6 | 27.1 | 28 | 27.4 | 26.9 | 27.8 | 28.2 |
| SD | 1.4 | 1.3 | 2.3 | 2.9 | 4.0 | 3.3 | 2.9 | 3.6 | 4.0 | |
| Range | 17.9–24.9 | 25–29.9 | 30.1–40.3 | 20–37.5 | 17.9–40.3 | 22.1–35 | 20–37.5 | 17.9–40.3 | 22.1–38 | |
| p value (Kruskal-Wallis test) | <0.001 | 0.32 | 0.16 | |||||||
| Plasma volume (liters) | Mean | 3.1 | 3.2 | 3.45 | 3.2 | 3.3 | 3.2 | 3.2 | 3.2 | 3.2 |
| SD | 0.13 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | |
| Range | 2.9–3.4 | 2.8–3.9 | 2.9–4.1 | 2.7–3.7 | 2.8–4.1 | 2.9–3.7 | 2.7–3.7 | 2.8–4.1 | 2.9–3.9 | |
| p value (Kruskal-Wallis test) | <0.001 | 0.21 | 0.43 | |||||||
| PSA concentration (ng/ml) | Mean | 12.8 | 14.1 | 14.2 | 6.4 | 14.2 | 31.3 | 6.1 | 14.1 | 32.6 |
| SD | 8.9 | 11.9 | 7.7 | 1.9 | 2.8 | 11.6 | 1.7 | 3.5 | 11.9 | |
| Range | 2.8–51.8 | 1.8–61.7 | 4.2–43.4 | 1.8–9.8 | 10–19.8 | 20.4–61.7 | 1.8–9.6 | 9–21.8 | 18–61.7 | |
| p value (Kruskal-Wallis test) | 0.28 | <0.001 | <0.001 | |||||||
| Gleason score | Median | 5 | 6 | 6 | 5 | 6 | 6 | 5 | 6 | 6 |
| p value (Kruskal-Wallis test) | 0.38 | 0.001 | 0.001 | |||||||
| PSA mass (μg) | Mean | 56.6 | 46.2 | 48.9 | 20.8 | 47 | 101.1 | 19.7 | 46.2 | 106.7 |
| SD | 27.4 | 39 | 25.1 | 6.37 | 10.3 | 37.1 | 5.6 | 11.3 | 36.5 | |
| Range | 31.9–156.6 | 6.5–196.6 | 13.7–129.5 | 6.5–32.7 | 30.3–71 | 64.2–196.6 | 6.5–29.8 | 30.3–69.6 | 70.7–196.6 | |
| p value (Kruskal-Wallis test) | 0.09 | <0.001 | <0.001 | |||||||
Cancer-specific survival
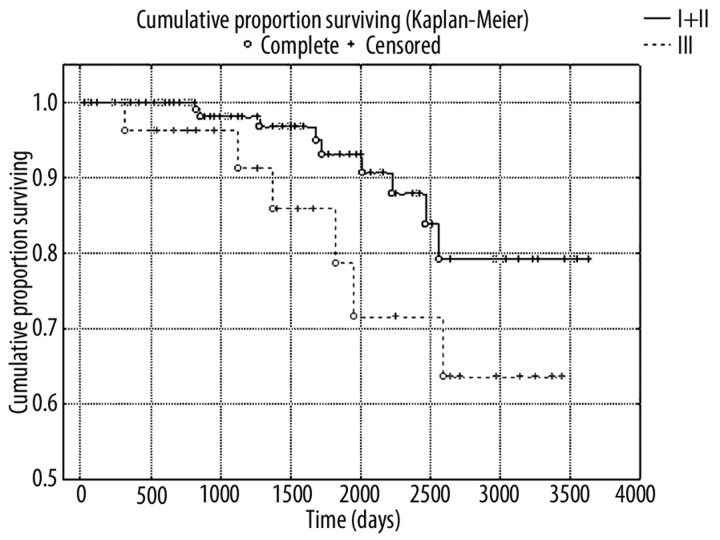
The study proved that the elevated preoperative value of PSA mass (p=0.02) is the factor that influences the cancer-specific survival of patients with prostate cancer after RP (Figure 1). Similarly higher values of PSA concentration had crucial impact on cancer-specific survival after RP (p=0.02)
Figure 1.
Kaplan-Meier analysis of cancer-specific survival in two groups of patients with high (≥70 μg) and low (<70 μg) values of PSA mass (I and II PSA mass groups combined due to lack of uncensored cases in group I) (log-rank test; p=0.02)
Odds ratio of biochemical recurrence after RP
The odds ratio (range) of biochemical recurrence, with the PSA mass increased 10 times, is equal to 10.9 (p<0.001 for the whole model) (Table 5). The odds ratio of biochemical recurrence, with the PSA concentration increased 10 times, is equal to 8.5 (p<0.001 for the whole model).
Table 5.
Model of logistic regression predicting biochemical recurrence after RP (p value=0.001).
| Odds ratio (range) | −95% confidence interval | +95% confidence interval | p value | |
|---|---|---|---|---|
| Age | 0.41 | 0.08 | 1.9 | 0.26 |
| BMI | 4.4 | 0.46 | 42.6 | 0.19 |
| PSA mass | 10.9 | 1.5 | 77.0 | 0.01 |
| Gleason score | 1.6 | 0.3 | 8.2 | 0.04 |
| pT3 | 2.5 | 1.1 | 5.7 | 0.02 |
| Positive surgical margin | 1.1 | 0.4 | 2.08 | 0.04 |
ROC curves
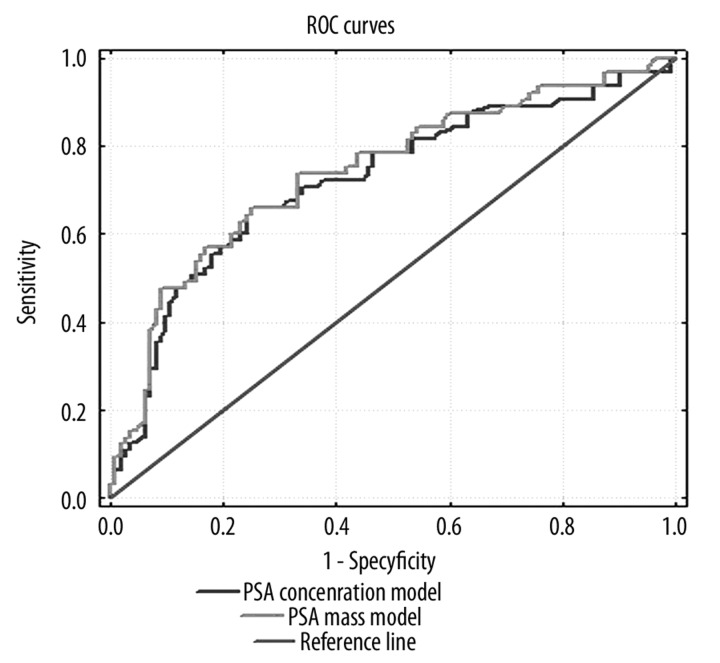
ROC curves for 2 models with PSA mass and PSA concentration showed an area under curve (AUC) of 0.74 and 0.69, respectively, for biochemical recurrence after RP (Figure 2). The difference (AUC) was statistically significant (p=0.04).
Figure 2.
The comparison of two ROC curves of models predicting biochemical recurrence after RP (p=0.04).
Discussion
There are various theories concerning the influence of obesity on the natural development, diagnostics or progression after radical treatment of prostate cancer. The 5-times increased percentage of biochemical recurrence observed in Afro-Americans, compared to Euro-Americans, is sometimes explained by a 3-times more frequent presence of overweight or obesity among the former [7]. Its influence is definitely negative, including the following:
difficulties in per rectum examination in obese patients [8],
dishormonose [9, 10] – abnormal hormone concentrations, which induces the intensification of diagnostics and at the same time postpones proper treatment,
comorbidities, which pushes the prostate diagnostics into the background and consequently patients suffer from more advanced forms of prostate cancer [11–13].
Some authors suggest another factor, namely, lower PSA concentration in obese patients [14,15]. The consequence of the aforesaid correlation may impact on prostate cancer diagnosis and evaluation of progression after its radical treatment. Other authors deny the abovementioned connection [16]. The authors who showed that obese patients are characterized by lower PSA concentration refer to the phenomenon of hemodilution. The supporters of this theory claim that obesity is characterized by a larger amount of circulating blood, so, theoretically, the constant PSA mass circulating in the organism would be dissolved in a large amount of plasma, resulting in a lower PSA concentration. This phenomenon has already been described [3,4]. However, our research did not show that the elevated BMI has a significant influence on the preoperative PSA concentration in the whole study group. There are 2 hypotheses to explain the inconsistency:
racial differences between the analyzed groups,
the fact that obese patients in our group had more advanced disease (pT3).
The following research has been done on a group of patients of Caucasian race, while the aforesaid research has been frequently based on ethnically heterogeneous groups. The cause of differences between the outcomes can result from the polymorphism of the androgen receptor, which causes higher PSA concentration in Afro-Americans, as well as statistically significant greater obesity in this group [17,18]. That fact may have influenced the results of the aforementioned authors, and therefore the correlation between PSA concentration and BMI was observed. The influence of ethnic differences can, of course, be dismissed by appropriate statistical manipulations; nevertheless, it seems that research done on homogenous groups is characterized by greater statistical power.
On the other hand, in our study group obese patients were more likely to have stage T3 disease. This surely influenced increased PSA concentration in this group of patients, despite the hemodilution phenomenon. This observation was confirmed after excluding patients with stage T3. This fact may hinder the utilization of PSA mass in practice, while its usefulness may be limited to organ-confined prostate cancers. However, we emphasize that analysis of ROC curves indicate good predictive value of PSA mass in the whole study group.
In comparison of both parameters (PSA concentration and the PSA mass) it must be stressed that the probability of biochemical recurrence after RP is better predicted by PSA mass, which surely results from the fact that the PSA mass includes the element eliminating the phenomenon of hemodilution. Despite the fact that both preoperative parameters “equally well” evaluate the progression after RP, the PSA mass seems to be a little more sensitive parameter (which is indicated by the difference in the odds ratio and AUC).
Conclusions
-
Increased preoperative value of the PSA mass is connected with:
more frequent cancer diagnosis of pT3 prostate cancer,
more frequent diagnosis of metastases in the surrounding lymph nodes,
more frequent recognition of the positive surgical margin,
shorter cancer-specific survival time,
higher percentage of progression.
The preoperative PSA mass is a better predictor of biochemical recurrence after RP than is PSA concentration.
Obese patients with organ-confined prostate cancer have lower PSA concentration due to the hemodilution phenomenon.
Footnotes
Source of support: The authors had no financial support
References
- 1.Kattan MW, Wheeler TM, Scardino PT. Postoperative nomogram for disease recurrence after radical prostatectomy for prostate cancer. J Clin Oncol. 1999;17:1499–507. doi: 10.1200/JCO.1999.17.5.1499. [DOI] [PubMed] [Google Scholar]
- 2.Kattan MW, Eastham JA, Stapleton AMF, et al. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766–71. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 3.Bañez LL, Hamilton RJ, Partin AW, et al. Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA. 2007;298:2275–80. doi: 10.1001/jama.298.19.2275. [DOI] [PubMed] [Google Scholar]
- 4.Vollmer RT, Humphrey PA. Tumor volume in prostate cancer and serum prostate-specific antigen: analysis from a kinetic viewpoint. Am J Clin Pathol. 2003;119:80–89. doi: 10.1309/UNAQ-JTFP-B1RQ-BQD4. [DOI] [PubMed] [Google Scholar]
- 5.Boer P. Estimated lean body mass as an index for normalization of body fluid volumes in humans. Am J Physiol. 1984;247:632–36. doi: 10.1152/ajprenal.1984.247.4.F632. [DOI] [PubMed] [Google Scholar]
- 6.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–71. [PubMed] [Google Scholar]
- 7.Spangler E, Zeigler-Johnson CM, Coomes M, et al. Association of obesity with tumor characteristics and treatment failure of prostate cancer in African-American and European American men. J Urol. 2007;178:1939–44. doi: 10.1016/j.juro.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 8.Bray GA. Obesity: the disease. J Med Chem. 2006;49:4001–7. doi: 10.1021/jm0680124. [DOI] [PubMed] [Google Scholar]
- 9.Hsing AW, Reichardt JK, Stanczyk FZ. Hormones and prostate cancer: current perspectives and future directions. Prostate. 2002;52:213–35. doi: 10.1002/pros.10108. [DOI] [PubMed] [Google Scholar]
- 10.Kaaks R, Lukanova A, Sommersberg B. Plasma androgens, IGF-1, body size, and prostate cancer risk: a synthetic review. Prostate Cancer Prostatic Dis. 2000;3:157–72. doi: 10.1038/sj.pcan.4500421. [DOI] [PubMed] [Google Scholar]
- 11.Gong Z, Agalliu I, Lin DW, et al. Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer. 2007;109:1192–202. doi: 10.1002/cncr.22534. [DOI] [PubMed] [Google Scholar]
- 12.Benez LL, Hamilton RJ, Vollmer RT, et al. Can hemodilution explain the lower PSA concentrations among obese men? J Urol. 2007;177(Supl.4):abs.4, 1418. [Google Scholar]
- 13.Rodriguez C, Patel AV, Calle EE, et al. Body mass index, height, and prostate cancer mortality in two large cohorts of adult men in the United States. Cancer Epidemiol Biomarkers Prev. 2001;10:345–53. [PubMed] [Google Scholar]
- 14.Werny DM, Thompson T, Saraiya M, et al. Obesity is negatively associated with prostate-specific antigen in U.S. men, 2001–2004. Cancer Epidemiol Biomarkers Prev. 2007;16:70–76. doi: 10.1158/1055-9965.EPI-06-0588. [DOI] [PubMed] [Google Scholar]
- 15.Baillargeon J, Pollock BH, Kristal AR, et al. The association of body mass index and prostate-specific antigen in a population-based study. Cancer. 2005;103:1092–95. doi: 10.1002/cncr.20856. [DOI] [PubMed] [Google Scholar]
- 16.Freedland SJ, Platz EA, Presti JC, Jr, et al. Obesity, serum prostate specific antigen and prostate size: implications for prostate cancer detection. J Urol. 2006;175:500–4. doi: 10.1016/S0022-5347(05)00162-X. [DOI] [PubMed] [Google Scholar]
- 17.Saraiya M, Kottiri BJ, Leadbetter S, et al. Total and percent free prostate-specific antigen levels among U.S. men, 2001–2002. Cancer Epidemiol Biomarkers Prev. 2005;14:2178–82. doi: 10.1158/1055-9965.EPI-05-0206. [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Meyers DA, Sterling DA, et al. Association studies of serum prostate-specific antigen levels and the genetic polymorphisms at the androgen receptor and prostate-specific antigen genes. Cancer Epidemiol Biomarkers Prev. 2002;11:664–69. [PubMed] [Google Scholar]