Summary
Background
Endothelial dysfunction is an independent predictor of future cardiac events.
Material/Methods
We evaluated the relationship between flow-mediated dilation (FMD) in brachial artery and coronary risk factors in 93 patients (70 males, mean age: 62±8 years) with ACS treated with primary angioplasty (PCI). The patients were divided into 2 subgroups: 43 patients with diabetes mellitus type 2 (DM) and 50 non-diabetics (non-DM). Patients were examined on the 3rd day after ACS and after 6 months. FMD on the 3rd day were significantly lower in DM than in non-DM (5.8±2.2% vs. 8.8±4.9%, p=0.0007) and after 6 months (6.2±2.6% vs. 9.4±4.4%, p<0.0001). It was also observed that the improvement of FMD in both groups after a 6-month follow-up inversely correlated with the increase of left ventricular end-diastolic volume (LVEDV) (r=−0.41, p<0.001).
Results
There was an inverse relationship between FMD and age (r=−0.26, p<0.01), BMI (r=−0.26, p<0,005), total cholesterol (r=−0.56, p<0.001) and LDL cholesterol (r=−0.53, p<0.001). There was no relationship between triglycerides, hypertension and history of smoking. In the DM group, FMD negatively correlated with HbA1c (r=−0.68, p<0.001). Restenosis rate was significantly higher in the DM group (19% vs. 6%, p<0.001) but there was no relationship between FMD and restenosis.
Conclusions
Impaired FMD is more significant in diabetics than in non-diabetic patients with ACS. Lack of improvement of FMD after acute coronary syndrome can be a predictor of detrimental left ventricular remodeling in patients with ACS.
Keywords: flow-mediated dilatation, endothelium, diabetes mellitus, acute coronary syndrome, left ventricular remodeling
Background
The vascular endothelium plays an integral role not only in the regulation of vascular tone, but also in the prevention and formation of thrombus and inflammation [1]. Endothelial dysfunction is associated with coronary risk factors and atherosclerosis, and it has close pathophysiological relation with acute coronary syndromes [2,3]. Endothelial dysfunction has been shown in patients with documented atherosclerosis, but it is also an early step in the pathogenesis of the atherosclerotic cascade [4,5]. Among various methods of assessing endothelial function, endothelium-dependent flow-mediated dilation is a non-invasive, highly reproducible, simple method based on high sensitivity ultrasound waves [4,6,7]. The left ventricular remodeling is a gradual change in the left ventricular size, morphology and function induced by the myocardial mechanics, neuro-hormones and genes after infarction [8,9]. It begins several hours after infarction and becomes severe 2 weeks after infarction and continues to proceed, and is associated with higher mortality and higher incidence of heart failure [8,10].
This study evaluated the relationship between flow-mediated dilation in brachial artery and coronary risk factors in diabetic and non-diabetic patients with acute coronary syndromes (ACS).
Material and Methods
Patient population
Ninety-three patients with troponin-positive ACS, ST-elevation, and non-ST elevation myocardial infarction treated with primary angioplasty with bare-metal stent implantation (PCI) (70 males, 23 females), ages 46–75 years (mean: 62±8), were recruited. Diagnosis of ACS was con-firmed angiographically as >70% stenosis in at least 1 major epicardial coronary artery, which was qualified for PCI. The patients were divided into 2 subgroups: 43 patients with diabetes mellitus type 2 and 50 non-diabetics. Diabetes mellitus (DM) diagnosis was established as a previous history of DM, use of antidiabetic agents, or on the basis of positive oral glucose tolerance test performed 1 month after ACS. All study subjects underwent a complete physical examination, biochemical tests, and echocardiography. Vascular endothelial function was measured in the brachial artery by flow-mediated dilatation (FMD) technique. Patients with coronary artery narrowing not suitable for PCI with not completely successful (TIMI III) blood flow after PCI, using a drug eluting stent, with left ventricular dysfunction (ejection fraction <45%) or past myocardial infarction, were excluded from the study. The study protocol was approved by the local Ethics Committee.
Echocardiographic and vascular study
All patients were examined on the third day after ACS, and follow-up appointments were scheduled 3 and 6 months after ACS. Doppler echocardiography was performed according to The American Society of Echocardiography guidelines, using Siemens Sequoia C512 equipment (Accson Simens, Mountain View, CA, USA). FMD was accomplished according to the guidelines for sonographic assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery [11]. Right brachial artery diameter was measured with an 8.0 MHz transducer. Scans were taken at rest, during reactive hyperemia (FMD flow-mediated dilation: endothelial-dependent stimulus to vasodilatation), then again at rest and after sublingual nitroglycerin (NTG: endothelium-independent vasodilatation). The skin was marked and arterial flow velocity was measured at rest using a pulsed Doppler signal. Blood flow through the brachial artery was altered with a sphygmomanometer cuff placed on the forearm 5–8 cm distal to the site of brachial artery measurement. By inflating the cuff to 250 mmHg, the distal circulation was stopped and the flow through the brachial artery was reduced. By deflating the cuff after 5 min of inflation, the flow through the brachial artery increased (reactive hyperemia). The brachial artery diameter was measured 60 s after cuff deflation, and 10 min later the second rest scan was recorded. Nitroglycerin (0.5 mg) was then administered sublingually, and the artery was scanned 5 min later. All images were analyzed by the same physician who was unaware of the clinical details. Artery diameter measurements were made at end-diastole (peak of R wave on electrocardiogram) using electronic calipers. Five cardiac cycles were analyzed, and measurements were averaged. Brachial artery diameter measurements after reactive hyperemia were taken 60 s after cuff deflation. FMD and NTG were calculated as follows:
where: VDhyperaemia – brachial artery diameter after cuff deflation, VDrest – brachial artery diameter at rest, VDntg – brachial artery diameter after nitroglycerin.
Biochemical tests
Lipid parameters (serum cholesterol, HDL cholesterol, LDL cholesterol, triglycerides) and glycated hemoglobin (HbA1c) were obtained using routine laboratory methods.
Statistical analysis
Statistical analyses were performed with StatsDirect statistical software (England: StatsDirect Ltd. 2008). Continuous variables are displayed as mean ± standard deviation and categorical variables were displayed as percentage. The Shapiro-Wilk test revealed non-normal distribution. Mann-Whitney U test, Wilcoxon rank test and Pearson correlation test were performed. The value of p<0.05 was considered as statistically significant.
Results
Demographic characteristics of the study population are presented in Table 1.
Table 1.
Demographic characteristics of the study population.
| DM (n=43) | Non-DM (n=50) | p value | |
|---|---|---|---|
| Age (years) | 61±8 | 62±8 | ns |
| BMI (kg/m2) | 31.2±4.3 | 30.7±4.6 | ns |
| History of smoking (%) | 78% | 76% | ns |
| Hypertension (%) | 68% | 66% | ns |
| LVEF (%) | 50.2±3.3 | 50.5±3.7 | ns |
| TG level (mmol/l) | 1.81±0.83 | 1.76±0.98 | ns |
| TC level (mmol/l) | 6.39±1.33 | 6.29±1.23 | ns |
| LDL-C level (mmol/l) | 4.48±1.19 | 4.17±1.12 | ns |
| HDL-C level (mmol/l) | 0.91±0.76 | 0.93±0.54 | ns |
| HbA1c (%) | 7.26±1.32 | -------- | ------- |
BMI – body mass index; LVEF – left ventricular ejection fraction; TC – total cholesterol; TG – triglyceride; HDL-C – high-density lipoprotein-cholesterol; LDL-C – low-density lipoprotein-cholesterol; HbA1c – glycated hemoglobin.
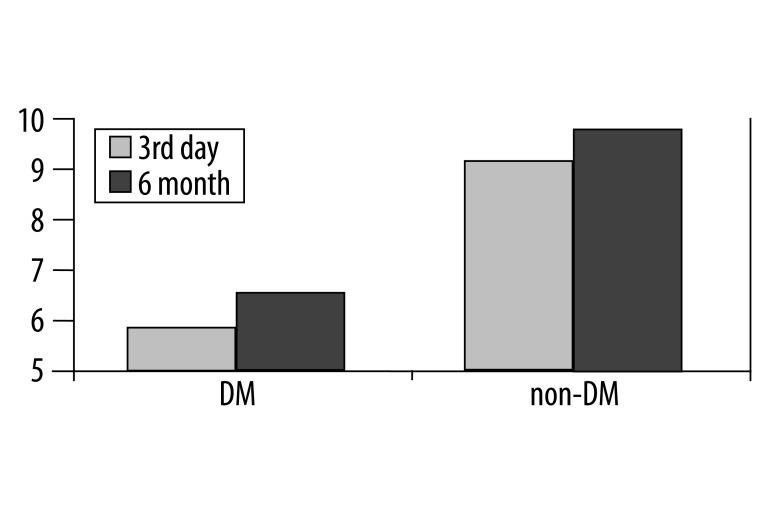
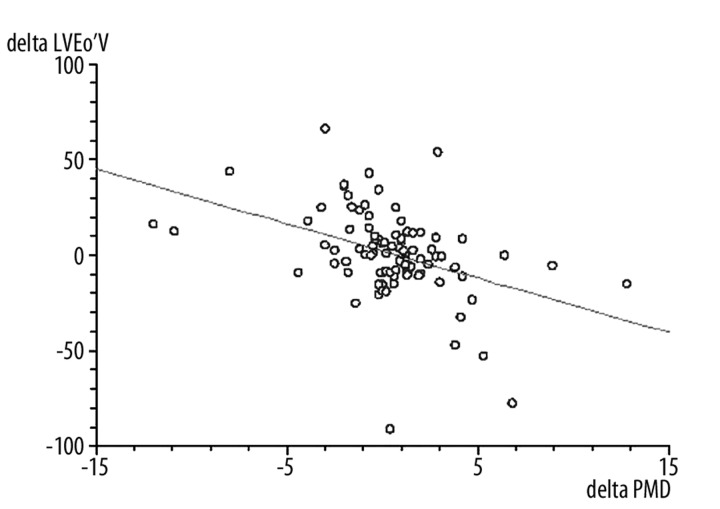
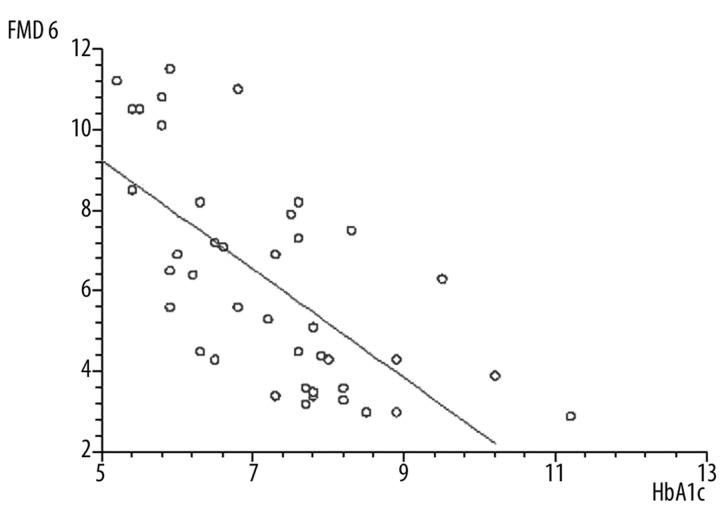
The values of FMD on the day 3 after ACS were significantly lower in patients with DM than in patients without DM (5.8±2.2% vs. 8.8±4.9%, p=0.0007), and this relationship was also significant after 6 months (6.2±2.6% vs. 9.4±4.4%, p<0.0001) (Figure 1). We did not observe any significant changes in FMD measured on day 3 after ACS and 6 months later in non-DM patients (8.8±4.9% vs. 9.4±4.4%, ns), but the patients with DM FMD significantly improved after 6 months (5.8±2.2% vs. 6.2±2.6%, p=0.04). The improvement of FMD in both groups after a 6-month follow-up inversely correlated with the increase of left ventricular end-diastolic volume (LVEDV) (r=−0.41, p<0.001) (Figure 2). There was no significant correlation between FMD and left ventricular ejection fraction 6 months after PCI. In the univariate analysis, there was an inverse relationship between FMD and age (r=−0.26, p<0.01), BMI (r=−0.26, p<0.005), total cholesterol level (r=−0.56, p<0.001) and LDL cholesterol level (r=−0.53, p<0.001). However, there was no relationship between triglycerides, hypertension and history of smoking. In the DM group, FMD negatively correlated with HbA1c (r=−0.68, p<0.001) (Figure 3).
Figure 1.
The values of flow-mediated dilatation (FMD) on day 3 and 6 months after acute coronary syndrome in patients with DM (DM) and patients without DM (non-DM).
Figure 2.
Negative correlation between the improvement of flow-mediated dilatation (FMD) and left ventricular end-diastolic volume (LVEdV) (r=−0.41, p<0.001). delta LVEdV = LVEdV after 6 months minus LVEdV on day 3; delta FMD = FMD after 6 months minus FMD on day 3.
Figure 3.
Negative correlation between flow-mediated dilatation (FMD) and glycated hemoglobin (HbA1c) in patients with diabetes mellitus (r=−0.68, p<0.001). FMD 6 – flow-mediated dilatation 6 months after acute coronary syndrome.
Distribution of coronary arteries undergoing PCI is presented in Table 2. Clinical examination revealed coronary artery restenosis, confirmed angiographically, in 8 patients with DM and 3 patients without DM (Table 3). Restenosis rate was significantly higher in the DM group (19% vs. 6%, p<0.001). There was no relationship between FMD and restenosis.
Table 2.
Coronary artery angioplasty (PCI).
| DM (n= 43) | Non-DM (n= 50) | |
|---|---|---|
| LAD | 19 | 33 |
| Cx | 11 | 7 |
| RCA | 13 | 10 |
LAD – left anterior descending artery; Cx – circumflex artery; RCA – right coronary artery.
Table 3.
Restenosis in coronary arteries.
| DM (n= 43) | Non-DM (n= 50) | |
|---|---|---|
| LAD | 4 | 2 |
| Cx | 3 | 0 |
| RCA | 1 | 1 |
LAD – left anterior descending artery; Cx – circumflex artery; RCA – right coronary artery.
Discussion
Numerous studies have investigated the relationship between endothelial dysfunction and coronary risk factors. Most revealed a negative correlation between FMD and hyperlipidemia, hypertension, blood pressure, age, BMI or smoking [6,12–14]. Our study analyzed FMD in ACS patients with and without DM, and showed that DM patients had a significantly lower FMD during ACS than did nondiabetics. Previous studies have also shown that DM was an independent factor of endothelial dysfunction [15–17]. We observed a significant improvement of FMD 6 months after ACS only in the DM group, but it did not change among patients without DM.
There are only a few published studies of FMD during ACS. Kartzis et al presented findings that impaired FMD in men who had acute coronary syndromes without ST-segment elevation was an independent predictor of future cardiovascular events such as cardiovascular death, myocardial infarction, stroke and unstable angina [18]. Similarly, Patti et al. [19] showed that impaired FMD independently predicted the occurrence of in-stent restenosis in patients undergoing PCI. This was the first prospective study demonstrating that impaired FMD is an independent predictor of in-stent restenosis in patients with single-vessel CAD undergoing PCI. Although there are several proofs of a close relationship between endothelial dysfunction and atherosclerosis [20,21], the role of endothelium in the process of restenosis after stent implantation is still unclear [22]. Previous studies have found an independent correlation between coronary endothelial function and risk of cardiovascular events [23], but there are no data on the possible association between endothelial dysfunction in the coronary circulation and the risk of restenosis. In our study we did not observe any correlation between in-stent restenosis and FMD. Although FMD was significantly lower in the DM group, and we also noted higher restenosis rate in this group, the statistical analysis did not reveal any correlation between restenosis and FMD. The presented relationship between FMD and different coronary risk factors (eg, DM, total cholesterol, LDL cholesterol, BMI and age) confirmed complex, multifactor pathogenesis of endothelial dysfunction. On the other hand, in-stent restenosis due to intimal hyperplasia occurs after PCI with stent dependently on various clinical, angiographic, and procedural features. Therefore, simple stratification of patients according to the risk of the development of in-stent restenosis is very difficult. The mechanisms involved in the pathogenesis of in-stent restenosis include: platelets and inflammatory cell activation due to procedural vascular injury with subsequent local release of cytokines and growth factors, leukocyte adherence, smooth muscle cell proliferation, and extracellular matrix synthesis [24]. FMD is predominantly influenced by NO synthesis and release from the endothelial monolayer. In addition, FMD depends on the local release from other endothelium-derived vasodilators [25]. Hafner et al could neither observe a difference in FMD values regarding restenosis after a 1-year observational period nor confirm FMD as an independent risk factor for restenosis in peripheral arterial disease [26].
The present study confirms the role of diabetes mellitus as a known risk factor for restenosis. It seems that FMD could be useful as an indicator of global cardiovascular risk in patients with coronary artery disease, rather than as a marker of coronary stent restenosis.
Another interesting result of this study is the relationship between the change of FMD and left ventricular end-diastolic volume (LVEDV) during the 6 months following ACS. We noted that the decline of FMD correlated with increase in LVEDV. Left ventricular dilation and increase in LVEDV is a surrogate of left ventricular remodeling used in many studies [8,10]. Nakamura et al found that in patients with heart failure, muscular conduit artery remodeling occurs with alterations in arterial wall elastic properties, and this vascular wall functional change may be related to endothelial dysfunction [27]. Otherwise, peripheral vascular function in patients with symptomatic heart failure is characterized by increased vascular tone at rest and impaired dilatory response to vasodilator stimuli. Previous studies showed that the mechanisms behind increased vascular tone are caused by activation of neurohumoral factors, such as the renin-angiotensin system, the sympathetic nervous system, and endothelin [28,29]. Most probably, we observed the decline of FMD resulting from neurohormonal activation in the group with left ventricular remodeling. Poelzl et al. found a correlation between FMD and the severity of CHF, and suggested that increased plasma levels in endothelin-1 might play a role in the vascular remodeling process [30]. Observations of Kitta et al. suggest that patients with CAD and persistently impaired FMD, despite optimized therapy to reduce known risk factors, have high risk of future cardiovascular events such as cardiac death, nonfatal myocardial infarction, recurrent and refractory angina pectoris requiring coronary revascularization, or ischemic stroke [31]. Meta-analysis of 14 studies recently published by Inaba et al provides strong evidence that the impairment of brachial FMD is significantly associated with future cardiovascular events [32]. Our findings offer further evidence of the prognostic utility of FMD assessment in acute coronary syndromes. Assessment of this non-invasive indicator of endothelial function is plausible, and adds valuable information for risk stratification and therapeutic strategy for patients with an acute coronary syndrome.
Conclusions
Impaired flow-mediated dilatation, which represents endothelial dysfunction, is more significant in diabetics than in non-diabetic patients with acute coronary syndrome. Aside from diabetes, the relationship between different coronary risk factors and endothelial dysfunction has been shown in many studies, but in the present study we also showed that the lack of improvement of flow-mediated dilatation after acute coronary syndrome can be a predictor of detrimental left ventricular remodeling in patients with acute coronary syndrome treated with coronary angioplasty. On the other hand, we did not observe any relationship between flow-mediated dilatation and restenosis. These results need to be validated with further large-scale and long-term studies.
Footnotes
Source of support: This work was supported by the Ministry of Science and Higher Education research grant No 2 P05B 050 30
References
- 1.Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323(1):27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 2.Martinovic I, Abegunewardene N, Seul M, et al. Elevated monocyte chemoattractant protein-1 serum levels in patients at risk for coronary artery disease. Circ J. 2005;69(12):1484–89. doi: 10.1253/circj.69.1484. [DOI] [PubMed] [Google Scholar]
- 3.Takase B, Hamabe A, Satomura K, et al. Comparable prognostic value of vasodilator response to acetylcholine in brachial and coronary arteries for predicting long-term cardiovascular events in suspected coronary artery disease. Circ J. 2006;70(1):49–56. doi: 10.1253/circj.70.49. [DOI] [PubMed] [Google Scholar]
- 4.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340(8828):1111–15. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 5.Ross R. The pathogenesis of atherosclerosis – an update. N Engl J Med. 1986;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- 6.Celermajer DS, Sorensen KE, Bull C, et al. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24(6):1468–74. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 7.Stout M. Flow-mediated dilatation: a review of techniques and applications. Echocardiography. 2009;26(7):832–41. doi: 10.1111/j.1540-8175.2009.00927.x. [DOI] [PubMed] [Google Scholar]
- 8.Gaudron P, Eilles C, Kugler I, Ertl G. Progressive left ventricular dysfunction and remodeling after myocardial infarction. Potential mechanisms and early predictors. Circulation. 1993;87(3):755–63. doi: 10.1161/01.cir.87.3.755. [DOI] [PubMed] [Google Scholar]
- 9.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101(25):2981–88. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 10.Bolognese L, Neskovic AN, Parodi G, et al. Left ventricular remodeling after primary coronary angioplasty: patterns of left ventricular dilation and long-term prognostic implications. Circulation. 2002;106(18):2351–57. doi: 10.1161/01.cir.0000036014.90197.fa. [DOI] [PubMed] [Google Scholar]
- 11.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 12.Benjamin EJ, Larson MG, Keyes MJ, et al. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation. 2004;109(5):613–19. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 13.Tanriverdi H, Evrengul H, Kuru O, et al. Cigarette smoking induced oxidative stress may impair endothelial function and coronary blood flow in angiographically normal coronary arteries. Circ J. 2006;70(5):593–99. doi: 10.1253/circj.70.593. [DOI] [PubMed] [Google Scholar]
- 14.Ishibashi Y, Takahashi N, Shimada T, et al. Short duration of reactive hyperemia in the forearm of subjects with multiple cardiovascular risk factors. Circ J. 2006;70(1):115–23. doi: 10.1253/circj.70.115. [DOI] [PubMed] [Google Scholar]
- 15.Kirma C, Akcakoyun M, Esen AM, et al. Relationship between endothelial function and coronary risk factors in patients with stable coronary artery disease. Circ J. 2007;71(5):698–702. doi: 10.1253/circj.71.698. [DOI] [PubMed] [Google Scholar]
- 16.Djaberi R, Beishuizen ED, Pereira AM, et al. Non-invasive cardiac imaging techniques and vascular tools for the assessment of cardiovascular disease in type 2 diabetes mellitus. Diabetologia. 2008;51(9):1581–93. doi: 10.1007/s00125-008-1062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simova II, Denchev SV, Dimitrov SI, Ivanova R. Endothelial function in patients with and without diabetes mellitus with different degrees of coronary artery stenosis. J Clin Ultrasound. 2009;37(1):35–39. doi: 10.1002/jcu.20532. [DOI] [PubMed] [Google Scholar]
- 18.Karatzis EN, Ikonomidis I, Vamvakou GD, et al. Long-term prognostic role of flow-mediated dilatation of the brachial artery after acute coronary syndromes without ST elevation. Am J Cardiol. 2006;98(11):1424–28. doi: 10.1016/j.amjcard.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 19.Patti G, Pasceri V, Melfi R, et al. Impaired flow-mediated dilation and risk of restenosis in patients undergoing coronary stent implantation. Circulation. 2005;111(1):70–75. doi: 10.1161/01.CIR.0000151308.06673.D2. [DOI] [PubMed] [Google Scholar]
- 20.Cox DA, Vita JA, Treasure CB, et al. Atherosclerosis impairs flow-mediated dilation of coronary arteries in humans. Circulation. 1989;80(3):458–65. doi: 10.1161/01.cir.80.3.458. [DOI] [PubMed] [Google Scholar]
- 21.Quyyumi AA. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med. 1998;105(1A):32S–39S. doi: 10.1016/s0002-9343(98)00209-5. [DOI] [PubMed] [Google Scholar]
- 22.Wu TC, Chen YH, Chen JW, et al. Impaired forearm reactive hyperemia is related to late restenosis after coronary stenting. Am J Cardiol. 2000;85(9):1071–76. doi: 10.1016/s0002-9149(00)00698-6. [DOI] [PubMed] [Google Scholar]
- 23.Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106(6):653–58. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 24.Ferns GA, Avades TY. The mechanisms of coronary restenosis: insights from experimental models. Int J Exp Pathol. 2000;81(2):63–88. doi: 10.1046/j.1365-2613.2000.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42(7):1149–60. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 26.Hafner F, Seinost G, Gary T, et al. Are flow-mediated vasodilatation and intima-media thickness of the brachial artery associated with restenosis after endovascular treatment of peripheral arterial occlusive disease? Eur Radiol. 2010;20(10):2533–40. doi: 10.1007/s00330-010-1801-z. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura M, Sugawara S, Arakawa N, et al. Reduced vascular compliance is associated with impaired endothelium-dependent dilatation in the brachial artery of patients with congestive heart failure. J Card Fail. 2004;10(1):36–42. doi: 10.1016/s1071-9164(03)00585-2. [DOI] [PubMed] [Google Scholar]
- 28.Katz SD, Krum H, Khan T, Knecht M. Exercise-induced vasodilation in forearm circulation of normal subjects and patients with congestive heart failure: role of endothelium-derived nitric oxide. J Am Coll Cardiol. 1996;28(3):585–90. doi: 10.1016/0735-1097(96)00204-5. [DOI] [PubMed] [Google Scholar]
- 29.Rudic RD, Shesely EG, Maeda N, et al. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest. 1998;101(4):731–36. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poelzl G, Frick M, Huegel H, et al. Chronic heart failure is associated with vascular remodeling of the brachial artery. Eur J Heart Fail. 2005;7(1):43–48. doi: 10.1016/j.ejheart.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Kitta Y, Obata JE, Nakamura T, et al. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol. 2009;53(4):323–30. doi: 10.1016/j.jacc.2008.08.074. [DOI] [PubMed] [Google Scholar]
- 32.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 2010;26(6):631–40. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]