Summary
Background
Functional classification systems generally divide children with cerebral palsy (CP) into mild, moderate, and severe types. Although depending on functional limitations, they do not seem to evaluate abnormal postural patterns in standing. Since the most asymmetrical patterns can be observed in hemiplegia, the goal of this case series study was to provide their objective analysis and to establish any potential clinical value for evaluation and management of CP.
Material/Methods
A group of 36 children (aged 5–10 years) with spastic hemiplegic CP, who could stand and ambulate independently, were selected. The photogrammetric and pedobarographic studies were obtained for the postural analysis in standing.
Results
Two different anti- and pro- gravitational postural patterns were identified. They seem not only to affect functional status and rehabilitation potential, but also clinical value for evaluation and management of CP hemiplegia.
Conclusions
The importance of strong study design cannot be overemphasized. The 2 different postural patterns indicate dissimilar compensatory tendencies, which may help in prognosis of deformity and functional outcomes of rehabilitation. The use of objective photogrammetric and the pedobarographic studies may also help to develop a more specific therapeutic intervention in order to facilitate the pattern leading towards better outcome (orthosis in the anti-gravitational postural pattern vs focal spasticity management in the pro-gravitational postural pattern).
Keywords: unilateral spastic cerebral palsy, alignment of the body segments, abnormal postural patterns
Background
The inclusion of postural evaluation into the physical examination of children with cerebral palsy (CP) is increasingly recommended by many specialists.
Bax et al. describes this condition as “…a group of disorders of the development of movement and posture causing activity limitation, that are attributed to non-progressive disturbances that occurred in the developing fetal or infant brain,” and recommends that “…all body regions – including head, trunk, shoulder girdle, pelvic girdle, and lower limbs – be described in terms of any impairment of movement or posture” [1]. As efficient postural control is important for the performance of voluntary skills, postural abnormalities could contribute to the delays and impairments seen in the motor skills of children with CP [2–6]. At the same time, abnormal postural attitudes might result from the need to cope with specific primary deficits such as poor balance control or muscle weakness, so that adaptive or substitutive postural changes will become part of the clinical picture of CP [7–14].
Children with CP often experience a reduced range of motion in joints and contractures of the hip, knee, and ankle muscles, which may contribute to atypical postural presentation in standing. This atypical alignment can also be expressed as a change in the position of the body with reference to gravity and the base of support (BOS). Asymmetric alignment in standing is often present in children with unilateral neurological lesions, such as unilateral spastic CP (spastic hemiplegia) [15]. Although the above observations appear to have remarkable clinical relevance (e.g., for a programming of targeted therapeutic interventions), the available data on functional impairment in CP are based on a limited number of studies aimed at describing postural conditions and possible disturbances of the different types of postural control mechanisms. Our study addressed these points, in particular the characteristics of postural alignment of body segments during standing.
The present study is the first part of a broader research project on complex functional assessment of CP children, titled “Postural control in sitting, standing and walking in children with CP.” The goal of the present study was to provide a descriptive analysis of postural patterns in children with spastic hemiplegia.
Material and Methods
Methodology
The protocol was approved by the Bioethical Committee of the Medical University of Silesia in Katowice, Poland. Prior to the study, legal guardians had signed an informed consent for every participating child.
Participants
Subjects were selected from children receiving therapy at 3 children’s rehabilitation centers in Silesia, Poland. The criteria for selection included spastic hemiplegia in children who were able to stand and ambulate independently. From 3 Silesian rehabilitation centers, we chose 41 CP children who met the criteria, and 36 parents gave consent for their children to participate in the study (18 girls and 18 boys; 20 with right and 16 with left hemiplegia). The baseline age ranged from 5 to 10 years (mean value: 8 years and 2 months). According to the Gross Motor Function Classification System (GMFCS), 82% of the participants were distributed in level I, and only 18% in level II. Each child was examined and diagnosed by a board certified neurologist. The evaluation based on GMFCS was established by the physical therapists who managed the individual cases.
Method
The research was conducted from June through December 2008 and consisted of the measurement and assessment of postural patterns in hemiplegic children during relaxed standing. The postural patterns refer to the relationship of the selected body segments, as well as to the position of the body with reference to gravity and BOS. Basic posturometric parameters, which determine both the position of the body and the relationship between body segments, were measured using Moiré fringe topography for 3-dimensional analysis of spine deformities [16], as well as trunk, shoulder, and pelvic disorders [17,18]. In medicine, the Moiré fringe topography is a method of 3-dimensional morphometry, in which contour maps (photograms) are produced on the body surface from the overlapping interference fringes created when a human body is illuminated by beams of coherent light coming from 2 different point sources (modified from Biology-online.org). These maps can be scanned into the computer and analyzed by software, which can calculate many parameters of spatial relationships of different body landmarks pertinent to the physical examination of body posture. The semi-automatic analysis of the maps (landmarks are manually marked on the body surface) allows us to quickly obtain quite detailed morphometric information on body posture [19]. The software can not only provide numeric data on length and depth of the spinal curvatures, but also can compute different clinically significant ratios between them (eg, compensatory index, kyphosis-to-lumbar lordosis ratio, frontal asymmetry index [15–19].
The research literature on Moiré fringe topography shows a high clinical value for evaluation and documentation of scoliosis [20]. It is an inexpensive, simple, noninvasive imaging technique with a significantly high correlation with Cobb angle, except for subjects with severe curvature or significant obesity [21–23]. Suzuki and Inami have also demonstrated significant correlations between many postural indicators of asymmetry [17,18]. Goldberg et al. have shown high Cobb angle correlation with rib hump and with Posterior Trunk Symmetry Index, estimating the sensitivity and specificity at 100% and 45%, respectively [24].
Procedures
The analysis of Moiré topograms begins with marking the following anatomical landmarks:
inferior scapular angles bilaterally (“scapular line”),
spinous processes from C7 to L5 along posterior median line (“spinal line”),
posterior superior iliac spine bilaterally (PSIS) (“pelvic line”).
The Moiré topograms were then analyzed using a semi-automated computer system provided by CQ Electronic System, Artur Œwierc, (Poland) to obtain [19]:
a compensatory index that presented the difference between the angle of kyphosis and lordosis (K-L),
the angle of scoliosis (SCOL),
the angle of inclination of the scapular line (SL) and pelvic lines (PL) against the horizontal axis,
he angle between the scapular and pelvic lines (SL/PL),
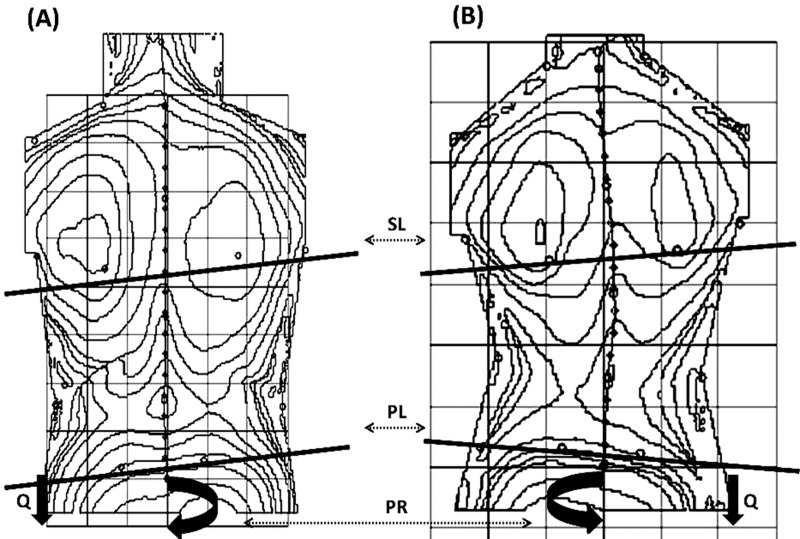
the angle of pelvic rotation in the horizontal plane (PR) (Figure 1).
Figure 1.
Position of particular body segments in 2 postural patterns, anti-gravitational and pro-gravitational, were measured in the right spastic hemiplegia using Moiré Topography for 3-dimensional analysis of scapular line (SL), pelvic line (PL), and pelvis rotation (PR), and the body weight distribution on the BOS showed the overloaded body side (Q).
All topograms were supplemented with a digital pictogram of each child’s lower extremities, taken posteriorly in the coronal plane. In addition, the analysis of the weight distribution on the base of support (BOS) between affected and unaffected body side, as well as their center of pressure (COP), were done simultaneously. A force plate PDM, ZEBRIS (Germany) with FootPrint software was applied for these types of pedobarographic measurements [15,25–28]. All measurements were taken twice. For statistical analysis, the mean values of tested parameters were selected. Since 1992, we have researched and utilized the Moiré fringe topography in children with scoliosis and cerebral palsy [29–31], and since 2000 have also studied the postural control mechanisms in these children [32–36].
Data analysis
The analysis covered the comparison between 2 groups of hemiplegic children, since they expressed 2 dissimilar postural patterns. Student’s t-test (Statistica 7.0) was used for statistical analysis.
Results
None of the subjects met the postural symmetry criteria in either the coronal plane in reference to the BOS or the median axis alignment to the BOS during quiet stance. The values and distribution of those asymmetries differed individually. Asymmetries were seen primarily on the affected body side. Some of the examined children demonstrated characteristic spatial relationships of the SL/PL ratio. Both were much higher than the respective contralateral (corresponding points) body segments (up to 20° of inclination of the scapular line and up to 40˚ of inclination of the pelvis line). However, the other children presented a quite different trend - the shoulder was on the same level or slightly above (0–5°), and the pelvis much farther below in relation to the respective contralateral segments (up to 34°).
Postural analysis in the horizontal plane showed a similar differentiation of abnormalities. The pelvic rotation angle on the affected side gave 2 opposite extreme values – from 34° of external rotation in one subgroup, to 40° of internal rotation in another subgroup. Postural analysis in the sagittal plane yielded the fewest abnormalities. The maximum values of the compensatory index (reflecting the dominance of kyphosis or lordosis in body posture) ranged from –35° to 41°, which allowed us to define one subgroup as kyphotic and another subgroup as lordotic, respectively. The evident postural disturbance of symmetry expressed itself as mechanical spinal axis deviation from the anatomical midline axis. In all cases, the lateral spinal curvatures were apparent, with convexity towards the unaffected side (from 7° to 38°, 17.11°±9.23). S-type scoliosis was found in 72% (65% of primary thoracic and 35% of primary lumbar), and C-type scoliosis located in either the lumbar or thoraco-lumbar region was detected in 28% of all study participants. All the examined children with the C-type had a tendency to stand with weight displaced to one side (the “overloaded side”). The body weight distribution on the BOS showed the “overloaded” leg received on average 61.56% ±8.92 of total body mass. Surprisingly, only half of the subjects tended to stand with weight displaced toward the non-involved and the other half toward the involved side. The proportional (percentage) distribution of body weight on BOS between the “overloaded” and the “underloaded” leg in independent standing was the criterion dividing subjects into 2 subgroups: 18 children (50% of the subjects) tended to stand with weight displaced toward the non-involved side, and 18 children (50% of the subjects) tended to stand with weight displaced toward the involved side. This breakdown strongly influenced the results of postural analysis.
Based on the weight bearing on the affected body side, the participants were divided into 2 subgroups according to the above criterion (50% and 50% of the subjects, respectively):
18 children with tendency to underload the affected body side (UNL children),
18 children with tendency to overload the affected body side (OVL children).
We observed that the type of distribution of the body mass between the affected and unaffected body sides determined the characteristic spatial relations of the shoulder/pelvic line, pelvis rotation, and type of scoliosis. The scapular and pelvic lines (SL and PL) were close to the parallel in the UNL children, and the angular values of the inclination ratio (SL/PL) had either positive or negative sign (i.e. +/+ or −/−). The scapular and pelvic lines (SL and PL) were far off the parallel in OVL children, and the angular values of the inclination ratio (SL/PL) had the opposite sign (ie, +/− or −/+) (Table 1). The 2 postural patterns differed in direction of the lateral spinal curvatures with reference to the overloaded body side. The direction of scoliosis in both types was with the convexity to the unaffected side of the body. However, the affected side was underloaded in UNL children and overloaded in OVL children (Table 1).
Table 1.
Inter – group comparison of the basic posturometric and pedobarographic* parameters.
| Pattern | ||||||
|---|---|---|---|---|---|---|
| Anti-gravitational (n=18) | Progravitational (n=18) | Antigravitational (n=18) | Progravitational (n=18) | T | p | |
| Min./max | Min./max | Mean (SD) | Mean (SD) | |||
| Scapular line | 6/20 | 0/18 | 11.30 (3.81) | 9.22 (4.66) | −1.74 | 1.7000 |
| Pelvic line | 5/18 | −20/2 | 11.33 (4.39) | −9.00 (6.83) | −11.66 | 0.0001 |
| Pelvis rotation | −34/−5 | 0/40 | −13.72 (9.37) | 12.40 (12.07) | 5.92 | 0.0001 |
| Compensatory index | −35/6 | 5/41 | −13.78 (11.04) | 15.04 (8.93) | 7.82 | 0.0004 |
| Angle of scoliosis | 2/22 | 12/38 | 11.28 (5.62) | 22.94 (8.47) | 4.64 | 0.0002 |
| Percentage distribution of body weight between*: | ||||||
| Unaffected side | 51/75 | 25/49 | 61.72 (8.04) | 39.28 (7.14) | −8.02 | 0.0002 |
| Affected side | 25/48 | 51/76 | 38.28 (8.03) | 61.28 (7.92) | 7.68 | 0.0001 |
p – comparison between group of anti- and pro-gravitational.
Based on the aforementioned relationships, 2 types of asymmetrical postural patterns were defined:
The pro-gravitational postural pattern (PGPP), which gives the impression of a “submission” to the force of gravity by overloading the affected side (an asymmetrical crouched posture) (Figure 1).
The anti-gravitational postural pattern (AGPP), which was marked with an increased activity against gravity by underloading the affected side (an excessive and involuntary gravity resistance, resulting in the asymmetrically extended posture) (Figure 1).
In all subjects expressing the PGPP, S-type scoliosis was noted, with the primary curve mainly in the thoracic spine (75% of subjects). C-type scoliosis predominated in the AGPP, and the remaining the S-type scoliosis were primary in the lumbar spine (80% and 20% of subjects, respectively). The scoliotic values of the Cobb angles were about 10 degrees higher in PGPP children than in AGPP children, and the observed differences were statistically significant (22.94±8.47° and 11.28±5.62°, respectively) (Table 1). In AGPP children, the affected side of the pelvis expressed a tendency toward the external rotation in the range of −5° to −34° (−13.72° ±9.37). In PGPP children, the affected side of the pelvis expressed a tendency toward internal rotation on the affected side, and could be seen in the range of 0° to 40° (12.40° ±12.07) (Table 1).
The difference in posturometric parameters between 2 postural patterns were also seen in the sagittal plane. The values of the compensatory index (the parameter reflecting a dominance of either thoracic kyphosis or lumbar lordosis) fluctuated in the range of −35° to 6° in AGPP children (indicating the lordotic type of body posture), and in the range of 5° to 41° in PGPP children (indicating the kyphotic type). Most of the above results illustrating the difference between the AGPP and PGPP were statistically significant. Assessment of the postural patterns was completed by descriptive analysis of body weight distribution between the affected and unaffected foot. In children with AGPP, the unaffected foot carried the majority of the body weight, while the affected foot “pushed off” (pes equinus) from the ground with the forefoot, and could bear on average only 37.6% of the total body weight. The same analysis in PGPP children showed a tendency for the weight to be displaced toward the medial aspect of the affected foot (pes valgus).
Discussion
Children with CP express many therapeutic problems for many various reasons. Unfortunately, neither complete recovery nor causal treatment is currently possible. The most important goal in therapy and management is to improve the child’s ability to perform functional tasks in the context of activities of daily living (ADL) [37–40]. The ability to become independent in ADL seems to be determined by postural control; therefore, most interventions also include postural elements (explicitly or implicitly) in order to improve this aspect of rehabilitation. Postural control has traditionally been divided into 2 categories: steady-state balance control (including the postural alignment with all the characteristics of body sway when standing quietly), and postural adjustments to externally and internally triggered perturbations with the requirement of anticipatory motor actions [41]. In this study, the postural patterns have been recognized in hemiplegic CP children. These patterns have also been defined based on the relationship of the selected body segments to one another, the position of the body with reference to gravity, and the distribution of the body mass between the affected and unaffected sides on the base of support (BOS). Although not included here, in our larger research study of hemiplegic CP children we also examined the relevance of compensatory mechanisms in the abnormalities of dynamic equilibrium (gait analysis) to the above described postural patterns.
Multiple studies have also demonstrated that abnormal standing postures can be conditioned either by reducing the range of motion in the relevant joints (in the case of bilateral spastic CP), or by compensating for weakness (in the case of unilateral spastic CP). This may indicate that postural disturbances do not necessarily result from primary impairment of the postural control systems, but are a consequence of specific or non-specific pathophysiological factors such as non-neural peripheral components or paresis [7,8,15,42–49]. These constraints often result in a crouched posture while standing [15]. The atypical postural alignment may also be expressed as a change in the position of the body with reference to gravity and the base of support. For example, the asymmetric alignment in standing is often characteristic of children with a unilateral neurological lesion, such as hemiplegia [15]. Only a few studies have examined the body weight distribution between the affected and unaffected sides. An experimental study has indicated that children with unilateral CP tend to displace their weight toward the non-involved side [15,50]. However, our current studies have shown that children with unilateral CP are not homogeneous in terms of the distribution of the body weight, and that these observations have applied only to certain subgroups of children with an anti-gravitational postural pattern (AGPP). Children expressing a pro-gravitational postural pattern (PGPP) tend to overload the affected side. Standing asymmetry in body weight distribution may lead to the characteristic position of the body with reference to gravity (postural equilibrium).
For the first time, the research on analysis of postural control with Moiré fringe topography has allowed us to describe and define the abnormal postural patterns in CP children with hemiplegia. Even though no reference standards for this type of research are available, one may assume that the abnormal postural equilibrium patterns seem to involve multiple pathophysiological mechanisms, which Cremona et al. proposed as: 1) the non-neural passive muscle-tendon properties (passive component), 2) central executive factors including impaired muscle activation (paretic component), 3) loss of selectivity in neuromuscular output (co-contraction component), 4) abnormal EMG recruitment (spastic component), 5) persistence of immature motor programs (immature component), and 6) central deficit of sensor motor integration [7,8]. The relevant abnormal postural patterns in children with CP, along with related adaptation and substitution for pathophysiological mechanisms, most of which are amenable to objective detection and descriptive analysis, have at least partially been recognized as potential targets for treatment. Some results have shown that each type of postural pattern may determine the most important signs and symptoms of compensation, adaptation, and substitution, which may help to establish the most effective treatment approach.
Our research has shown that excessive pelvic lift, pelvic external rotation, and Pes equinovarus on the affected side were most typical for the anti-gravitational postural pattern (AGPP). We could appreciate this mechanism when AGPP children were pushing the forefoot of the affected leg away from the BOS center, as abnormal spasticity could be observed. This is perhaps due to deficits in the structure of the somatic receptors, and/or processing of somatic sensation, or deficits in sensory-motor integration [51–60]. One may imagine that overloading the unaffected body side creates some mechanical advantage, not only for the children’s safety, but also for postural development when they attempt to inhibit the progression of postural asymmetry. Most importantly, it prevents progression of lateral spinal curvature, since the direction of the body weight transfer is crucial in the pathogenesis of scoliosis. The chronic pathological facilitation of the foot flexion has an undesired and very disadvantageous shortening of the gastrocnemius muscle and subsequent foot deformity (Pes equinus). The above described asymmetrical alignment seems to be a strategy that typically develops to compensate for weakness in the paretic leg [15].
Our studies have shown that PGPP is accompanied by many additional negative factors affecting posturogenesis. They result mainly from abnormal hypotonic muscular tone and chronic changes in the mechanical load onto the paretic side (trunk, pelvic and lower limb on the paretic side) under static and dynamic conditions. The excessive submission to gravity on this side has been manifested by excessive internal rotation and pelvic obliquity in the sagittal plane. The excessive overloading of the paretic body side toward the concavity of lateral spinal curvature poses a further risk for the progression of scoliosis. Additionally, the displacement of body mass center and the abnormal passive muscle-tendon properties in children with this postural pattern may develop bone and joint abnormalities, most commonly in later stages of dysfunction. This would typically affect the foot (pronation of the midfoot-forefoot complex), the tibio-fibular and femoral segments (rotational and torsional deformities, and trochanter head anteversion), the hip joint (dislocation, subluxation due to acetabular dysplasia), and the spine (kyphosis, lordosis, and scoliosis). Structural bone and joint deformities are believed to result from chronic changes in the mechanical load on the specific body segments under static and dynamic conditions [61], which in turn can be largely due to changes in tone and stiffness of the muscles spanning the relevant joints [62–64].
The results reported above indicate that dysfunctional postural control is a key feature in children with unilateral CP; however the degree of postural disorder varied greatly within this group. There are now several diverse treatment options that may influence the postural patterns, and therefore control of posture in everyday functional performance, but the scientific basis for the majority of interventions has not been fully established. We postulate that identification of the compensatory postural pattern in CP in the early stage of its course may help to control the direction of compensation during therapy toward optimal postural patterns, and therefore maximize function and ADL. Because the measurements of the Moiré topography and Cobb angle are highly correlated, Moiré fringe topography can reduce the child’s exposure to radiation. It is an inexpensive, noninvasive, relatively fast, and easy to use diagnostic tool, which may provide clinicians with objective data on evaluation and prognosis without requiring long and expensive training. The topographs also provide a basis for clearly identifying which part of the spine is involved, as well as changes during therapy, if, as described in this study, anatomical landmarks are used as points of reference. The availability of sensitive, reliable, and valid instruments and methods for evaluation and assessment of children with CP should help to verify the effectiveness of different therapeutic methods and to establish the duration of therapeutic intervention, which in turn may improve the quality and cost effectiveness of rehabilitation.
Conclusions
Children with CP hemiplegia demonstrate abnormalities in the arrangement of individual body segments (ie, shoulders, pelvis, and spine) in all the anatomical planes. These abnormalities can be measured objectively.
The asymmetry of body posture is mainly determined by mechanisms to compensate for a deficit in the abnormal postural equilibrium, which is expressed in a particular postural pattern.
Two fundamental stereotypes of postural pattern can be recognized in children with hemiplegic CP: the anti-gravitational and the pro-gravitational postural pattern.
These postural patterns can be described in terms of basic posturometric parameters in conjunction with values of body mass distribution between the affected and the unaffected sides.
The availability of an objective, specific, reliable, and validated diagnostic method may help to evaluate and establish not only the effectiveness of different therapeutic approaches, but also the therapeutic progress or plateau.
Although the results of our study are very promising, further research, with stronger study design and a larger number of subjects, is needed.
Footnotes
Source of support: Departmental sources
References
- 1.Bax M, Goldstein M, Rosenbaum P, et al. Proposed definition and classification of cerebral palsy. Dev Med Child Neurol. 2005;47:571–76. doi: 10.1017/s001216220500112x. [DOI] [PubMed] [Google Scholar]
- 2.Diener HC, Horak FB, Nashner LM. Influence of stimulus parameters on human postural responses. J Neurophysiol. 1988;59:1888–905. doi: 10.1152/jn.1988.59.6.1888. [DOI] [PubMed] [Google Scholar]
- 3.Allum JH, Honegger F. Interactions between vestibular and proprioceptive inputs triggering and modulating human balance-correcting responses differ across muscles. Exp Brain Res. 1988;121:478–94. doi: 10.1007/s002210050484. [DOI] [PubMed] [Google Scholar]
- 4.Brogren E, Forssberg H, Hadders-Algra M. Influence of two silting positions on postural adjustments in children with spastic diplegia. Dev Med Child Neurol. 2001;43:5–46. doi: 10.1017/s0012162201000974. [DOI] [PubMed] [Google Scholar]
- 5.Horak FB, Diener HC, Nashner LM. Influence of central set on human postural responses. J Neurophysiol. 1989;62:841–53. doi: 10.1152/jn.1989.62.4.841. [DOI] [PubMed] [Google Scholar]
- 6.Wilk M, Pąchalska M, Lipowska M, et al. Speach intelligibility in cerebral palsy children attending an art. therapy program. Med Sci Monit. 2010;16(5):222–31. [PubMed] [Google Scholar]
- 7.Crenna P, Inverno M, Frigo C, et al. Pathophysiologica profile of gait in children cerebral palsy. In: Forssberg H, Hirshfeld H, editors. Movement Disorders in Children, Medicine and Science. Vol. 36. Basel: Karger; 1992. pp. 186–98. [Google Scholar]
- 8.Crenna P, Inverno M. Objective detection of pathophysiological factors contributing to gait disturbance in supraspinal lesions. In: Fedrizzi E, Avanzini G, Crenna P, editors. Motor Development in Children. London: J Libbey; 1994. pp. 103–18. [Google Scholar]
- 9.Darrah J. Typical or compensatory movement strategies: how do you choose? Dev Med Child Neurol. 2003;S94(45):31–32. [Google Scholar]
- 10.Forssberg H, Hirschfeld H. Postural adjustments in sitting humans following external perturbations: muscle activity and kinematics. Exp Brain Res. 1994;97:515–27. doi: 10.1007/BF00241545. [DOI] [PubMed] [Google Scholar]
- 11.Hadders-Algra M, Brogren E, Forssberg H. Ontogeny of postural adjustments during sitting in infancy: variation, selection and modulation. J Physiol. 1996;493:273–88. doi: 10.1113/jphysiol.1996.sp021382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hadders-Algra M, Brogren E, Katz-Salamon M, Forssberg H. Periventricular leucomalacia birth have different detrimental effects on postural adjustments. Brain. 1999;122:727–40. doi: 10.1093/brain/122.4.727. [DOI] [PubMed] [Google Scholar]
- 13.Polensek S, Sterk C, Tusa R. Screening for vestibular disorders: A study of clinicians’ compliance with recommended practice. Med Sci Monit. 2008;14(5):238–42. [PubMed] [Google Scholar]
- 14.Kerppers II, Arisawa EAL, Oliveira LVF, et al. Heart rate variability in individuals with cerebral palsy. Arch Med Sci. 2009;5(1):45–50. [Google Scholar]
- 15.Shumway-Cook A, Horak F. Balance Rehabilitation in the Neurologic Patient: Course Syllabus. Seattle: NERA; 1992. [Google Scholar]
- 16.Stokes AF, Armstrong JG, Moreland MS. Spinal Deformity and Back Surface Asymmetry in Idiopathic Scoliosis. J Orthop Resear. 1988;6:129–37. doi: 10.1002/jor.1100060117. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki N, Inami K, Ono T, et al. Analysis of Posterior Trunk Symmetry Index (POSTI) In Scoliosis. Part1. Res Spinal Deform. 1999;59:81–84. [Google Scholar]
- 18.Inami K, Suzuki N, Ono T, et al. Analysis of posterior trunk symmetry index (POTSI) in scoliosis. Part 2. Res Spinal Deform. 1999;59:85–88. [Google Scholar]
- 19.Mínguez M, Buendía M, Cibrián RM, et al. Quantifier variables of the back surface deformity obtained with a noninvasive structured light method: evaluation of their usefulness in idiopathic scoliosis diagnosis. Eur Spine J. 2007;16:73–82. doi: 10.1007/s00586-006-0079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willner S. Moiré topography for the diagnosis and documentation of scoliosis. Acta Orthop Scand. 1979;50:295–302. doi: 10.3109/17453677908989770. [DOI] [PubMed] [Google Scholar]
- 21.Ruggerone M, Austin JH. Moiré topography in scoliosis. Correlations with vertebral lateral curvature as determined by radiography. Phys Ther. 1986;66:1072–77. doi: 10.1093/ptj/66.7.1072. [DOI] [PubMed] [Google Scholar]
- 22.El-Sayyad MM. Comparison of Roentgenography and Moiré Topography for Quantifying Spinal Curvature. Physical Therapy. 1986;66:1078–82. doi: 10.1093/ptj/66.7.1078. [DOI] [PubMed] [Google Scholar]
- 23.Neugebauer H. The different methods of measuring the curve of a scoliotic spine. In: Drerup B, et al., editors. Moiré Fringe Topography and Spinal Deformity. Stuttgart: Gustav Fischer Verlag; 1983. pp. 17–269. [Google Scholar]
- 24.Goldberg JC, Kaliszer M, Moore DP, et al. Surface topography, Cobb angles and cosmetic change in scoliosis. Spine. 2001;26:55–63. doi: 10.1097/00007632-200102150-00005. [DOI] [PubMed] [Google Scholar]
- 25.Ferdjallah M, Harris GF, Smith P, Wertsch JJ. Analysis of postural control synergies during quiet standing in healthy children and children with cerebral palsy. Clin Biomech. 2002;17:203–10. doi: 10.1016/s0268-0033(01)00121-8. [DOI] [PubMed] [Google Scholar]
- 26.Rose J, Wolff DR, Jones VK, et al. Postural balance in children cerebral palsy. Dev Med Child Neurol. 2002;44:58–63. doi: 10.1017/s0012162201001669. [DOI] [PubMed] [Google Scholar]
- 27.Kilburn K, Thornton J. Prediction equations for balance measured as sway speed by head tracking with eyes open and closed. Occup Environ Med. 1995;52:544–46. doi: 10.1136/oem.52.8.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kilburn K, Warshaw R, Hanscom B. Balance measured by head (and trunk) tracking and a force platform in chemically (PCB and TCE) exposed and referent subjects. Occup Environ Med. 1994;51:381–85. doi: 10.1136/oem.51.6.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Domagalska M, Nowotny J, Szopa A, et al. Możliwości autokorekcji i autoelongacji oraz nawyk postawy u dzieci i młodzieży z bocznymi skrzywieniami kręgosłupa. Postępy Rehabilitacji. 1992;1:25–31. [in Polish] [Google Scholar]
- 30.Szopa A, Domagalska M. Kryteria prawidłowej postawy ciała w świetle badań czynnościowych układu oddechowego. In: Œlężyński J, editor. Zeszyty metodyczno-naukowe. Vol. 2. Katowice: AWF; 1993. pp. 179–86. [in Polish] [Google Scholar]
- 31.GwoŸdziowski D, Saulicz E, Nowotny J, et al. Trójpłaszczyznowa automatyczna ocena postawy ciała u osób z bocznymi skrzywieniami kręgosłupa. In: Œlężyński J, editor. Postawa ciała człowieka i metody jej oceny. Katowice: AWF; 1992. pp. 61–66. [in Polish] [Google Scholar]
- 32.Szopa A, Domagalska M, Nowotny J. Distribution of body mass on the support base in cerebral motor disorders children as an expression of antigravity unabilities. Polish Journal of Physiotherapy. 2007;7:250–57. [Google Scholar]
- 33.Domagalska M, Szopa A, Czupryna K, Nowotny J. Compensatory body segments dislocations in frontal plane in children with cerebral palsy. Polish Journal of Physiotherapy. 2005;5:127–33. [Google Scholar]
- 34.Domagalska M, Nowotny J, Szopa A, et al. Compensatory body segments dislocations in sagital plane in cerbral palsied children. Physiotherapy. 2005;2(Supp 1):56–57. [Google Scholar]
- 35.Domagalska M, Czupryna K, Szopa A, Nowotny J. Postural problems of children with cp based on hemiparesis. Polish Journal of Physiotherapy. 2008;8:253–59. [Google Scholar]
- 36.Szopa A, Domagalska M, Czupryna K, Płaszewski M. Postural consequences of muscle tone disorders in children with cerebral palsy (hemiparesis) Polish Journal of Physiotherapy. 2007;7:241–49. [Google Scholar]
- 37.Bartlett DJ, Palisano RJ. A multivariate model of determinants of motor change for children with cerebral palsy. Phys Ther. 2000;80:598–614. [PubMed] [Google Scholar]
- 38.Bower F, Michell D, Burnett MA, et al. Randomized controlled trial of physiotherapy in 56children with cerebral palsy followed for 18 months. Dev Med Child Neurol. 2001;43:4–15. doi: 10.1017/s0012162201000020. [DOI] [PubMed] [Google Scholar]
- 39.Ketelaar M, Vermeer A, Hart HA, et al. Effects of a functional therapy program on motor abilities of children with cerebral palsy. Phys Ther. 2001;81:1534–45. doi: 10.1093/ptj/81.9.1534. [DOI] [PubMed] [Google Scholar]
- 40.Ahl LE, Granat T, Johansson E, Carlberg EB. Functional therapy for children with cerebral palsy: an ecological approach. Dev Med Child Neurol. 2005;47:613–19. [PubMed] [Google Scholar]
- 41.Jones GM. Posture. In: Kandel E, Schwartz J, Jessell T, editors. Principles of Neural Science. 4th ed. McGraw-Hill; 2000. pp. 816–21. [Google Scholar]
- 42.Fulford GE, Brown JK. Position as a cause of deformity in children with cerebral palsy. Dev Med Child Neurol. 1976;18:305–14. doi: 10.1111/j.1469-8749.1976.tb03652.x. [DOI] [PubMed] [Google Scholar]
- 43.Tardieu C, Tarbary J, Heut de la Tour EA, et al. The relationship between sarcomere length in the soleus and tibialis anterior and the articular angle of the tibiacalcaneum in cats during growth. J Anat. 1977;124:581–88. [PMC free article] [PubMed] [Google Scholar]
- 44.Delp SL. What causes increased muscle stiffness in cerebral palsy? Muscle Nerve. 2003;27:131–32. doi: 10.1002/mus.10284. [DOI] [PubMed] [Google Scholar]
- 45.Fridén J, Lieber RL. Spastic muscle cells are shorter and stiffer than normal cells. Muscle and Nerve. 2003;26:157–64. doi: 10.1002/mus.10247. [DOI] [PubMed] [Google Scholar]
- 46.Graham HK. Mechanisms of deformity. In: Scrutton D, Damiano D, Mayston M, editors. Management of the Motor Disorders in Children with Cerebral Palsy. 2nd ed. London: Mac Keith Press; 2004. pp. 105–29. [Google Scholar]
- 47.Hägglund G, Andersson S, Duppe HA, et al. The first ten years experience of a population-based prevention programme. J Bone Joint Surg. 2005;87:95–101. [PubMed] [Google Scholar]
- 48.Scrutton D, Baird J, Smeeton N. Hip dysplasia in bilateral cerebral palsy: incidence and natural history in children aged 18 months to 5 years. Dev Med Child Neurol. 2001;43:586–600. doi: 10.1017/s0012162201001086. [DOI] [PubMed] [Google Scholar]
- 49.Østensjø S, Brogren Carlberg E, Vøllestad NK. Motor impairments in young children with cerebral palsy: relationship to gross motor function and everyday activities. Dev Med Child Neurol. 2004;46:580–89. doi: 10.1017/s0012162204000994. [DOI] [PubMed] [Google Scholar]
- 50.Woollacott MH, Shumway-Cook A. Postural dysfunction during standing and walking in children withcerebral palsy: what are the underlying problems and what new therapies might improve balance? Neural Plast. 2005;12:211–19. doi: 10.1155/NP.2005.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tachdjian MO, Minear WL. Sensory disturbances in the hands of children with cerebral palsy. J Bone Join Surg. 1958;40:85–90. [PubMed] [Google Scholar]
- 52.Van Heest AE, House J, Putnam M. Sensibility deficiencies in the hands of children with spastic hemiplegia. J Hand Surg. 1993;18A:278–81. doi: 10.1016/0363-5023(93)90361-6. [DOI] [PubMed] [Google Scholar]
- 53.McLaughlin J, Felix SD, Nowbar SA, et al. Lower extremity sensory function in children with cerebral palsy. Pediatr Rehab. 2005;8:45–52. doi: 10.1080/13638490400011181. [DOI] [PubMed] [Google Scholar]
- 54.Uvebrant P. Hemiplegie cerebral palsy. Aetiology and outcome. Acta Paediatr Scand Suppl. 1988;77(345):1–100. doi: 10.1111/j.1651-2227.1988.tb14939.x. [DOI] [PubMed] [Google Scholar]
- 55.Bolanos AA, Bleck EE, Firestone P, Young L. Comparison of stereognosis and two-point discrimination testing of the hands of children with cerebral palsy. Dev Med Child Neurol. 1989;31:371–76. doi: 10.1111/j.1469-8749.1989.tb04006.x. [DOI] [PubMed] [Google Scholar]
- 56.Yekutiel M, Jariwala M, Stretch P. Sensory deficit in the hands of children with cerebral palsy: a new-look at assessment and prevalence. Dev Med Child Neurol. 1994;36:619–24. doi: 10.1111/j.1469-8749.1994.tb11899.x. [DOI] [PubMed] [Google Scholar]
- 57.Cooper J, Majnemer A, Rosenblatt B, Binibaum R. The determinants of sensory deficits in children with hemiplcgic cerebral palsy. J Child Neurol. 1995;10:300–9. doi: 10.1177/088307389501000412. [DOI] [PubMed] [Google Scholar]
- 58.Krumlinde-Sundholm L, Eliasson A-C. Comparing tests of tactile sensibility: aspects relevant to their use in testing children with spastic hemiplegia. Dev Med Child Neurol. 2002;44:604–12. doi: 10.1017/s001216220100264x. [DOI] [PubMed] [Google Scholar]
- 59.Thickbroom GW, Byrnes ML, Archer SAA, et al. Differences in sensory and motor cortical organization following brain in jury early in life. Ann Neurol. 2001;49:320–27. [PubMed] [Google Scholar]
- 60.Staudt M. Reorganization of the developing human brain following periventricular white matter lesions. Neurosci Biobehav Rev. 2007;31:1150–56. doi: 10.1016/j.neubiorev.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 61.Zhang P, Tanaka SM, Jiang HA, et al. Diaphyseal bone formation in murine tibiae in response to knee loading. J Appl Physiol. 2006;100:1452–59. doi: 10.1152/japplphysiol.00997.2005. [DOI] [PubMed] [Google Scholar]
- 62.Chambers HG. Advances in cerebral palsy. Curr Opin Orthop. 2002;13:424–31. [Google Scholar]
- 63.Duffy CM, Cosgrove AP. The foot in cerebral palsy. Current Orthopaedics. 2002;16:104–13. [Google Scholar]
- 64.Horstmann HM, Bleck EE. Orthopaedic Management in Cerebral Palsy. 2nd ed. London: Press; 2007. [Google Scholar]