Summary
Background
The incidence of hepatocellular carcinoma (HCC) in Japan has still been increasing. The aim of the present study was to analyze the epidemiological trend of HCC in the western area of Japan, Kyushu.
Material/Methods
A total of 10,010 patients with HCC diagnosed between 1996 and 2008 in the Liver Cancer study group of Kyushu (LCSK), were recruited for this study. Cohorts of patients with HCC were categorized into five year intervals. The etiology of HCC was categorized to four groups as follows; B: HBsAg positive, HCV-RNA negative, C: HCV-RNA positive, HBsAg negative, B+C: both of HBsAg and HCV-RNA positive, nonBC: both of HBsAg and HCV-RNA negative.
Results
B was 14.8% (1,485 of 10,010), whereas 68.1% (6,819 of 10,010) had C, and 1.4% (140 of 10,010) had HCC associated with both viruses. The remaining 1,566 patients (15.6%) did not associate with both viruses.
Cohorts of patients with HCC were divided into six-year intervals (1996–2001 and 2002–2007). The ratio of C cases decreased from 73.1% in 1996–2001 to 64.9% in 2002–2007. On the other hand, B and -nonBC cases increased significantly from 13.9% and 11.3% in 1996–2001 to 16.2% and 17.6% in 2002–2007, respectively.
Conclusions
The incidence of hepatocellular carcinoma associated with hepatitis C infection decreased after 2001 in Kyushu area. This change was due to the increase in the number and proportion of the HCC not only nonBC patients but also B patients.
Keywords: hepatitis virus, hepatocellular carcinoma, Japan
Background
The three leading causes of death in Japan are malignancy neoplasms, cardiovascular diseases, and cerebrovascular diseases. Since 1981, malignant neoplasms have been the leading cause of death in Japan. For the last 30 years, liver cancer has been the third leading cause of death from malignant neoplasms in men. In women, liver cancer has ranked fifth during the past decade [1]. Hepatocellular carcinoma (HCC) accounts for 85% to 90% of primary liver cancers[2] and the age-adjusted HCC mortality rate has increased in recent decades in Japan [3]. Similarly, a trend of increasing rates of HCC has been reported from several developed countries in North America, Europe and Asia [4,5]. HCC often develops in patients with liver cirrhosis caused by hepatitis B virus (HBV), hepatitis C virus (HCV), excessive alcohol consumption, or nonalcoholic fatty liver disease. Of the hepatitis viruses which cause HCC, HCV is predominant in Japan [6–9].
Although the age-adjusted incidence of HCC has increased in Japan, sequential changes in etiology of HCC patients between 2001 and 2008 are not fully understood [10]. To clarify factors affecting epidemiological changes in Japanese HCC patients, especially the recent trend of HCC, we analyzed the epidemiological trend of HCC in the western area of Japan, Kyushu area.
Material and Methods
Patients
A total of 10,010 patients with HCC diagnosed between 1996 and 2008 in the Liver Cancer study group of Kyushu (LCSK), were recruited for this study. The diagnosis of HCC was based on AFP levels and imaging techniques including ultrasonography (USG), computerized tomography (CT), magnetic resonance imaging (MRI), hepatic angiography (HAG), and/or tumor biopsy. The diagnostic criteria for HCC were either a confirmative tumor biopsy or elevated AFP (≥20 ng/mL) and neovascularization in HAG and/or CT.
Etiology of HCC
A diagnosis of chronic HCV infection was based on the presence of HCV-RNA detected by polymerase chain reaction (PCR), whereas diagnosis of chronic HBV infection was based on the presence of hepatitis B surface antigen (HBsAg). The etiology of HCC was categorized to four groups as follows; B: HBsAg positive, HCV-RNA negative, C: HCV-RNA positive, HBsAg negative, B+C: both of HBsAg and HCV-RNA positive, nonBC: both of HBsAg and HCV-RNA negative.
Statistical analysis
The data were analyzed by the Mann-Whitney test for the continuous ordinal data, the ×2 test with Yates’ correction and the Fisher exact test for the association between two qualitative variables. The standard deviation was calculated based on the binomial model for the response proportion. P<0.05 was considered statistically significant.
Results
Clinical features of the studied patients
A total of 10,010 patients with HCC were diagnosed at our study group from 1996 to 2008. Table 1 show that the proportion of patients diagnosed with B was 14.8% (1,485 of 10,010), whereas 68.1% (6,819 of 10,010) had C, and an additional 1.4% (140 of 10,010) had HCC associated with both viruses. The remaining 1,566 patients (15.6%) did not associate with both viruses. In analysis of patients in HCC by category, the median age of patients at diagnosis of B was 57 years old significant younger than other types HCC(C: 69, nonBC: 70,.B+C 65 years old).
Table 1.
The characteristic of HCC patients during the period of 1996–2008.
| Age (y.o.) | B | C | nonB | B+C | Total | ||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | ||
| 0– | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 |
| 10– | 4 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 7 |
| 20– | 6 | 2 | 1 | 0 | 1 | 1 | 0 | 0 | 11 |
| 30– | 31 | 5 | 4 | 0 | 11 | 3 | 2 | 0 | 56 |
| 40– | 204 | 22 | 130 | 12 | 32 | 15 | 12 | 0 | 427 |
| 50– | 507 | 66 | 728 | 145 | 167 | 32 | 31 | 6 | 1,682 |
| 60– | 287 | 118 | 1836 | 741 | 411 | 102 | 35 | 13 | 3,543 |
| 70– | 140 | 64 | 1775 | 947 | 483 | 133 | 22 | 14 | 3,578 |
| 80– | 9 | 18 | 271 | 214 | 97 | 65 | 1 | 4 | 679 |
| 90– | 0 | 0 | 9 | 5 | 9 | 2 | 0 | 0 | 58 |
| Total | 1,189 | 296 | 4,754 | 2,065 | 1,211 | 355 | 103 | 37 | 10,010 |
| 1,485 (4.8%) | 6,819 (68.1%) | 1,566 (15.6%) | 140 (1.4%) | ||||||
| Median | 57 | 63 | 67 | 70 | 68 | 70 | 61 | 68 | 67 |
| 57 | 69 | 70 | 65 | ||||||
| Mean | 56 | 64 | 68 | 71 | 69 | 71 | 62 | 68 | 67 |
| 58 | 68 | 68 | 63 | ||||||
| Range | 1–87 | 14–89 | 27–94 | 0–93 | 28–96 | 17–90 | 36–82 | 55–82 | 0–96 |
| 1–89 | 0–94 | 17–96 | 36–82 | ||||||
Age: B vs. C p≤0.001; B vs. B+C p≤0.001; B vs. nonBC p≤0.001; C vs. BC p≤0.001; C vs. nonBC p=0.043; BC vs. nonB+C p≤0.001. IQR – interquartile range; SD – standard deviation.
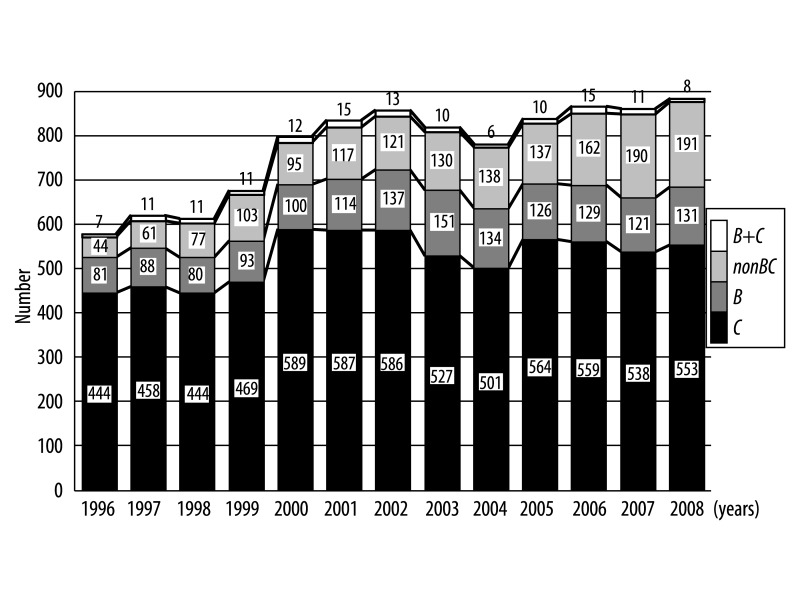
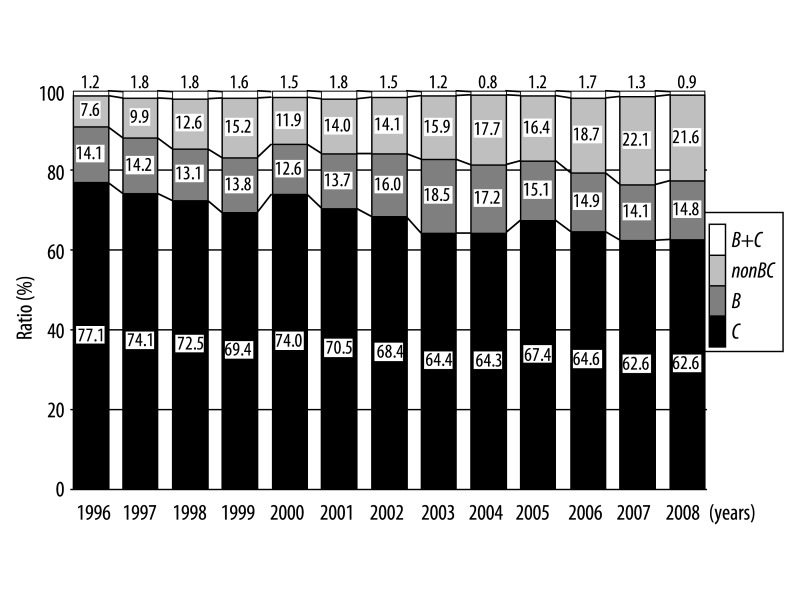
As shown in Figures 1 and 2, the number and ratio of B cases remained unchanged from1996 to 2001 and thereafter increased and plateaued, whereas C rapidly increased from 1996 to 2000 and thereafter decreased and plateaued. In addition, the number and ratio of the nonBC cases has increased continued gradually and continued in this study period.
Figure 1.
Sequential changes in the number of HCC patients categorized by etiology during the period 1996–2008.
Figure 2.
Sequential changes in the ratio of HCC patients categorized by etiology during the period 1996–2008.
Change of etiology in patients with HCC during the period 1996–2007 with 6-years intervals
Cohorts of patients with HCC were divided into six-year intervals (1996–2001 and 2002–2007). Table 2 show that the incident rate of C decreased significantly from 73.1% in 1996–2001 to 64.9% in 2002–2007 (1996–2001 vs. 2002–2007, p=<0.001). On the other hand, the incident rate of B and -nonBC increased significantly from 13.9% and 11.3% in 1996–2001 to 16.2% and 17.6% in 2002–2007, respectively. Not only the incident rate but also number of B and -nonBC became larger in same 6 years periods.
Table 2.
Change of etiology in patients with HCC during the period 1996–2007 with 6-years intervals.
| Period | 1996–2001 | 2002–2007 | P value | |
|---|---|---|---|---|
| Number | 3,023 | 4,173 | ||
| Sex | Male | 2,162 | 2,849 | |
| Female | 861 | 1,324 | ||
| Ratio (male/female) | 2.5 | 2.2 | 0.003 | |
| Age (y.o.) (IQR) | 66 (14) | 69 (12) | <0.001 | |
| Hepatitis virus (%) | B | 13.9 | 16.2 | |
| C | 73.1 | 64.9 | ||
| B+C | 1.7 | 1.3 | ||
| nonBC | 11.3 | 17.6 | 0.001 | |
QR – interquartile range.
Table 3 shows that male/female ratio of C and -nonBC decreased significantly from 2.2 and 4.0 in 1996–2001 to 1.8 and 2.7 in 2002–2007, respectively (p≤0.001). The ratio became clearly smaller, indicates an increase in female patients with C and nonBC. On the other hand, the male/female ratio of B patients did not significantly change during the period. The median age at diagnosis of B, C, and nonBC in six-year intervals were significant increase from 56 to 58, from 67 to 71 and from 68 to 71 years of age during the period.
Table 3.
The median age and male/female ratio of HCC patients during the period of 1996–2007.
| Period | 1996–2001 | 2002–2007 | P value | |
|---|---|---|---|---|
| B | ||||
| Age (y.o.) (IQR) | 56 (14) | 58 (15) | 0.001 | |
| Sex | Male | 331 | 519 | |
| Female | 88 | 157 | ||
| Ratio (male/female) | 3.8 | 3.3 | 0.391 | |
| C | ||||
| Age (y.o.) (IQR) | 67 (9) | 71 (11) | <0.001 | |
| Sex | Male | 1,524 | 1,753 | |
| Female | 687 | 955 | ||
| Ratio (male/female) | 2.2 | 1.8 | 0.002 | |
| nonBC | ||||
| Age (y.o.) (IQR) | 68 (12) | 71 (13) | <0.001 | |
| Sex | Male | 273 | 534 | |
| Female | 69 | 201 | ||
| Ratio (male/female) | 4.0 | 2.7 | 0.012 | |
QR – interquartile range.
Discussion
Our study was the twenty-three major liver center-based study designed to examine the sequential change in the background of HCC patients during the past 13 years, 1996–2008. More than 80% of our patients had chronic HBV or HCV infections. During this observation period, the number and proportion of HCC-C reached a peak in 2000 and thereafter decreased and became stabilized. Previous studies from Japan reported that the proportion of the HCC patients with HCV infection had been increased and reached a plateau in the period of 1981–2001 [1,3,10–12]. However, in our study, the number and proportion of the HCC patients with HCV infection cases decreased in 2001–2008. The reason may be explained as follows; interferon therapy for chronic hepatitis C may have been associated with a decreased incidence of HCC [13–17]. Oral supplementation with a oral branched-chain amino acids has been useful in the prevention HCC [18]. Finally, the chronically HCV-infected population is aging in Japan. Yoshizawa et al. reported that age-specific prevalence for the presence of HCVAb among ~300,000 voluntary blood donors from Hiroshima in 1999 clearly increased with the age, reaching the highest proportion of 7% in individuals who were more than 70 years old [10,19]. In this study, the median age of the HCC patients with HCV infection steadily increased from 67 to 71 years of age during the studied period. In a word, HCV infected people become older with years in Japan and they were regarded as a high risk for HCC.
The prevalence rate of HBV in Kyushu area has been reported to be higher than other area in Japan [1]. In Kyushu area, 95% of patients with chronic HBV infection had HBV genotype C except for Okinawa [20]. HBV genotype C is thought to be associated with higher incidence of HCC compared with other HBV genotypes [21]. In the present study, the incident rate of HCC patients with HBV infection became larger in this study period. To explain this change, we must consider from two viewpoints. The one is that the number of patients with HCC caused by HCV infection decreased, the other is that the proportion of chronic HBV infected patients who have reached the age of developing HCC is relatively high as described below.
Nationwide health survey for HBsAg in the over 40 years of age population had been done between 2002 and 2006 in Japan. This survey reports indicated that the average HBsAg prevalence was 1.2% in the total Japanese population patients with chronic HBV infection [10] and the age-specific prevalence of HBsAg was higher in the group aged between 50 (1.4%) and 55 years (1.5%). In the HCC patients with HBV genotype C, the mean age was 55 years in Japan [20]. This overlap between age-specific prevalence and hepatocellular carcinogenic age would be associated with the increase of HCC patients with HBV infection. Nucleoside analogue reverse transcriptase inhibitor (NARTI) therapy effectively reduces the incidence of HCC in chronic hepatitis B patients [22,23]. However, Interferon therapy for chronic hepatitis C started from 1992, whereas NARTI therapy for HBV started from 2000 in Japan [24,25]. Hence, HBV associated HCC will probably decrease in Japan during the next 10 to 20 years.
The survey of HCC patients associated with nonBC infection in Japan was conducted by Inuyama Hepatitis Research Group from 1995 to 2003. The ratio of HCC patients with nonBC accounted 9.3% [1]. In the present study, the ratio of HCC patients with nonBC was 14.1%. Furthermore, the number and the proportion of HCC patients with nonBC have been gradually increasing in the periods. The current two studies account for the increase in number and proportion of HCC patients with nonBC. First, Lai et al. reported that type 2 diabetes increases the risk of developing HCC in those who are HCV negative or have a high level of total cholesterol [26]. Second, Nakano et al. reported that epidemiological studies on diabetes mellitus revealed that the number of patients with diabetes mellitus is gradually increasing in Japan along with development of car society and westernization of food intake. Since prevalence of diabetes mellitus increases with aging, proportion of individuals with diabetes mellitus aged over 60 has exceeded two-third of estimated total number of patients (7.40 million in 2002) in Japan where aging of society is rapidly progressing [27]. In a word, the number of type 2 diabetes people is increasing in Japan and they were regarded as a high risk for HCC. Then, the number and the proportion of HCC patients with nonBC have been increased recent twelve years in Japan.
It is known that 2 to 4 decades of chronic HCV infection are required to develop cirrhosis and subsequent HCC [28–31]. The number of HCC cases has increased in Japan, because individuals infected with HCV during the past have grown old and have reached the cancer-bearing age. The prevalence of HCV infection in young Japanese individuals is low and the incidence of HCVAb is very low because of preventative actions against HCV infection such as the screening of blood products for HCV and the use of sterile medical equipment [32]. Additionally, we showed that the number and proportion of patients with HCC-C cases decreased, whereas the number and ratio of HCC-nonBC steadily increased during the studied period. These findings may be expected that the incidence of HCC patients with nonBC in Japan may continue to increase even after the consequence of the HCV epidemic level off, a country that is far advanced with regard to HCC patients with HCV infection, in the near future.
Conclusions
In summary, HCC patients had increased from 1996 to 2000 and this increase was originated from HCC patients with HCV infection. The number and proportion of HCC patients with HCV infection reached a peak in 2000 and thereafter decreased and became stabilized. The incidence of hepatocellular carcinoma associated with hepatitis C infection decreased after 2001 in Kyushu area. This change was due to the increase in the number and proportion of the HCC not only nonBC patients but also B patients.
Footnotes
Source of support: Departmental sources
References
- 1.Umemura T, Kiyosawa K. Epidemiology of hepatocellular carcinoma in Japan. Hepatol Res. 2007;37(Suppl 2):S95–100. doi: 10.1111/j.1872-034X.2007.00169.x. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 3.Kiyosawa K, Tanaka E. Characteristics of hepatocellular carcinoma in Japan. Oncology. 2002;62:5–7. doi: 10.1159/000048269. [DOI] [PubMed] [Google Scholar]
- 4.McGlynn KA, Tsao L, Hsing AW, et al. International trends and patterns of primary liver cancer. Int J Cancer. 2001;94:290–96. doi: 10.1002/ijc.1456. [DOI] [PubMed] [Google Scholar]
- 5.Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Hamasaki K, Nakata K, Tsutsumi T, et al. Changes in the prevalence of hepatitis B and C infection in patients with hepatocellular carcinoma in the Nagasaki Prefecture, Japan. J Med Virol. 1993;40:146–49. doi: 10.1002/jmv.1890400212. [DOI] [PubMed] [Google Scholar]
- 7.Kato Y, Nakata K, Omagari K, et al. Risk of hepatocellular carcinoma in patients with cirrhosis in Japan. Analysis of infectious hepatitis viruses. Cancer. 1994;74:2234–38. doi: 10.1002/1097-0142(19941015)74:8<2234::aid-cncr2820740805>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Shiratori Y, Shiina S, Imamura M, et al. Characteristic difference of hepatocellular carcinoma between hepatitis B- and C- viral infection in Japan. Hepatology. 1995;22:1027–33. doi: 10.1016/0270-9139(95)90605-3. [DOI] [PubMed] [Google Scholar]
- 9.Shiratori Y, Shiina S, Zhang PY, et al. Does dual infection by hepatitis B and C viruses play an important role in the pathogenesis of hepatocellular carcinoma in Japan? Cancer. 1997;80:2060–67. [PubMed] [Google Scholar]
- 10.Kiyosawa K, Umemura T, Ichijo T, et al. Hepatocellular carcinoma: recent trends in Japan. Gastroenterology. 2004;127:S17–26. doi: 10.1053/j.gastro.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Taura N, Yatsuhashi H, Hamasaki K, et al. Increasing hepatitis C virus-associated hepatocellular carcinoma mortality and aging: Long term trends in Japan. Hepatol Res. 2006;34:130–34. doi: 10.1016/j.hepres.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Taura N, Hamasaki K, Nakao K, et al. Aging of patients with hepatitis C virus-associated hepatocellular carcinoma: long-term trends in Japan. Oncol Rep. 2006;16:837–43. [PubMed] [Google Scholar]
- 13.Nishiguchi S, Kuroki T, Nakatani S, et al. Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet. 1995;346:1051–55. doi: 10.1016/s0140-6736(95)91739-x. [DOI] [PubMed] [Google Scholar]
- 14.Nishiguchi S, Shiomi S, Nakatani S, et al. Prevention of hepatocellular carcinoma in patients with chronic active hepatitis C and cirrhosis. Lancet. 2001;357:196–97. doi: 10.1016/S0140-6736(00)03595-9. [DOI] [PubMed] [Google Scholar]
- 15.Kasahara A, Hayashi N, Mochizuki K, et al. Risk factors for hepatocellular carcinoma and its incidence after interferon treatment in patients with chronic hepatitis C. Osaka Liver Disease Study Group. Hepatology. 1998;27:1394–402. doi: 10.1002/hep.510270529. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda K, Saitoh S, Arase Y, et al. Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: A long-term observation study of 1,643 patients using statistical bias correction with proportional hazard analysis. Hepatology. 1999;29:1124–30. doi: 10.1002/hep.510290439. [DOI] [PubMed] [Google Scholar]
- 17.Makiyama A, Itoh Y, Kasahara A, et al. Characteristics of patients with chronic hepatitis C who develop hepatocellular carcinoma after a sustained response to interferon therapy. Cancer. 2004;101:1616–22. doi: 10.1002/cncr.20537. [DOI] [PubMed] [Google Scholar]
- 18.Muto Y, Sato S, Watanabe A, et al. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2005;3:705–13. doi: 10.1016/s1542-3565(05)00017-0. [DOI] [PubMed] [Google Scholar]
- 19.Yoshizawa H. Hepatocellular carcinoma associated with hepatitis C virus infection in Japan: projection to other countries in the foreseeable future. Oncology. 2002;62:8–17. doi: 10.1159/000048270. [DOI] [PubMed] [Google Scholar]
- 20.Orito E, Mizokami M. Hepatitis B virus genotypes and hepatocellular carcinoma in Japan. Intervirology. 2003;46:408–12. doi: 10.1159/000075000. [DOI] [PubMed] [Google Scholar]
- 21.Orito E, Mizokami M. Differences of HBV genotypes and hepatocellular carcinoma in Asian countries. Hepatol Res. 2007;37:S33–35. doi: 10.1111/j.1872-034X.2007.00101.x. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto A, Tanaka E, Rokuhara A, et al. Efficacy of lamivudine for preventing hepatocellular carcinoma in chronic. Hepatol Res. 2005;32(3):173–84. doi: 10.1016/j.hepres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Shi J, Wang YP, et al. Milder liver cirrhosis and loss of serum HBeAg do not imply lower risk for. Med Sci Monit. 2009;15(6):CR274–79. [PubMed] [Google Scholar]
- 24.Hayashi N, Takehara T. Antiviral therapy for chronic hepatitis C: past, present, and future. J Gastroenterol. 2006;41(1):17–27. doi: 10.1007/s00535-005-1740-7. [DOI] [PubMed] [Google Scholar]
- 25.Kumashiro R, Kuwahara R, Ide T, et al. Subclones of drug-resistant hepatitis B virus mutants and the outcome of breakthrough hepatitis in patients treated with lamivudine. Intervirology. 2003;46(6):350–54. doi: 10.1159/000074991. [DOI] [PubMed] [Google Scholar]
- 26.Lai MS, Hsieh MS, Chiu YH, Chen TH. Type 2 diabetes and hepatocellular carcinoma: A cohort study in high prevalence area of hepatitis virus infection. Hepatology. 2006;43:1295–302. doi: 10.1002/hep.21208. [DOI] [PubMed] [Google Scholar]
- 27.Nakano T, Ito H. Epidemiology of diabetes mellitus in old age in Japan. Diabetes Res Clin Pract. 2007;77(Suppl 1):S76–81. doi: 10.1016/j.diabres.2007.01.070. [DOI] [PubMed] [Google Scholar]
- 28.Deuffic S, Poynard T, Valleron AJ. Correlation between hepatitis C virus prevalence and hepatocellular carcinoma mortality in Europe. J Viral Hepat. 1999;6:411–13. doi: 10.1046/j.1365-2893.1999.00178.x. [DOI] [PubMed] [Google Scholar]
- 29.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–50. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 30.Planas R, Balleste B, Antonio Alvarez M, et al. Natural history of decompensated hepatitis C virus-related cirrhosis. A study of 200 patients. J Hepatol. 2004;40:823–30. doi: 10.1016/j.jhep.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Davila JA, Morgan RO, Shaib Y, et al. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: a population-based study. Gastroenterology. 2004;127:1372–80. doi: 10.1053/j.gastro.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki F, Tanaka J, Moriya T, et al. Very low incidence rates of community-acquired hepatitis C virus infection in company employees, long-term inpatients, and blood donors in Japan. J Epidemiol. 1996;6:198–203. doi: 10.2188/jea.6.198. [DOI] [PubMed] [Google Scholar]