Summary
Background
Glucagon-like peptide-1(GLP-1), released from enteroendocrine cells of the intestine, exerted cardiovascular protective effect. Circulating endothelial progenitor cells (EPCs) play an important role in maintaining endothelial integrity regulating neovascularization and reendothelialization after endothelial injury. Vascular endothelial growth factor (VEGF) is an important cytokine in the process of EPCs vascular differentiation and proliferation.
Material/Methods
This study was designed to investigate the association between VEGF changes and the proliferation/differentiation function of EPCs in the presence of GLP-1.
Results
We demonstrated that GLP-1 markedly enhanced the EPCs proliferation and expression of EC-specific markers, and simultaneously upregulated VEGF secretion in EPCs. Exogenous VEGF augmented EPCs proliferation/differentiation abilities in a dose-dependent manner. However, all of the beneficial effects of GLP-1 were suppressed by anti-VEGFmAb or the KDR-specific tyrosine kinase inhibitor SU1498.
Conclusions
These findings suggest that GLP-1 improves VEGF generation, which contributed to improvement of EPCs biological function, partly by tyrosine kinase KDR. VEGF is a necessary intermediate, mediating the effects of GLP-1 on EPCs. These changes offer a novel explanation that upregulation EPCs bioactivities may be one of the mechanisms of GLP-1 cardiovascular protective effect.
Keywords: glucagon-like peptide-1 (GLP-1), endothelial progenitor cells (EPCs), VEGF
Background
Glucagon-like peptide-1(GLP-1), released from enteroendocrine cells of the intestine, is a regulatory factor of various cells and tissues. Recent studies showed that GLP-1 improved the vascular endothelial cell dysfunction and exerted cardiovascular protective effect [1]. Endothelial dysfunction is essentially the damage of injury-repair process homeostatic equilibrium. Circulating endothelial progenitor cells (EPCs) derived from bone marrow were isolated and characterized in 1997 [2], altering our understanding of endothelial function and new blood vessel growth. Circulating EPCs play an important role in maintaining endothelial integrity regulating neovascularization and reendothelialization after endothelial injury [3,4]. Therefore, we hypothesized that GLP-1 is involved in protection of vascular endothelium by improving the biologic function of EPCs.
EPCs share characteristics of both hematopoietic stem cells and endothelial cells, such as expression of endothelial markers and the ability to differentiate into mature endothelium [5]. Cytokines is known to participate in the process of EPCs differentiation into endothelial cells [6]. Vascular endothelial growth factor VEGF is identified as a key regulators of EPCs bifurcation. VEGF-induced mobilization of bone marrow-derived EPCs resulted in increased differentiated EPCs in vitro and augmented corneal neovascularization in vivo [7,8]. These findings thus establish a novel role for VEGF in postnatal neovascularization, which complements its known impact on angiogenesis. Given VEGF regulatory role in both angiogenesis and vasculogenesis in EPCs, we observe the effects of GLP-1 on biological function and VEGF generation of EPCs to explore the probable mechanism of the cardiovascular protective effect of GLP-1.
Material and Methods
Isolation, cultivation, and characterization of EPCs
EPCs were obtained by isolating peripheral mononuclear cells from blood of healthy volunteers by use of Ficoll density centrifugation. Recovered cells were washed twice with PBS. Unselected mononuclear cells were plated on fibronectin-coated culture dishes (Biocoat; BectonDickinson Labware) at a density of 106 cells/ml in Medium 199 (Invitrogen), incubated by M199 medium (Hyclone) consisting of 20% fetal bovine serum, 100 units/ml penicillin/streptomycin (Invitrogen). Three days after seeding, nonadherent cells were removed by washing with PBS, and the media were reapplied. Adherent cells were identified as EPCs.
Characterization of cultured EPCs
At the end of the growth curve, cultured cells were characterized to confirm their endothelial phenotype in 6 randomly selected subjects. The cells were fixed with 4% paraformaldehyde for 30 min, then washed twice with phosphate-buffered saline (PBS) and stained with primary antibody (1:300): monoclonal antibodies against CD133/KDR. To have further methodological confirmation of the endothelial phenotype, cultured EPCs were detached CD133/CD34. Sections were incubated at 4 degrees Celsius overnight; followed by overnight incubation with secondary antibody (1:100) at 4 degrees Celsius. After washing twice with PBS, images of the stained cells were viewed with a laser scanning confocal microscope. Double-positive cells were identified as differentiating EPCs.
GLP-1 treatment of EPCs
EPCs were pretreated with and without different concentrations of GLP-1 1,10,20 nM for 72 h. Three groups of EPCs were treated with VEGF (Sigma) 10, 20,30ng/ml, an important cytokine in the process of vascular endothelial cell differentiation and maturation. To further confirm the role of VEGF in EPCs biology, 1 group of EPCs was treated with 5 μmol/l SU1498 (Sigma), a specific inhibitor of KDR signaling pathway. Another group of EPCs was treated with anti-VEGFmAb (Sigma) 100 ng/ml.
EPCs proliferation
Adherent cells were detached by 0.25% trypsin solution and collected 500 μl cell suspension to count cell number. EPCs were plated in 96-well human fibronectin-coated plates and 10 μl MTT (Sino-American Biotechnology Co) at 5mg/ml were added into each well and incubated for 4 hours at 37 degrees Celsius. After removing medium and adding 200μL dimethylsulfoxide (DMSO) into each well to dissolve the formazan by pipetting up and down several times, the optical density (OD) value of EPCs were determined by universal microplate spectrophotometer (SHIMADZU).
RT-PCR polymerase chain reaction assay
RNA was harvested with Trizol kit (Sigma) from cell according to the manufacturer’s instructions. mRNA was reverse transcribed to cDNA with the RNA PCR Core kit (Sino-American Biotechnology Co). Primer sequences are the following: GAPDH forward: 5′-CCATCACCATCTTCCAGGAG-3′, reverse: 5′-CCTGCTTCACCACCTTCTTG-3′: Flt-1 forward: 5′-ATTTGTGATTTTGGCCTTGC-3′, reverse: 5′-CAGGCTCATGAACTTGAAAGC-3′; KDR forward: 5′-AGACCAAAGGGGCACGATTC-3′, reverse: 5′-CAGCAAAACACCAAAAGACCAGAC-3′; VE-cadherin forward: 5′-GCTGAAGGAAAACCAGAAGAAGC-3′, reverse: 5′-TCGTGATTATCCGTGAGGGTAAAG-3′; eNOS forward: 5′-AGCGAGTGAAGGCGACAA-3′, reverse: 5′-TCCACGGACGAGCAAAGG-3′. The amplified product was sampled every other cycle and electrophoresed on 15 g/L agarose gel. Belts were observed and photos were taken by a gel image analysis system. GAPDH was calculated as a parameter of relative mRNA levels. The PCR primers were synthesized by Shanghai Sangon Biological Engineering Technology Co.
Measurement of VEGF in EPCs
VEGF in cell-culture medium was measured using the commercially available VEGF sandwich immunoassay kit (Quantikine human VEGF ELISA kits [R&D Systems] according to the manufacturer’s instructions.
The cell smears were obtained after centrifugation of EPCs and fixed with 4% paraformaldehyde for 30 min. SP immunocytochemistry was carried out to determine the VEGF protein levels according to the manufacturer’s instructions (Santa Cruz Biotechnology). Images of the stained cells were viewed with inverted microscope and analyzed by the Medical Image Analysis system (HMIA S-2000).
Statistical analysis
The data were generally expressed as means ±SD. SPSS software version 11.0 was used for statistical analyses. Statistical significance among mean values was evaluated by one-way ANOVA tests for measurement data, and LSD-t test for comparison between each other. Differences were considered significant when p value was less than 0.05.
Results
Characterization of EPCs
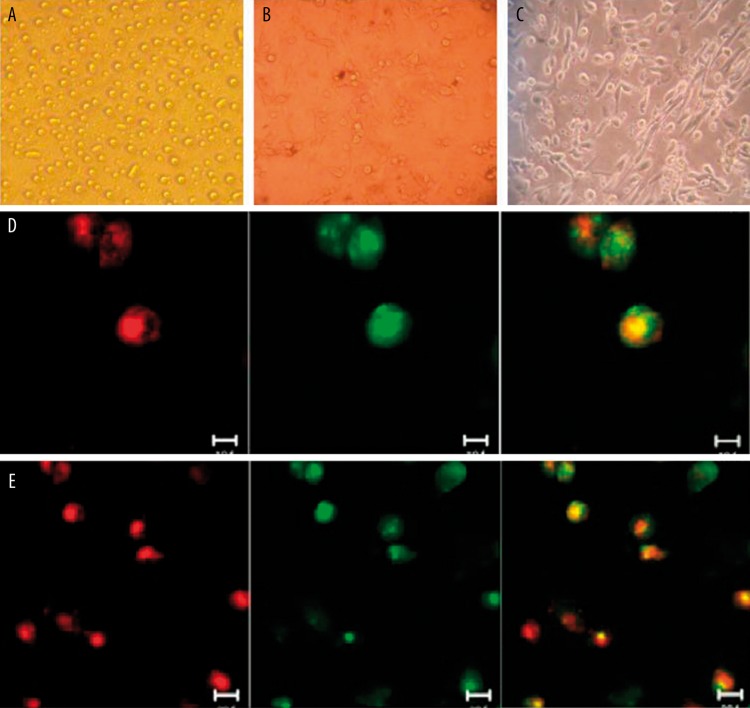
EPCs were originated from peripheral blood MNCs of healthy subjects as previously described. EPCs were cultured on fibronectin, a fraction of cells adhered to fibronectin, whereas others still suspended in culture medium (Figure 1A). Numerous spindle-shaped cells were observed 7 days in culture (Figure 1B). On day 14 of culture the majority of typical spindle-shaped endothelial-like cells were found and connected end-to-end to exhibit cord-like structure (Figure 1C).
Figure 1.
Morphology and characterization of endothelial progenitor cells (EPCs) from peripheral blood. (A) Mononuclear cells (MNCs) were isolated and plated on fibronectin-coated culture dish on the second day. (B) 7 days after plating, adherent EPCs with spindle-shape and cell colony were shown. (C) EPCs joined end to end exhibited cord-like structure. Identification of EPCs. (D) Double-positive for CD133 and CD34; (E) Double-positive for CD133 and KDR.
Identification of EPCs
After mononuclear cells were isolated from peripheral blood for 7 days, a laser scanning confocal microscope was used to detect the surface markers of adherent cells. Images of the double-staining cells for both CD133 and CD34 were viewed: positive cells for CD133 are red; positive cells for CD34 are green; yellow double-positive cells were identified as differentiating EPCs (Figure 1D). EPCs were further confirmed as cells double-positive for CD133 uptake and KDR binding affinity. Double-positive cells were identified as differentiating EPCs (Figure 1E). Laser scanning confocal microscope analysis revealed that EPCs expressed endothelial markers CD34 and KDR, which are considered critical markers of outgrowth endothelial cell-producing EPCs. Stem cell marker CD133 was still expressed.
GLP-1 increase proliferation of EPCs
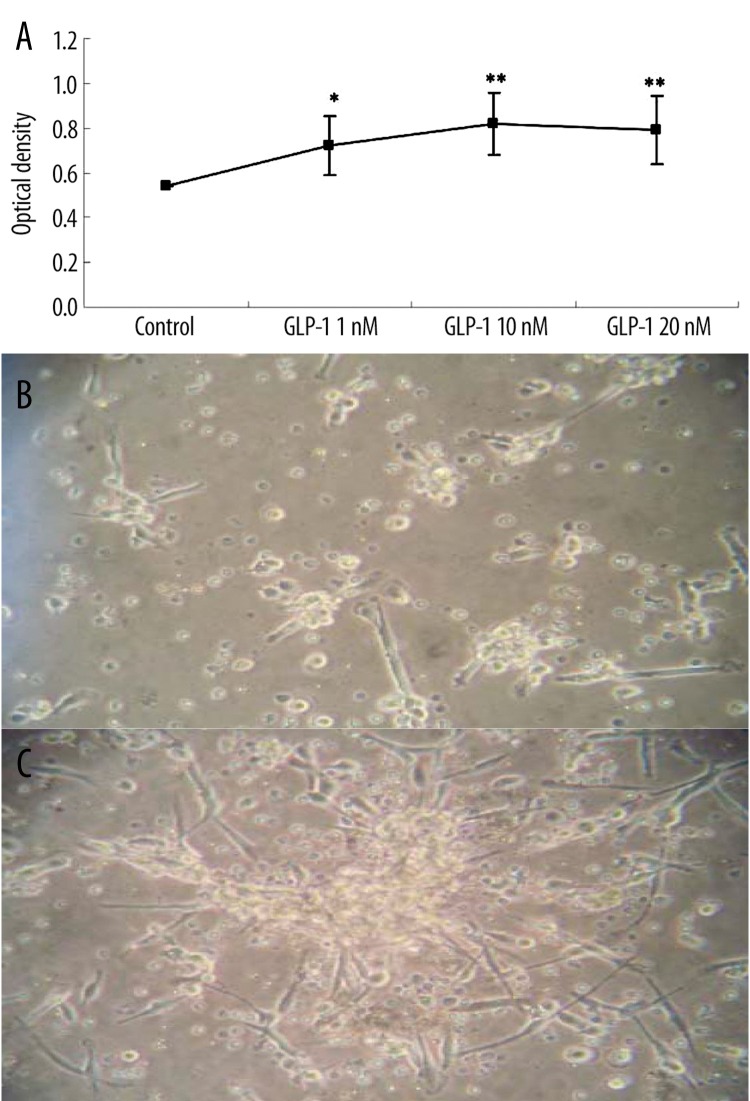
After seeding MNCs on wells, cells were incubated with different concentrations of GLP-1 for 72 hours. The effect of GLP-1 on EPCs proliferation was analyzed by MTT assay. GLP-1 concentration dependently improved EPCs proliferation activity, which became apparent at 1nmol/l (Figure 2).
Figure 2.
Effects of glucagon-like peptide-1 (GLP-1) on proliferation of EPCs. GLP-1 increases EPCs proliferation capacity. Cells were isolated and incubated with different concentrations of GLP-1 for 72 hours. MTT assay was also performed for EPCs proliferation activity, normalized to cells incubated in control medium. Data are expressed as means ±SE; n=6, *P < 0.05 vs. control. **P<0.01 vs. control (Figure 2A). Cell colony formation was observed in EPCs cultured with GLP-1 10nM (Figure 2C) different from control (Figure 2B).
Effects of GLP-1 on the expression of KDR, FLT-1, VE-cadherin eNOS mRNA in EPCs
Next, we investigated GLP-1 capable of improving the functional capacity of differentiation of EPCs. KDR, Flt-1, VE-cadherin and eNOS are key EC-lineage markers. The mRNA levels of KDR, Flt-1, VE-cadherin and eNOS were determined by using RT-PCR. GLP-1 modulated the expression of these EC-specific markers in EPCs in a dose-dependent manner at 72 hours (Figure 3). Pretreatment with GLP-1 significantly increased the KDR, Flt-1, VE-cadherin and eNOS expression. These results indicate that GLP-1 accelerates the differentiation of EPCs into endothelial cells.
Figure 3.
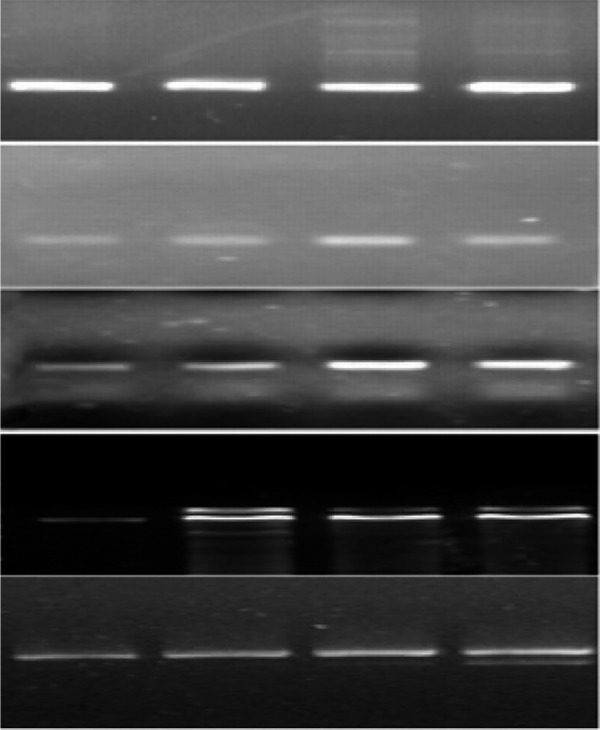
GLP-1 markedly increased KDR, Flt-1, VE-cadherin and eNOS mRNA Levels in a dose-dependent manner.1: Control group, 2: GLP-1 1 nM group; 3: GLP-1 10 nM group; 4: GLP-1 20 nM group.
GLP-1 augmented the VEGF generation
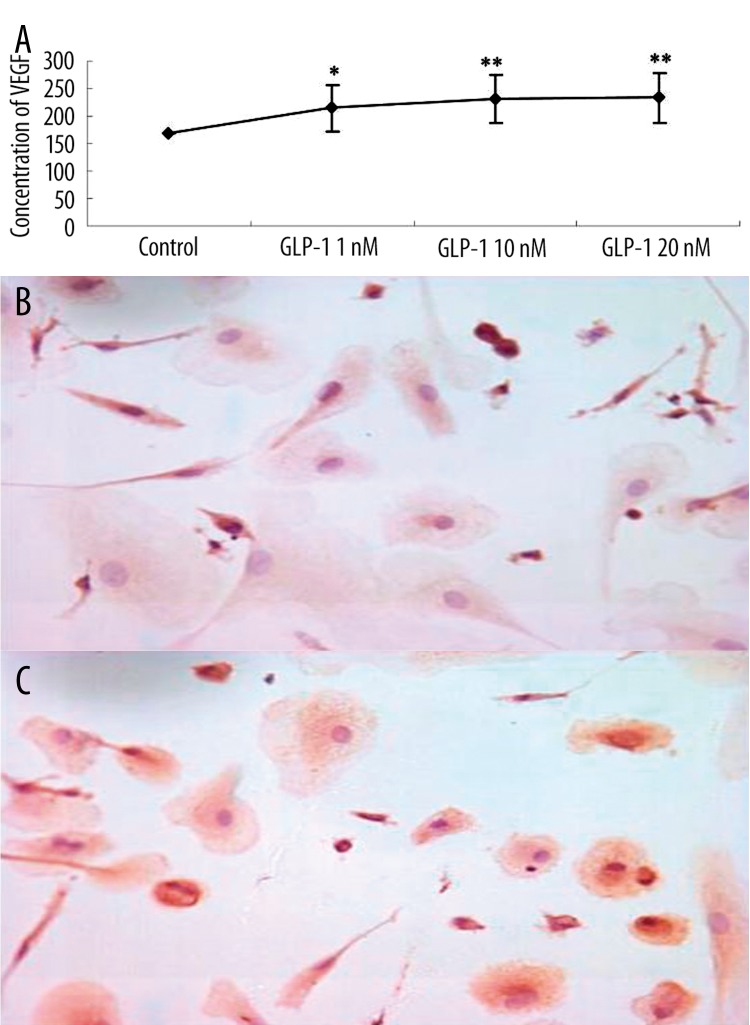
EPCs generated and released VEGF by autocrine or paracrine, which play an important role in the process of vascular endothelial cell differentiation and maturation. We therefore investigated the effects of GLP-1 on VEGF generation in EPCs. As shown in Figure 4A and C, GLP-1 augmented the VEGF generation from EPCs. The concentration of VEGF in GLP-1 culture media on 72 hours was significantly greater in the GLP-1 group than in the control group. Immunocytochemical examination revealed that EPCs expressed VEGF protein intensively in GLP-1-conditioned EPCs (Figure 4C). Thus, GLP-1 significantly augmented VEGF production and release from EPCs during EPCs differentiation.
Figure 4.
(A) VEGF concentration in culture medium was significantly greater in the GLP-1 group than in the control group. Data are expressed as means ±SE; n=6, *P<0.05 vs. control. **P<0.01 vs. control. (B) Immunocytochemical examination revealed weakly positive expression of VEGF in control group. (C) Strongly positive expression of VEGF in GLP-1 10 nM group (SP, ×600).
VEGF improve proliferation and differentiation of EPCs
To elucidate the role of VEGF released from EPCs on their self-differentiation, we examined the effects of exogenous rhVEGF in (10,20,30 ng/mL) on the proliferation and differentiation of EPCs [6]. Exogenous VEGF significantly enhanced the proliferation and expression of FLT-1, KDR, VE-cadherin, eNOS mRNA of EPCs.
Increase of VEGF autocrine plays an important role in the proliferation and differentiation of EPCs
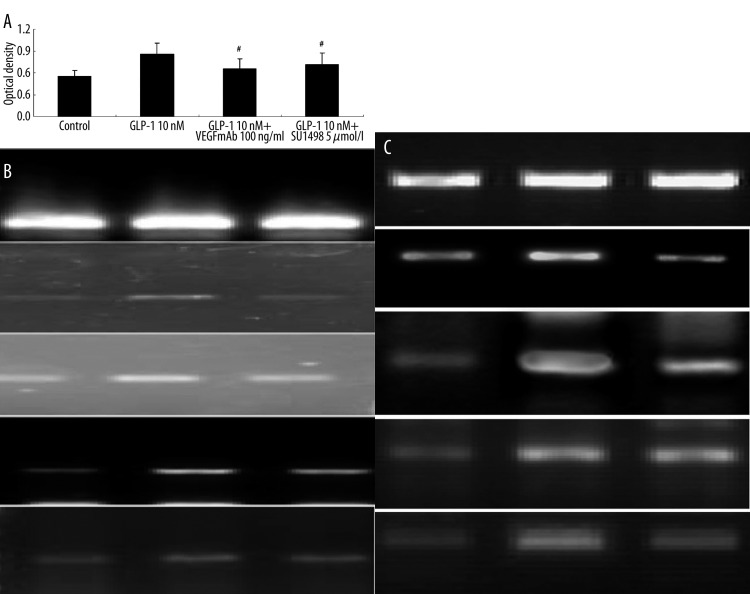
To test our hypothesis that GLP-1 influences proliferation and differentiation of EPCs increase of VEGF, we examined the effects of the anti-VEGFmAb, the tyrosine kinase inhibitor genistein, and the KDR-specific receptor tyrosine kinase inhibitor, SU1498, on the activity of EPCs [9]. Both of these 2 agents potently inhibited the proliferation of EPCs pre-incubated with GLP-1. Moreover, the anti-VEGFmAb and SU1498 significantly suppressed FLT-1, KDR, VE-cadherin and eNOS mRNA expression of EPCs cultured by GLP-1, indicating VEGF and KDR may mediate the improvement effect of GLP-1 on EPCs. GLP-1 downregulated EPCs by modulating VEGF secretion.
Discussion
Previous studies have shown that GLP-1 secreted from the gut is an incretin that has diverse actions, including promotion of propagation and differentiation of pancreatic islet B cells, increased secretion of insulin, inhibition of food and water intake, gastric emptying, and stimulation of neuroendocrine responses characteristic of visceral illness [10,11]. In the present study we demonstrated that exposure of cultured EPCs to GLP-1 improved the proliferation activity. KDR, Flt-1, VE-cadherin and eNOS are key EC-lineage markers. In addition, a greater number of GLP-1-conditioned EPCs expressed EC-lineage markers, FLT-1, eNOS, VE-cadherin and KDR compared with control, suggesting that GLP-1 accelerates the differentiation of EPCs into endothelial cells. Studies indicated that endothelial function is correlated with the number of circulating EPCs [12,13]. It is thought that regulation of the number and function of EPCs directly influences the maintenance and development of atherosclerosis. It is clinically important to estimate the degree of EPCs bioactivity and to increase the EPCs bioactivity by appropriate interventions [14]. Thus, these results suggest that GLP-1 potentially improved the endothelial cell dysfunction via regulating EPCs. In agreement with our study, numerous findings suggest a role for GLP-1 cardiovascular protective effect. GLP-1 potentially improved the endothelial cell dysfunction associated with premature atherosclerosis identified in type 2 diabetic patients [15]. Furthermore, GLP-1 has antihypertensive and cardiac and renal protective effects in rats fed a high salt diet [16]. Infusion of GLP-1 in subjects with type 2 diabetes and stable coronary disease has shown beneficial effects on flow-mediated vasodilatation [17].
We next examined the potential mechanisms by which GLP-1 enhanced proliferation and differentiation of EPCs. It has been shown that EPCs and endothelial cells produced and released cytokine or growth factor by autocrine or paracrine, such as VEGF, CP-1, FGF, TGF1 [18]. We observed that EPCs intervened by GLP-1, VEGF mRNA expression, cellular VEGF and supernatant VEGF were increased markedly, demonstrating that GLP-1 improves VEGF autocrine or paracrine in EPCs. VEGF is an important cytokine in the process of vascular endothelial cell differentiation and maturation. The increase of VEGF autocrine induced by GLP-1 may in turn influence the classical process of angiogenesis, namely the proliferation, migration and survival of mature endothelial cells. Many studies have established that ectogenesis VEGF can promote the EPCs endothelial cell differentiation, which agrees with the present study. Our study also indicated that EPCs cultivated from different sources showed a marked expression of growth factors such as VEGF, HGF, and IGF-1 [19,20]. These results support the notion that GLP-1 improves the function of EPCs through upregulating VEGF generation.
To minimize the possibility that our observations were the result of nonspecific pharmacological effects, we employed anti-VEGFmAb and exogenous VEGF to elucidate the role of autocrine VEGF released from EPCs. First, anti-VEGFmAb, blocking the endogenic VEGF, potently suppressed the proliferation function of EPCs. Simultaneously, the expressions of EC-lineage markers were decreased in the GLP-1 group, indicating the inhibition of differentiation of EPCs into endothelial cells. Second, exogenous VEGF enhanced both proliferation and differentiation activity of EPCs in a dose-dependent manner, consistent with the report that exogenous VEGF administration significantly enhanced the differentiation of PB-MNCs into EPCs. Thus, by using anti-VEGFmAb and exogenous VEGF, our data conclusively demonstrates that GLP-1 results in increase of VEGF generation, which appears to mediate beneficial effects of GLP-1 on EPCs. These novel findings suggested that VEGF is potentially the key effector linking GLP-1 to the improvement of EPCs.
It has been suggested that VEGF elicits the endothelialization and angiogenic function in EPCs mainly through the receptor tyrosine kinase KDR and Flk-1. That is, VEGF induces EPCs proliferation and increases PB-MNCs differentiation into EPCs via the KDR/Flk-1. Moreover, FLT-1 and KDR are important markers of maturated vascular endothelial cells [21]. Expression of the VEGF receptors KDR and Flt-1 in EPCs gradually increased with the duration of cell GLP-1 culture. These findings indicate that cells exposed to GLP-1 acquire enhanced responsiveness to endogenous VEGF. Studies have shown the VEGF-KDR signaling pathway also induces KDR expression as a positive feedback mechanism [22]. Thus, augmented VEGF release might have enhanced the expression of KDR on EPCs. To further clarify the mechanism, we tested the KDR-specific tyrosine kinase inhibitor SU1498 on the proliferation and differentiation of EPCs. SU1498 suppressed the proliferation and expression of EC-lineage markers. These findings clearly suggest that the VEGF generated from EPCs, and the VEGF signaling through the KDR receptor tyrosine kinase, play important roles in the differentiation of PB-MNCs into EPCs. Thus activation of KDR, as well as the increase in its expression, may be involved in the mechanism by which GLP-1 stimulates EPCs proliferation and differentiation activities.
Taken together, these results indicate that GLP-1 improves VEGF generation, which contributed to improvement of EPCs biological function, partly by tyrosine kinase KDR. VEGF is a necessary intermediate mediating the effects of GLP-1 on EPCs. These changes offer a novel explanation that upregulation EPCs bioactivities may be one of the mechanisms of GLP-1 cardiovascular protective effect.
Figure 5.
Exogenous addition of VEGF significantly enhanced the proliferation (A) and expression of FLT-1, KDR, VE-cadherin, eNOS mRNA in EPCs in a dose-dependent manner. (B). Data are expressed as means ±SE; n=6, *P<0.05 vs. control. **P<0.01 vs. control.1: Control group; 2: VEGF 10 ng/mL group; 3: VEGF 20 ng/mL group 4 VEGF 30 ng/mL group.
Figure 6.
(A) Both anti-VEGF mAb and SU1498 significantly decreased proliferation ability of EPCs cultured in GLP-1 10nM. Data are expressed as means ±SE; n=6, #P<0.05 vs. GLP-1 10 nM. (B) The neutralizing anti-VEGF mAb significantly inhibited expression of these EC-specific markers KDR, Flt-1, VE-cadherin and eNOS (1: Control group; 2: GLP-1 10nM group; 3: GLP-1 10nM + VEGFmAb 100 ng/ml group). (C) The KDR-specific tyrosine kinase inhibitor SU1498 significantly suppressed KDR, Flt-1, VE-cadherin and eNOS expression in EPCs (1: Control group, 2: GLP-1 10nM group, 3: GLP-1 10nM + SU1498 5 μM group).
Footnotes
Source of support: Departmental sources
References
- 1.Liu HB, Dear AE, Knudsen LB, Simpson RW. A long-acting glucagon-like peptide-1 analogue attenuates induction of plasminogen activator inhibitor type-1 and vascular adhesion molecules. J Endocrinol. 2009;201(1):59–66. doi: 10.1677/JOE-08-0468. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–67. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 3.Balbarini A, Barsotti MC, Di Stefano R, et al. Circulating endothelial progenitor cells characterization, function and relationship with cardiovascular risk factors. Curr Pharm Des. 2007;13(16):1699–713. doi: 10.2174/138161207780831329. [DOI] [PubMed] [Google Scholar]
- 4.van Royen N, Hoefer I, Buschmann I, et al. Effects of local MCP-1 protein therapy on the development of the collateral circulation and atherosclerosis in Watanabe hyperlipidemic rabbits. Cardiovasc Res. 2003;57:178–85. doi: 10.1016/s0008-6363(02)00615-6. [DOI] [PubMed] [Google Scholar]
- 5.Bompais H, Chagraoui J, Canron X, et al. Human endothelial cells derived from circulating progenitors display specific functional properties as compared to mature vessel wall endothelial cells. Blood. 2003;20:20. doi: 10.1182/blood-2003-08-2770. [DOI] [PubMed] [Google Scholar]
- 6.Li Wei, Shiyu Y, Zhong H, et al. Growth factors enhance endothelial progenitor cell proliferation under high-glucose conditions. Med Sci Monit. 2009;15(12):BR357–63. [PubMed] [Google Scholar]
- 7.Kalka C, Masuda H, Takahashi T, et al. Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000;86:1198–202. doi: 10.1161/01.res.86.12.1198. [DOI] [PubMed] [Google Scholar]
- 8.Papayannopoulou T. Current mechanistic scenarios in hematopoietic stem/progenitor cell mobilization. Blood. 2004;103:1580–85. doi: 10.1182/blood-2003-05-1595. [DOI] [PubMed] [Google Scholar]
- 9.Boguslawski G, McGlynn PW, Harvey KA, et al. SU1498, an Inhibitor of Vascular Endothelial Growth Factor Receptor 2, Causes Accumulation of Phosphorylated ERK Kinases and Inhibits Their Activity in Vivo and in Vitro. J. Biol. Chem. 2004;279(2):5716–24. doi: 10.1074/jbc.M308625200. [DOI] [PubMed] [Google Scholar]
- 10.David A, D’Alessio, Darleen A, et al. Seeley new ways in which GLP-1 can regulate glucose homeostasis. J Clin Invest. 2005;115(12):3406–8. doi: 10.1172/JCI27207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Perry T, Kindy MS, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Neuroscience. 2009;106(4):1285–90. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loomans CJ, de Koning EJ, Staal FJ, et al. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–99. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 13.Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:e1–e7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 14.Urbich C, Dimmeler S. Endothelial progenitor cells characterization and role in vascular biology. Circ Res. 2004;95:343–53. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 15.Nystrom T, Gutniak MK, Zhang Q, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004;287:E1209–15. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- 16.Yu M, Moreno C, Hoagland KM, et al. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens. 2003;21(6):1125–35. doi: 10.1097/00004872-200306000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Nyström T. The potential beneficial role of glucagon-like peptide-1 in endothelial dysfunction and heart failure associated with insulin resistance[J] Horm Metab Res. 2008;40(9):593–606. doi: 10.1055/s-0028-1082326. [DOI] [PubMed] [Google Scholar]
- 18.Hur J, Yoon CH, Kim HS, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–93. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 19.Pipp F, Heil M, Issbrucker K, et al. VEGFR-1-selective VEGF homologue PIGF is arteriogenic: evidence for a monocyte-mediated mechanism. Circ Res. 2003;92:378–85. doi: 10.1161/01.RES.0000057997.77714.72. [DOI] [PubMed] [Google Scholar]
- 20.Crosby JR, Kaminski WE, Schatteman G, et al. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res. 2000;87:728–30. doi: 10.1161/01.res.87.9.728. [DOI] [PubMed] [Google Scholar]
- 21.Gill M, Dias S, Hattori K, et al. Vascular trauma induces rapid but transient mobilization of VEGFR2+ AC133+ endothelial precursor cells. Circ Res. 2001;88:167–74. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 22.Shen BQ, Lee DY, Gerber HP, et al. Homologous up-regulation of KDR/Flk-1 receptor expression by vascula rendothelial growth factor in vitro. J Biol Chem. 1998;273:29979–85. doi: 10.1074/jbc.273.45.29979. [DOI] [PubMed] [Google Scholar]