Summary
Background
Resistance to antimicrobial agents among Staphylococcus aureus is an increasing problem. Two common genes responsible for resistance to macrolide, lincosamide and streptogramin B (MLSB) antibiotics are the ermA and ermC genes. Three resistance phenotypes have been detected to these antibiotics: strains containing cMLSB (constitutive MLSB) and iMLSB (inducible MLSB), which are resistant to macrolide, lincosamide and streptogramin B antibiotics, and MS, which is only resistant to macrolide and streptogramin B antibiotics. The aim of this study was to determine the prevalence of MLSB phenotypes and genotypes in erythromycin-resistant strains of S. aureus isolated from patients in 4 university hospitals in Tehran, Iran.
Material/Methods
S. aureus strains were isolated from various clinical specimens and identified by routine phenotypic methods and PCR for nuc gene. Erythromycin resistance was determined by disk diffusion testing. Prevalence of MLSB phenotypes was determined by use of the D-test. ermA and ermC genes were detected by PCR.
Results
Altogether, 126 erythromycin-resistant strains of S. aureus were detected. Prevalence of cMLSB, iMLSB and MS resistance phenotypes were 92.8%, 6.4%, and 0.8%, respectively; 60.3% of strains had ermA gene and 54.8% ermC gene; 61 strains (48.4%) contained 2 studied erm genes and 42 strains (33.3%) did not have any studied erm genes.
Conclusions
Due to the high prevalence of clindamycin resistance among S. aureus isolated from patients in Iran, we recommend clindamycin therapy only after proper antimicrobial susceptibility testing.
Keywords: clindamycin, D-test, ermA, ermC, erythromycin, Staphylococcus aureus
Background
Macrolide, lincosamide and streptogramin B (MLSB) antibiotics have different structure, but similar mode of action. These antibiotics inhibit bacterial protein synthesis by binding to 23s rRNA in 50S ribosomal subunits [1]. Erythromycin (a macrolide) and clindamycin (a lincosamide) are widely used in treatment of Staphylococcus aureus infections [2,3]. Clindamycin represents an attractive option for several reasons. Firstly, good oral absorption of clindamycin makes it suitable for outpatient therapy or as follow-up after intravenous therapy. Secondly, it has high tissue penetration (except for the central nervous system) and accumulation in abscesses, and no need for renal dosing adjustments. Thirdly, clindamycin can be used as an alternative antibiotic in patients allergic to penicillin. Fourthly, community-acquired methicillin-resistant S. aureus, which has rapidly emerged in recent years as a cause of skin and soft-tissue infections, has shown susceptibility to clindamycin. Finally, it has been shown that clindamycin inhibits the production of toxins and virulence factors in gram-positive organisms through inhibition of protein synthesis [2,4]. However, resistance to erythromycin and clindamycin is increasing among clinical isolates of S. aureus worldwide [3].
Three mechanisms have been reported for resistance to MLSB antibiotics: target site modification, efflux of antibiotics, and drug modification [1]. Methylation of the A2058 residue, located in the conserved domain V of 23s rRNA, takes place in target-site modification and prevents the binding of MLSB antibiotics to their ribosomal target. This phenomenon leads to cross-resistance to these antibiotics, and produces the MLSB phenotype that was encoded by erythromycin ribosome methylases (erm) genes [5,6]. Among the 4 major classes of erm genes (ermA, ermB, ermC and ermF) in different bacteria, ermA and ermC are the primary genes responsible for MLSB resistance in S. aureus [1,2,5].
On the other hand, MLSB phenotype can be constitutive (rRNA methylase is always produced) or inducible (methylase is produced only in the presence of an inducing agent) [1,7]. While strains with constitutive MLSB resistance (cMLSB) phenotypes can be detected by routine disk diffusion testing, strains with inducible MLSB resistance (iMLSB) phenotypes show resistance to erythromycin and sensitivity to clindamycin, similar to strains containing the MS phenotype, which had resistance to only macrolide and streptogramin B, not to clindamycin. Therefore, a special disk diffusion method, the D-test, was developed for the detection of iMLSB. In this test, an erythromycin disk was placed in close proximity to a clindamycin disk. In iMLSB-resistant strains, resistance to clindamycin is induced by diffusion of erythromycin through the agar, and leads to flattening of the clindamycin zone of inhibition adjacent to the erythromycin disk (a D-shaped zone), while MS phenotype-containing strains form a circular zone around the clindamycin disk [2].
There are reports of clinical failures of clindamycin in treating patients with iMLSB resistance phenotype [8–12], attributed to selection for a mutation in the macrolide-responsive promoter region upstream of the erm gene and emergence of cMLSB-resistant isolates [1], leading some investigators to recommend that clindamycin therapy be avoided for S. aureus isolates that display the iMLSB resistance phenotype [1,9,11]. On the other hand, labeling all erythromycin-resistant S. aureus as clindamycin-resistant may prevent the use of clindamycin in cases where it would be effective therapy [2]. Thus, accurate detection of iMLSB-resistant strains is very important.
The present investigation was undertaken to determine the prevalence of cMLSB, iMLSB and MS resistance phenotypes and primary erm genes (ermA and ermC) in 126 erythromycin-resistant S. aureus isolates from patients in Tehran, Iran.
Material and Methods
Bacterial strains
All Staphylococcus aureus isolates from various clinical samples (wounds, abscesses, urine, blood, sterile body fluids, and respiratory tract samples), identified from January to June 2008 in 4 university hospitals (3 general hospitals and 1 burn hospital) in Tehran, Iran, were included in this study. Multiple isolates from the same patient were excluded, even when the site of infection was different. Identification of the isolates as S. aureus was based on colony and microscopic morphology, growth on mannitol salt agar and fermentation of mannitol, and production of catalase, coagulase, and deoxyribonuclease. Moreover, amplification of the species-specific nuc gene was used to confirm phenotypic identification of S. aureus isolates, as described below. Confirmed S. aureus strains were stored at −70°C in nutrient broth plus 15% glycerol.
Phenotypic determination of antibiotic resistance
Disk diffusion testing was used to determine antibiotic resistance according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI), with S. aureus ATCC 25923 as control. For detecting erythromycin and clindamycin resistance, 15 μg erythromycin disks and 2 μg clindamycin disks (purchased from Mast Co., Merseyside, UK) were used. Interpretation of the diameters of zones of inhibition was as follows: for erythromycin =23 mm; S, 14–22 mm; I, =13 mm; R, and for clindamycin =21 mm; S, 15–20 mm; I, =14 mm; R [13]. Intermediate resistant strains were considered resistant. Erythromycin-resistant S. aureus strains were selected for further studies.
D-testing was performed for erythromycin-resistant S. aureus strains according to the guidelines of the CLSI. Suspension equivalent to 0.5 McFarland of each freshly cultured isolate in normal saline was prepared and used for inoculation of Mueller-Hinton agar (Merck-Hampshire, England) plates. Erythromycin and clindamycin disks were placed on inoculated plates 15 mm apart (edge-to-edge). Plates were read after 18 h of incubation at 35°C and the shape of the clindamycin zone was verified. Strains resistant to both antibiotics were considered to have cMLSB resistance. Strains with flattening of the susceptible zone of inhibition to clindamycin adjacent to the erythromycin disk (D-shape) were considered to contain iMLSB-resistance, while strains with circular zones were considered to contain MS resistance [2].
Polymerase Chain Reaction (PCR)
DNA was extracted from all erythromycin-resistant S. aureus strains by rapid DNA extraction method [14]. Five colonies of each isolate’s overnight growth on brain-heart infusion agar were suspended in 300 μl of sterile distilled water and heated for 15 min at 100°C. After centrifugation at 14 000 rpm for 5 min, supernatant was collected and used as the DNA template in each PCR run.
PCR was performed with primers specific for ermA, ermC, mecA and nuc genes according to previous studies [3,15,16]. Primers were purchased from Cinnagen, Iran, and their sequences, thermal cycling profile and PCR fragment size are shown in Table 1. PCR reaction was performed in a 20 μl volume, and 2 μl of DNA template was added to 18 μl of PCR mixture consisting of 2 μl of PCR buffer (10×), 1 μl of MgCl2 (50mM), 4 μl of dNTPs (1mM), 1 μl of each primers (10 Pmol), 0.25 μl of Taq DNA polymerase (5 u/μl) and 8.75 μl of double-distilled water. DNA amplification was carried out in a thermocycler (Touchgene Gradient, Techne, UK). In each thermal cycling profile, there was an initial denaturation step at 94°C for 5 min, and a final extension step at 72°C for 5 min. After PCR amplification, 5 μl of PCR product was subjected to agarose gel electrophoresis (2% agarose, 1× Tris-acetate-EDTA, 100 V, 100 min). The gel was stained with ethidium bromide, and a PCR fragment was visualized using a gel documentation system by comparison with a molecular size marker (100 bp ladder, Eurobio, UK). Positive control strains for ermA and ermC donated by Mohammad Emaneini (Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran) and molecular grade water instead of template DNA as the negative control were included in each run. S. aureus ATCC29213 (without ermA, ermC, and mecA genes) was also used as a negative control.
Table 1.
Primers sequence, thermal cycling profile and size of amplified PCR fragment in each PCR reaction.
| Gene | Primers sequences | No. of PCR cycles (condition) | No. of nucleotide for amplified PCR fragment | Reference |
|---|---|---|---|---|
| nuc | 5′-GCGATTGATGGTGATACGGTT-3′ 5′-AGCCAAGCCTTGACGAACTAAAGC-3′ |
30 (1 min at 94°C, 1 min at 50°C, 2 min at 72°C) | 279 | [15] |
| mecA | 5′-GTAGAAATGACTGAACGTCCGATAA-3′ 5′-CCAATTCCACATTGTTTCGGTCTAA-3′ |
30 (30 s at 94°C, 30 s at 52°C, 30 s at 72°C) | 310 | [15] |
| ermA | 5′-GTTCAAGAACAATCAATACAGAG-3′ 5′-GGATCAGGAAAAGGACATTTTAC-3′ |
35 (30 s at 94°C, 30 s at 52°C, 1 min at 72°C) | 421 | [3,16] |
| ermC | 5′-GCTAATATTGTTTAAATCGTCAATTCC-3′ 5′-GGATCAGGAAAAGGACATTTTAC-3′ |
35 (30 s at 94°C, 30 s at 52°C, 1 min at 72°C) | 572 | [3,16] |
Results
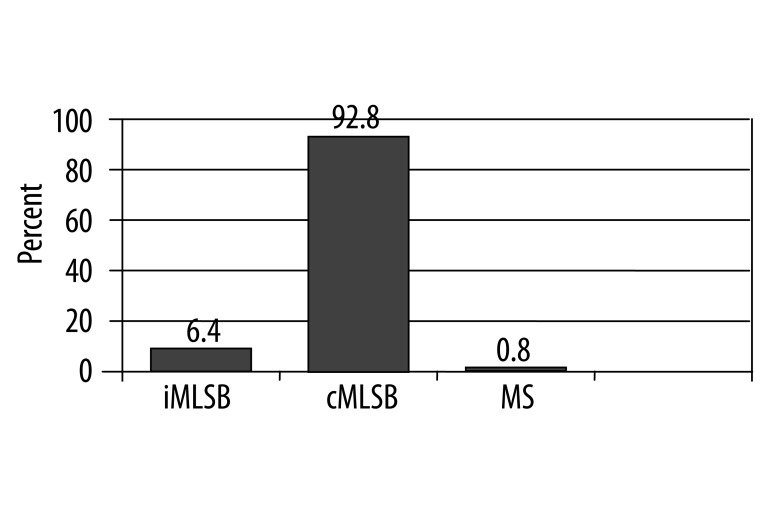
During the 6-month study period, 186 Staphylococcus aureus strains were isolated from patients admitted to 4 university hospitals in Tehran, Iran. As expected, all phenotypically detected S. aureus strains showed species-specific nuc gene amplification in PCR, as reported by Zhang K., et al. [15]. In disk diffusion testing, 126 strains (67.7%) showed resistance to erythromycin and were selected for further study. From these, 117 strains (92.9%) showed resistance to clindamycin in disk diffusion testing and 9 strains were clindamycin-susceptible. In D-testing, 117 strains (92.9%) exhibited cMLSB resistance, 8 strains (6.3%) iMLSB, and 1 strain (0.8%) had MS resistance phenotype (Figure 1). Therefore, from 9 clindamycin susceptible strains in disk diffusion testing, 8 strains had iMLSB and 1 strain had MS resistance phenotypes.
Figure 1.
The result of D-test for erythromycin-resistant S. aureus strains.
In PCR testing, 76 and 69 strains (60.3% and 54.8%) showed ermA and ermC genes amplification, respectively; 84 strains (66.7%) contained 1 or 2 of the studied erm genes and 42 strains (33.3%) did not contain any studied erm genes. Both genes (ermA and ermC) were co-present in 61 strains (48.4%). Table 2 shows the difference in MLSB resistance phenotypes in relation to presence of ermA and ermC genes. In strains with cMLSB and iMLSB resistance phenotypes, the most prevalent genotype was ermA + ermC, while strains with the MS resistance phenotype had only the ermA gene.
Table 2.
Difference of MLSB resistance phenotypes regarding presence of studied erm genes.
| Gene | No. of strains with resistance phenotype | ||
|---|---|---|---|
| cMLSB (n=116) | iMLSB (n=8) | MS (n=1) | |
| ermA alone | 10 | 2 | 1 |
| ermC alone | 7 | 0 | 0 |
| ermA + ermC | 61 | 5 | 0 |
| without ermA and ermC | 39 | 1 | 0 |
The mecA gene was detected in 86 strains by PCR, thus 68.3% of strains were considered methicillin-resistant Staphylococcus aureus (MRSA). Most strains with the cMLSB resistance phenotype were MRSA (69.2%). Also, among 8 strains with iMLSB resistance phenotypes, 5 strains were MRSA, while 1 strain with the MS resistance phenotype was MSSA.
Of 86 MRSA strains, 70 strains contained the ermA gene, while in 40 MSSA strains only 6 strains contained this gene. In addition, 64 MRSA strains and 5 MSSA strains contained ermC genes. Prevalence of the ermA gene in MRSA and MSSA strains was 81.4% and 15%, respectively, and for the ermC gene prevalence was 74.4% and 12.5%, respectively. ermA and ermC genes were co-present in 58 (67.4%) of MRSA strains and in 3 (7.5%) of the MSSA strains. Therefore, ermA and ermC genes were more common in MRSA erythromycin-resistant strains than in MSSA strains (Table 3).
Table 3.
Distribution of ermA and ermC genes among studied strains.
| Gene | No. (%) in | ||
|---|---|---|---|
| MRSA (n=86) | MSSA (n=40) | total strains (n=126) | |
| ermA alone | 12 (14%) | 3 (7.5%) | 15 (11.9%) |
| ermC alone | 6 (7%) | 2 (5%) | 8 (6.4%) |
| ermA + ermC | 58 (67.4%) | 3 (7.5%) | 61 (48.4%) |
| without ermA and ermC | 10 (11.6%) | 32 (80%) | 42 (33.3%) |
Discussion
The increasing frequency of S. aureus infections and their antimicrobial resistance have led to renewed interest in the use of MLSB antibiotics, especially clindamycin, for treatment of these infections in many countries [17]. For appropriate therapy decision-making, accurate susceptibility data are important. However, only a few published articles are available on the prevalence of erythromycin and clindamycin resistance in Iranian isolates of S. aureus. Moreover, false susceptibility results for clindamycin may be obtained if isolates are not tested for iMLSB resistance by D-testing, because it cannot be determined using standard susceptibility tests [2]. Recognition of this type of resistance is important because treatment of patients harboring iMLSB-resistant S. aureus with clindamycin leads to the development of constitutive resistance, subsequently leading to therapeutic failure [8–12].
In this study, resistance to erythromycin (67.7%) was higher than in studies from other countries, such as the study of Schmitz et al. on S. aureus isolated from patients in 20 European university hospitals with rates of 39% [18]. In another study in Tehran, resistance to erythromycin in clinical isolates of S. aureus was also high (56.2%) [19]. Most erythromycin-resistant strains (92.9%) in this study also showed clindamycin resistance and were MRSA (68.3%).
In the present study, prevalence of cMLSB, iMLSB and MS resistance phenotypes among erythromycin-resistant S. aureus was 92.9%, 6.3% and 0.8%, respectively. There is only 1 previous study of MLSB resistance among S. aureus isolated in Iran for comparison [20], reporting 9.7% of S. aureus strains isolated from patients in Milad Hospital, Tehran, had the iMLSB resistance phenotype. In the present study, the cMLSB resistance phenotype was more common than the iMLSB resistance phenotype among erythromycin – resistant S. aureus isolates, in agreement with the results of the 3-year study by Spiliopoulou et al. on 173 erythromycin-resistant S. aureus strains isolated from patients in a university hospital in Greece, which reported 61.3%, 30.6% and 7.5% prevalence to cMLSB, iMLSB and MS resistance phenotypes, respectively [16]. Higher prevalence of cMLSB than iMLSB resistance phenotype among S. aureus isolates were also reported in other studies [2,4,18]. Low prevalence of the MS resistance phenotype seen in the present study has also been shown in other studies performed in Turkey, the neighboring country of Iran [17,21]; although in the previously mentioned European study [18], it was relatively high (13%). Such differences in the MLSB resistance pattern could be caused by differences in guidelines for drug usage in Iran, where MLSB antibiotics are widely used in treating S. aureus infections. However, since the occurrence of iMLSB varies widely by hospital and geographic region [22], it is necessary to perform the D-zone test for erythromycin-resistant, clindamycin-susceptible S. aureus isolates [23].
We studied the distribution of 2 erm genes (ermA and ermC) among erythromycin-resistant S. aureus isolates. These genes were reported as being the most prevalent genes responsible for resistance to MLSB antibiotics within S. aureus strains in other studies, including the study by Lina et al in French hospitals [3]. In the present study, ermA and ermC were also prevalent in erythromycin-resistant S. aureus isolates (60.3% and 54.8%, respectively), although there was no significant difference between their presence. It should be noted the prevalences of these genes were variable in different studies, and in some studies ermA was more prevalent than ermC, while in other studies the reverse was found. In research by Martineau et al, the prevalence of ermA and ermC in erythromycin-resistant S. aureus strains was 21% and 10.2%, respectively [24]. Schmitz et al. [25] found ermA was more prevalent than ermC (67% and 23%, respectively). On the other hand, 16% and 84% of S. aureus strains isolated in Denmark were carrying ermA and ermC, respectively [26]. In the study by Spiliopoulou et al. [16], ermC was more prevalent than ermA (70% and 22%, respectively).
A notable finding of the present study was the co-existence of ermA and ermC in a significant number of erythromycin-resistant S. aureus strains (48.4%), similar to results from 2 studies in Turkey (37.5% and 18.6%), and in contrast to 2 studies in European countries (0.6% and 3%) [16,25,27,28]. We also found that a significant number of erythromycin-resistant S. aureus isolates (33.3%) did not carry ermA and ermC, therefore other genes also have a significant role in resistance to erythromycin.
We found prevalence of 81.4% for MRSA strains and 15% for MSSA isolates for ermA. Prevalence of ermC in MRSA and MSSA isolates were 74.4% and 12.5%, respectively; therefore, both ermA and ermC were more prevalent among MRSA than MSSA isolates in Tehran, Iran. These results regarding predominance of the ermA among MRSA isolates are consistent with previous reports [25,29], but predominance of the ermC among MRSA isolates has not been reported, except from Greece [16]. However, prevalence of these genes among MRSA and MSSA strains were variable in different studies. A multicenter study in 24 European university hospitals [25] revealed that in S. aureus, the ermA gene was more common among MRSA isolates (88%) than in MSSA isolates (38%). In contrast, ermC was more common in MSSA (47%) than in MRSA (5%). Otsuka et al. [29] found the ermA gene was also predominant among erythromycin-resistant isolates of MRSA compared to MSSA strains (95.0% and 53.3%, respectively), while the ermC gene were more prevalent among MSSA than MRSA strains (42.0% and 11.5%, respectively). High prevalence of erm genes in MRSA strains emphasizes the need for performing antimicrobial susceptibility testing when clindamycin is considered for use in treatment of infections caused by MRSA.
Conclusions
Although there are some reports of MLSB resistance phenotypes in Iranian isolates of S. aureus, to our knowledge this is the first report of the involved genes in Iran. We found ermA + ermC related resistance was the most prevalent in erythromycin-resistant S. aureus isolates, and constitutive resistance was particularly predominant among MRSA strains.
Acknowledgements
This paper is the result of an M. Sc. student thesis and was financially supported by the Research Council of Shahed University. We also thank Dr. Mohammad Emaneini, Faculty member of Tehran University of Medical Sciences, Tehran, Iran, for providing control strains.
Footnotes
Source of support: Research Council of Shahed University
References
- 1.Leclercq R. Mechanisms of resistance to macrolides and lincosamides: Nature of the resistance elements and their clinical implications. Clin Infect Dis. 2002;34:482–92. doi: 10.1086/324626. [DOI] [PubMed] [Google Scholar]
- 2.Fiebelkorn KR, Crawford SA, McElmeel ML, Jorgensen JH. Practical disk diffusion method for detection of inducible clindamycin resistance in Staphylococcus aureus and coagulase-negative staphylococci. J Clin Microbiol. 2003;41:4740–44. doi: 10.1128/JCM.41.10.4740-4744.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lina G, Quaglia A, Reverdy ME, et al. Distribution of genes encoding resistance to macrolides, lincosamides, and streptogramins among staphylococci. Antimicrob Agents Chemother. 1999;43(5):1062–66. doi: 10.1128/aac.43.5.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasten MJ. Clindamycin, metronidazole, and chloramphenicol. Mayo Clin Proc. 1999;74:825–33. doi: 10.4065/74.8.825. [DOI] [PubMed] [Google Scholar]
- 5.Weisblum B. Erythromycin resistance by ribosome modification. Antimicrob Agents Chemother. 1995;39:577–85. doi: 10.1128/AAC.39.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts MC, Sutcliffe J, Courvalin P, et al. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob Agents Chemother. 1999;43:2823–30. doi: 10.1128/aac.43.12.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daurel C, Huet C, Dhalluin A, et al. Differences in potential for selection of clindamycin-resistant mutants between inducible erm(A) and erm(C) Staphylococcus aureus genes. J Clin Microbiol. 2008;46(2):546–50. doi: 10.1128/JCM.01925-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levin TP, Suh B, Axelrod P, et al. Potential clindamycin resistance in clindamycin-susceptible, erythromycin-resistant Staphylococcus aureus: report of a clinical failure. Antimicrob Agents Chemother. 2005;49(3):1222–24. doi: 10.1128/AAC.49.3.1222-1224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drinkovic D, Fuller ER, Shore KP, et al. Clindamycin treatment of Staphylococcus aureus expressing inducible clindamycin resistance. J Antimicrob Chemother. 2001;48(2):315–16. doi: 10.1093/jac/48.2.315. [DOI] [PubMed] [Google Scholar]
- 10.Frank AL, Marcinak JF, Mangat PD, et al. Clindamycin treatment of methicillin-resistant Staphylococcus aureus infections in children. Pediatr Infect Dis J. 2002;21:530–34. doi: 10.1097/00006454-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Siberry GK, Tekle T, Carroll K, Dick J. Failure of clindamycin treatment of methicillin-resistant Staphylococcus aureus expressing inducible clindamycin resistance in vitro. Clin Infect Dis. 2003;37(9):1257–60. doi: 10.1086/377501. [DOI] [PubMed] [Google Scholar]
- 12.Panagea S, Perry JD, Kate Gould F. Should clindamycin be used as treatment of patients with infections caused by erythromycin-resistant staphylococci? J Antimicrob Chemother. 1999;44:581–82. doi: 10.1093/jac/44.4.581. [DOI] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility tests; Fifteenth informational supplement. 1. Vol. 25. Clinical and Laboratory Standards Institute; Wayne, Pennsylvania, USA: 2005. CLSI document M100—S15. [Google Scholar]
- 14.Perez-Roth E, Claverie-Martin F, Villar J, Mendez-Alvarez S. Multiplex PCR for simultaneous identification of Staphylococcus aureus and detection of methicillin and mupirocin resistance. J Clin Microb. 2001;39:4037–41. doi: 10.1128/JCM.39.11.4037-4041.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang K, Sparling J, Chow BL, et al. New quadriplex PCR assay for detection of methicillin and mupirocin resistance and simultaneous discrimination of Staphylococcus aureus from coagulase-negative staphylococci. J Clin Microbiol. 2004;42(11):4947–55. doi: 10.1128/JCM.42.11.4947-4955.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spiliopoulou I, Petinaki E, Papandreou P, Dimitracopoulos G. erm(C) is the predominant genetic determinant for the expression of resistance to macrolides among methicillin-resistant Staphylococcus aureus clinical isolates in Greece. J Antimicrob Chemother. 2004;53(5):814–17. doi: 10.1093/jac/dkh197. [DOI] [PubMed] [Google Scholar]
- 17.Yilmaz G, Aydin K, Iskender S, et al. Detection and prevalence of inducible clindamycin resistance in staphylococci. J Med Microbiol. 2007;56:342–45. doi: 10.1099/jmm.0.46761-0. [DOI] [PubMed] [Google Scholar]
- 18.Schmitz FJ, Verhoef J, Fluit AC. Prevalence of resistance to MLS antibiotics in 20 European university hospitals participating in the European SENTRY surveillance programme. Sentry Participants Group. J Antimicrob Chemother. 1999;43:783–92. doi: 10.1093/jac/43.6.783. [DOI] [PubMed] [Google Scholar]
- 19.Saderi H, Owlia P, Habibi M. Mupirocin resistance among Iranian isolates of Staphylococcus aureus. Med Sci Monit. 2008;14(10):BR210–13. [PubMed] [Google Scholar]
- 20.Rahbar M, Hajia M. Inducible clindamycin resistance in Staphylococcus aureus: a cross-sectional report. Pak J Biol Sci. 2007;10(1):189–92. doi: 10.3923/pjbs.2007.189.192. [DOI] [PubMed] [Google Scholar]
- 21.Delialioglu N, Aslan G, Ozturk C, et al. Inducible clindamycin resistance in staphylococci isolated from clinical samples. Jpn J Infect Dis. 2005;58:104–6. [PubMed] [Google Scholar]
- 22.Schreckenberger PC, Ilendo E, Ristow KL. Incidence of constitutive and inducible clindamycin resistance in Staphylococcus aureus and coagulase-negative staphylococci in a community and a tertiary care hospital. J Clin Microbiol. 2004;42:2777–79. doi: 10.1128/JCM.42.6.2777-2779.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zelazny AM, Ferraro MJ, Glennen A, et al. Selection of strains for quality assessment of the disk induction method for detection of inducible clindamycin resistance in Staphylococci: a CLSI collaborative study. J Clin Microbiol. 2005;43(6):2613–15. doi: 10.1128/JCM.43.6.2613-2615.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martineau F, Picard FJ, Lansac N, et al. Correlation between the resistance genotype determined by multiplex PCR assays and the antibiotic susceptibility patterns of Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 2000;44(2):231–38. doi: 10.1128/aac.44.2.231-238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitz FJ, Sadurski R, Kray A, et al. Prevalence of macrolide-resistance genes in Staphylococcus aureus and Enterococcus faecium isolates from 24 European university hospitals. J Antimicrob Chemother. 2000;45(6):891–94. doi: 10.1093/jac/45.6.891. [DOI] [PubMed] [Google Scholar]
- 26.Westh H, Hougaard DM, Vuust J, Rosdahl VT. Erm genes in erythromycin-resistant Staphylococcus aureus and coagulase-negative staphylococci. APMIS. 1995;103:225–32. [PubMed] [Google Scholar]
- 27.Aktas Z, Aridogan A, Kayacan CB, Aydin D. Resistance to macrolide, lincosamide and streptogramin antibiotics in staphylococci isolated in Istanbul, Turkey. J Microbiol. 2007;45(4):286–90. [PubMed] [Google Scholar]
- 28.Gul HC, Kilic A, Guclu AU, et al. Macrolide-lincosamide-streptogramin B resistant phenotypes and genotypes for methicillin-resistant Staphylococcus aureus in Turkey, from 2003 to 2006. Pol J Microbiol. 2008;57(4):307–12. [PubMed] [Google Scholar]
- 29.Otsuka T, Zaraket H, Takano T, et al. Macrolide-lincosamide-streptogramin B resistance phenotypes and genotypes among Staphylococcus aureus clinical isolates in Japan. Clin Microbiol Infect. 2007;13(3):325–27. doi: 10.1111/j.1469-0691.2006.01632.x. [DOI] [PubMed] [Google Scholar]