Summary
Background
Acute decompensation heart failure (ADHF) remains a cause of hospitalization in patients with end-stage congestive HF. The administration of levosimendan in comparison with a standard therapy in CHF patients admitted for ADHF was analysed.
Material/Methods
Consecutive patients admitted for ADHF (NYHA class III–IV) were treated with levosimendan infusion 0.1 μg/kg/min or with furosemide infusion 100–160 mg per day for 48 hours (control group). All subjects underwent determination of brain natriuretic peptide (BNP), non-invasive cardiac output (CO), and echocardiogram at baseline, at the end of therapy and 1 week after therapy.
Results
Seven patients admitted for 20 treatments in 16 months (age 66 years; mean admission/year 5.4) were treated with levosimendan and compared with 7 patients admitted for 15 treatments (age 69.1 years; mean admission/year 6.1). At the end of levosimendan therapy, BNP decreased (from 679.7±512.1 pg/ml to 554.2±407.6 pg/ml p=0.03), and 6MWT and LVEF improved (from 217.6±97.7 m to 372.2±90.4 m p=0.0001; from 22.8±9.1% to 25.4±9.8% p=0.05). Deceleration time, E/A, E/E’, TAPSE, pulmonary pressure and CO did not change significantly after levosimendan therapy and after 1 week. At follow-up, only 6-min WT and NYHA class showed a significant improvement (p=0.0001, p=0.001 respectively). The furosemide infusion reduced NYHA class and body weight (from 3.4±0.6 to 2.3±0.5 p=0.001; from 77.5±8.6 kg to 76±6.6 kg p=0.04), but impaired renal function (clearances from 56.3±21.9 ml/min to 41.2±10.1 ml/min p=0.04).
Conclusions
Treating end-stage CHF patients with levosimendan improved BNP and LVEF, but this effect disappeared after 1 week. The amelioration of 6MWT and NYHA class lasted longer after levosimendan infusion.
Keywords: brain natriuretic peptide, end-stage heart failure, levosimendan
Background
Levosimendan is a pharmacological agent that exerts positive inotropic effect by binding to cardiac troponin C in a calcium-dependent manner and sensitizing myofilaments to calcium without increasing myocardial oxygen consumption [1–3]. Levosimendan also has vasodilatory properties through its facilitation of an adenosine-triphosphate-dependent potassium channel opening [4] and anti-ischemic effects [5]. In clinical studies the infusion of levosimendan increased cardiac output, reducing cardiac filling pressures, and was correlated to an improvement of cardiac symptoms and prognosis (death and hospitalization for congestive heart failure [CHF]) [6,7]. Previous experiences [7–9] suggest that a single 24-hour levosimendan infusion in patients suffering severe CHF due to left ventricular dysfunction induces beneficial hemodynamic effects, relief of symptoms and reduction in short-term morbidity and mortality compared with placebo or dobutamine. However, the largest randomized trail (SURVIVE) [10] showed an initial reduction in B-type natriuretic peptide (BNP) in the levosimendan vs. the dobutamine group, but failed to demonstrate a significant reduction of all-cause mortality or secondary clinical outcomes. Nevertheless, different single-centre observations regarding the intermittent infusion of levosimendan in severe CHF have reported an improvement of left ventricular performance, relief of symptoms and prolonged short-term survival without an increase in incidence of cardiac arrhythmias [11–14].
The objective of this study was to analyse the feasibility and efficacy of levosimendan infusion in end-stage CHF patients admitted for repetitive acute decompensation (ADHF) episodes with evidence of clinical hyperhydration, comparing these patients with a control group treated traditionally with an infusion of furosemide in order to reduce the fluid overload. The effects of the 2 different strategies on plasma BNP, echocardiographic parameters and functional variables [NYHA class, 6-min walking test (6MWT) and non-invasive cardiac output (CO)] were analysed.
Material and Methods
Patients
This single-centre prospective study, approved by the local ethics committee, included end-stage (stage D AHA/ACC) patients >18 years old, admitted into the Heart Failure Unit from October 2008 to February 2010 with the diagnosis of ADHF (NYHA functional class III to IV). Patients were included when presenting at admission all the following criteria: symptoms of CHF according to the accepted criteria in the literature [15,16], refractory to the usual pharmacological treatments; NYHA class III or IV due to a deterioration of symptoms (at least 1 class) despite optimum oral therapy; echocardiographic evidence of systolic and/or diastolic dysfunction (see below); a cardiac index (CI) ≤2.5 L/min/m2; and clinical fluid overload (≥2 findings of congestion as elevated jugular venous pressure, pulmonary rales, hepatomegaly, orthopnea, paroxysmal nocturnal dyspnoea, abdominal bloating). The exclusion criteria were: childbearing potential; CHF related to restrictive or hypertrophic cardiomyopathy or to uncorrected stenotic valvular disease; concomitant unstable angina or myocardial infarction; systolic blood pressure below 85 mmHg; severe renal failure (clearance creatinine <30 ml/min); administration of inotropes in the last week; and absence of a written consent that authorized the levosimendan infusion. The protocol of levosimendan infusion did not provide a loading dose, being administered intravenously at the dosage of 0.1 μg/kg/min for 24–36 hours in order to complete the dosage of 12.5 mg. The reduction of systolic blood pressure <80 mmHg, tachycardia with heart rate >140/min, and symptomatic hypotension were considered criteria for reducing dosage of levosimendan or suspending it for 30–60 min until the dose-limiting event had resolved. In the control group, an infusion of 100–160 mg per day of furosemide for 48 hours was administered. The dose of concomitant medications was held constant unless urgent modifications were required by the clinical status in both groups. The therapy prescribed in those patients included angiotensin-converting enzyme inhibitors (enalapril, ramipril), angiotensin receptor blockade (candesartan, losartan) in case of enalapril/ramipril intolerance, beta-blockers (metoprolol, bisoprolol or carvedilol), digoxin, loop diuretic and spironolactone at low dose. For beta-blockers, angiotensin-converting enzyme inhibitors and angiotensin receptor blockade, the patients’ maximum tolerated dose was used, after an adequate titration period. Before the infusion of levosimendan/furosemide, after ending (within 6 hours) and at 1-week follow-up all the parameters scheduled were measured. In the levosimendan group, a measurement of non-invasive CO during the infusion (between 12 and 24 hours) was obtained. The evaluation of health-related quality of life was obtained at 1-week follow-up using the Minnesota Living with Heart Failure Questionnaire (MLHFQ) in all patients [17].
The objective of this prospective study was to evaluate the immediate and short-term effects of a levosimendan infusion (without a loading dose) on plasma BNP, non-invasive CO, echocardiographic parameters, renal function and quality of life in ADHF episodes in end-stage CHF patients, comparing this treatment with a furosemide infusion.
Doppler echocardiography
Echocardiograms were performed with a Vivid 7 computed sonography system (GE Medical Systems, Waukesha, Wisconsin, USA) according to the recommendations of the American Society of Echocardiography [18]. Two-dimensional apical 2- and 4-chamber views were used for volume measurements; LVEF was calculated with a modified Simpson’s method using biplane apical (2- and 4-chamber) views. The LV end-diastolic volume and the LV end-systolic volume were recorded. All echo examinations were performed by expert operators blinded to the results of BNP assay; the intra-observer variability in the evaluation of LVEF was found to be <5%. Echocardiographic measurements including LV end-diastolic diameter, and the diastolic thickness of the ventricular septum and the posterior LV wall were determined according to the American Society of Echocardiography recommendations [18]. Systolic dysfunction was defined as a level of LVEF <50%. The definition of restrictive filling pattern was a predefined modification of classifications used in prior studies (19): E/A ≥2, DT ≤150 msec, S/D ratio <1, and AR >35 cm/sec. All these criteria were verified to define the restrictive filling pattern. The Doppler sample was set 1–2 mm under the free edges of the mitral valve using the apical 4-chamber projection; an average of 5 beats was considered. In patients suffering from atrial fibrillation at the time of the echocardiogram, the diastolic function was classified as: 1) restrictive pattern (DT ≤150 msec), or 2) indeterminate (DT >150 msec). The pulmonary artery pressure (PAP) was obtained by determining the peak velocity of the tricuspid regurgitation jet, adding 5 or 10 mmHg as right atrial pressure according to right atrial size, severity of regurgitation and appearance of the inferior vena cava. From Doppler tissue imaging of the annulus, the E′ wave (early annular velocity opposites in direction to the mitral inflow) was determined and the ratio E/E′ calculated [20]. The right ventricle function was investigated using the M-mode echocardiography obtaining the tricuspid annular plane systolic excursion (TAPSE) [21].
Biochemical assays
All blood samples were collected by venipuncture and immediately analysed with the bedside Triage B type natriuretic fluorescence immunoassay (Biosite Diagnostics, La Jolla, CA, USA). The Triage Meter is used to measure BNP concentration by detecting a fluorescent emission that reproduces the amount of BNP in the blood. After the addition of 250 μl of whole blood to the disposable device, cells were filtered and separated from the plasma with BNP, which entered a reaction chamber containing fluorescent BNP antibodies. After 2-min incubation, the BNP-antibody mixture migrated to an area containing immobilised antibodies and remained fixed. The unbound fluorescent antibodies were washed away by the excess sample fluid. The Triage Meter then measured the fluorescent intensity of the BNP assay area. The assay results were complete in 15 minutes. The creatinine clearance was calculated using the MDRD formula.
Non-invasive cardiac output
For the measurement of non-invasive cardiac output (CO), an inert gas rebreathing method (Innocor, Innovison A/S, Odense, Denmark) was used. The system utilised a N2O (blood soluble gas) and SF6 (blood insoluble gas) enriched with O2 of 0.5% and 0.1%, respectively. Tidal volume was progressively increased in the closed circuit to match the physiologic increase. Use the SF6 allowed measuring the volume of lungs, valve and rebreathing bag. N2O concentration decreases during the rebreathing manoeuvre, with a rate proportional to pulmonary blood flow. Three to 4 respiratory cycles were needed to obtain N2O washout. Absence of pulmonary shunt was defined as arterial O2 saturation >98% (blood sample obtained from the arterial line). In the absence of a pulmonary shunt, pulmonary blood flow=CO. This method has been proven to be closely correlated with thermodilution (R=0.93) and the direct Fick method (R=0.94) [22].
Statistical analysis
Continuous variables were expressed as mean ±standard deviation (SD). Inter-group differences in continuous variables were evaluated using 2-tailed t test for unpaired data; differences between baseline and follow-up were evaluated by 2-tailed t test for paired data. Differences in non-continuous variables were evaluated using non-parametric tests as needed (Wilcoxon, Mann-Whitney). Distribution of categorical variables between groups was evaluated by chi-square with Yates correction. Statistical significance was set at p≤0.05. Analyses were performed using SPSS software for Windows, release 7.5, SPSS Inc., Chicago, USA.
Results
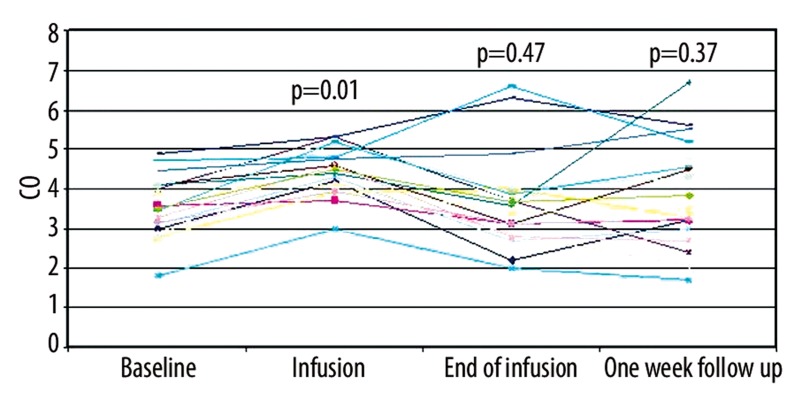
Seven patients (6 males; age 66.3±4.4) were admitted 20 times (range 1–6) for ADHF during the study (which lasted 16 months) and treated with a levosimendan infusion; 7 patients (5 males; age 69.1±3.9) did not agree to the levosimendan infusion and were admitted 15 times (range 1–4) for ADHF being treated with furosemide (control group). All subjects signed an informed consent. All subjects of the levosimendan group and 6 (85.7%) in the furosemide group had an implantable cardiac defibrillator with a pace-maker for cardiac resynchronization therapy. All patients of the 2 groups could not be included into a cardiac transplant protocol due to age, comorbidity (severe asthma) or recent history of malignant neoplasm. Two patients were evaluated for the implantation of a ventricular assistant device as a destination therapy and then excluded for severe right ventricular dysfunction. The etiology of the CHF was ischemic in 6/7 patients in the levosimendan group and in 3/7 in the furosemide group. Table 1 summarizes the comparison between the main parameters at baseline in the 2 groups. The effects caused by the levosimendan or furosemide infusion on renal function, blood pressure, echocardiographic parameters, functional status and cardiac output are described in Tables 2 and 3, respectively. In patients treated with levosimendan, a supplementary measurement of CO, cardiac index (CI) and stroke volume (SV) during the infusion (>12 hour) was provided. CO improved from 3.6±0.9 l/min to 4.5±0.7 l/min during infusion (p=0.001), reducing at the end of infusion and at 1-week follow-up (3.8±1.4 l/min and 4.1±1.5 l/min, p=0.5 and p=0.4 respectively). Similarly, CI and SV ameliorated during infusion (from 1.8±0.4 l/min/m2 to 2.3±0.3 l/min/m2 p=0.001; from 50.3±11.3 ml to 58.4±10 ml p=0.01) maintaining their improvement after the end of infusion (1.9±0.7 l/min/m2 p=0.4; 56.6±24.3 ml p=0.3) and at 1-week follow-up (2.1±0.7 l/min/m2 p=0.3; 56.6±24.2 ml p=0.3) (Figure 1). The analysis of the MLHFQ revealed a better health-related quality of life 1 week after levosimendan infusion (27.2±15.3 vs. 40.9±17.5, p=0.05).
Table 1.
Comparison between main parameters at baseline in the levosimendan group (7 patients/20 treatments) and control group (7 patients/15 treatments).
| Levosimendan group | Control group | p | |
|---|---|---|---|
| Age | 66.3±4.2 | 69.1±3.9 | 0.1 |
| Admission/year | 5.4±2.6 | 6.1±3.2 | 0.6 |
| BMI | 27.5±1.8 | 27.1±1.9 | 0.7 |
| Body weight (kg) | 81.5±7.4 | 77.5±8.6 | 0.2 |
| Furosemide (mg/die) | 92.1±39.1 | 115.5±44.6 | 0.2 |
| Bisoprolol (mg/die) | 1.6±1.1 | 2.3±2.1 | 0.2 |
| Enalapril (mg/die) | 4±1.9 | 7.2±8.3 | 0.1 |
| Spironolactone (mg/die) | 36.3±5.7 | 41.6±12.5 | 0.1 |
| Creatinine (mg/dl) | 1.7±0.5 | 1.7±0.7 | 0.9 |
| Clearances creat (ml/min/1.73 m2) | 74.5±29.7 | 56.3±21.9 | 0.06 |
| GFR (ml/min/1.73 m2) | 49.4±27.5 | 40.8±15.6 | 0.4 |
| 6MWT (m) | 217.6±97.7 | 164.4±49 | 0.06 |
| BNP (pg/ml) | 713.7±521.1 | 982.5±594.8 | 0.1 |
| Systolic blood pressure (mmHg) | 103.6±7.6 | 110.4±17.9 | 0.13 |
| Diastolic blood pressure (mmHg) | 63.1±7.1 | 63.6±8.1 | 0.8 |
| Heart rate (beats/min) | 74.5±8.1 | 76.1±9.2 | 0.5 |
| LVEF (%) | 22.8±9.1 | 37±13.4 | 0.003 |
| PAP (mmHg) | 38.2±16.2 | 37.4±15.3 | 0.9 |
| NYHA class | 2.9±0.8 | 3.4±0.7 | 0.1 |
| CO (l/min) | 3.7±0.9 | 3.1±0.5 | 0.1 |
| CI (l/min/m2) | 1.8±0.4 | 1.6±0.2 | 0.2 |
GFR – glomerular filtration rate; 6MWT – six-minute walking test; BNP – B-type brain natriuretic peptide; LVEF – left ventricular ejection fraction; PAP – pulmonary artery pressure; NYHA – New York Heart Association; CO – cardiac output; CI – cardiac index.
Table 2.
Comparison between main parameters at baseline, at the end of treatment and at 1-week follow-up in the levosimendan group (7 patients/20 treatments).
| Baseline | End of treatment | 1-week | |
|---|---|---|---|
| Body weight (kg) | 81.5±7.4 | 80.5±7.2 | 79.8±8.8 |
| Potassium (mEq/l) | 4.3±0.7 | 4.2±0.3 | 4.5±0.4 |
| Sodium (mEq/l) | 137.4±4.1 | 136.1±4.8 | 138.3±4.1 |
| Creatinine (mg/ml) | 1.7±0.5 | 1.5±0.4 | 1.6±0.5 |
| Clearances creat (ml/min/1.73 m2) | 74.5±29.7 | 73.5±24.6 | 73.7±32 |
| GFR (ml/min/1.73 m2) | 49.4±27.5 | 53.6±24.8 | 50.8±26.8 |
| 6MWT (m) | 217.6±97.7 | 372.3±90.4** | 401.4±83.7** |
| BNP (pg/ml) | 713.7±521.1 | 554.2±407.6* | 592.4±462 |
| SBP (mmHg) | 104±7.8 | 102.5±10.3 | 106.6±7.4 |
| DBP (mmHg) | 63.5±7.4 | 62.5±9.5 | 66.4±5.6 |
| LVEF (%) | 22.8±9.1 | 25.4±9.8* | 22.4±8.2 |
| LVEDV (ml) | 204.1±53 | 221.2±56.4* | 202.9±51.4 |
| LVESV (ml) | 160.4±50 | 166.8±81.4 | 157.1±41.2 |
| DT (ms) | 168.4±81.4 | 170.8±53.6 | 149.5±37.3 |
| E/A | 2.6±1.4 | 3.1±1.9 | 2.5±1.3 |
| E/E’ | 22.9±7.1 | 24.6±13.6 | 25.8±15.6 |
| TAPSE (mm) | 19.9±2.4 | 20.2±1.2 | 19.9±1.3 |
| PAP (mmHg) | 38.2±16.2 | 35.9±13.3 | 34.9±14.9 |
| NYHA class | 2.9±0.8 | 2.1±0.3** | 2.1±0.2** |
| CO (l/min) | 3.7±0.9 | 3.8±1.4 | 4.1±1.5 |
| CI (l/min/m2) | 1.8±0.4 | 1.9±0.7 | 2±0.7 |
GFR – glomerular filtration rate; 6MWT – six-minute walking test; BNP – B-type brain natriuretic peptide; SBP – systolic blood pressure; DBP – diastolic blood pressure; LVEF – left ventricular ejection fraction; LVEDV – left ventricular end-diastolic volume; LVESV – left ventricular end-systolic volume; DT – deceleration time; TAPSE – tricuspid annular plane systolic excursion; PAP – pulmonary artery pressure; NYHA – New York Heart Association; CO – cardiac output; CI – cardiac index;
p<0.05
p<0.01.
Table 3.
Comparison between main parameters at baseline, at the end of treatment and at 1-week follow-up in the control group (7 patients/15 treatments).
| Baseline | End of treatment | 1-week | |
|---|---|---|---|
| Body weight (kg) | 77.5±8.6 | 76±6.6* | 72.7±6.7* |
| Potassium (mEq/l) | 4.8±0.3 | 4.1±0.5** | 3.9±0.8** |
| Sodium (mEq/l) | 144±0.4 | 145.5±0.5* | 144.±0.5 |
| Creatinine (mg/ml) | 1.7±0.5 | 1.6±0.8 | 2.2±0.8** |
| Clearances creat (ml/min/1.73 m2) | 56.3±21.9 | 41.2±10.1* | 32.5±14.2** |
| GFR (ml/min/1.73 m2) | 40.8±15.6 | 52.5±27.1 | 37.5±26.4 |
| 6MWT (m) | 164.4±49 | 161.2±42.2** | 135±17** |
| BNP (pg/ml) | 982.5±594.8 | 657.5±129.9** | 624.5±135.1** |
| SBP (mmHg) | 123.7±23.2 | 116.2±15* | 125.1±12.2 |
| DBP (mmHg) | 65±7.8 | 60±10.7 | 67.5±9.5 |
| LVEF (%) | 37±13.4 | 35.6±14.8* | 36.4±12.8 |
| LVEDV (ml) | 168.4±69.3 | 166.1±63 | 167.2.±68.4 |
| LVESV (ml) | 127.1±27.2 | 124.7±81.5 | 125.7±31.2 |
| DT (ms) | 148.8±47.5 | 155±44.1 | 149.1±47.9 |
| E/A | 2.4±0.8 | 2.4±0.7 | 2.5±0.93 |
| E/E′ | 24.3±3.6 | 24.7±3.1 | 24.9±3.6 |
| TAPSE (mm) | 17.6±2.3 | 18±2.6 | 17.8±2.1 |
| PAP (mmHg) | 37.4±15.3 | 31.8±11.6 | 35.9±15.4 |
| NYHA class | 3.4±0.6 | 2.3±0.5** | 2.2±0.2** |
| CO (l/min) | 3.1±0.5 | 3.1±0.9 | 3.2±0.7 |
| CI (l/min/m2) | 1.6±0.2 | 1.6±0.5 | 1.5±0.7 |
GFR – glomerular filtration rate; 6MWT – six-minute walking test; BNP – B-type brain natriuretic peptide; SBP – systolic blood pressure; DBP – diastolic blood pressure; LVEF – left ventricular ejection fraction; LVEDV – left ventricular end-diastolic volume; LVESV – left ventricular end-systolic volume; DT – deceleration time; TAPSE – tricuspid annular plane systolic excursion; PAP – pulmonary artery pressure; NYHA – New York Heart Association; CO – cardiac output; CI – cardiac index;
p<0.05
p<0.01.
Figure 1.
Time-course of cardiac output in patients infused with levosimendan infusion (7 patients/20 treatments).
At 12-lead electrocardiogram, 2 (28.6%) subjects in the levosimendan group and 4 (57.2%) in furosemide the group were in permanent atrial fibrillation.
The study lasted 16 months; during this period appropriate AICD shocks of ventricular tachycardia or ventricular fibrillation after the levosimendan infusion were registered in 2 patients (28.6%) (1 patient died a non-cardiac death for sepsis after admission in Intensive Care Unit). Two patients (28.6%) in the control group died of cardiac death due to worsening of CHF and multi-organ failure, and in 1 subject an appropriate AICD shock was registered.
Discussion
This non-randomized single-centre study analysed the feasibility and effects of levosimendan infusion in end-stage CHF admitted for repetitive ADHF. The severity of clinical status of this population was underlined by the unfavourable prognosis (mortality rate 21.4% in 16 months) and the high rate of readmission/year. Treating end-stage CHF patients, new therapeutic approaches should be proven in terms of safety, efficacy in improving functional status, quality of life, and reduction of hospital readmission. Long-term administration of dobutamine or PDE inhibitors failed to demonstrate significant clinical benefits and the meta-analysis of Rapezzi et al. [23], based on 21 randomized trials, proved that the continuous administration of β-adrenergic agonists or PDE inhibitors increased mortality. The repetitive administration of levosimendan in advanced CHF patients has been reported to improve symptoms and left ventricular systolic function [14], reduce NT-proBNP and immune activation (Interleukin-6 and C-reactive protein) [13], and increase 45-day survival compared to dobutamine [12].
In our experience the levosimendan infusion during an ADHF improved the NYHA class and the 6MWT, maintaining these effects at 1-week follow-up. In the furosemide group, an amelioration of NYHA class but not of 6MWT was observed. Moreover, CHF patients treated with levosimendan showed a better quality of life at MLHFQ at 1-week follow-up (p=0.05). The MLHFQ has been recently evaluated as being the most correlated with NYHA class, 6MWT and functional status in CHF patients [24], exploring both the physical domain and emotional/psychological aspects of quality of life.
Plasma BNP was significantly reduced at the end of the levosimendan infusion (p=0.03), but returned to baseline after 1 week (p=0.08), while the reduction of volume overload with furosemide significantly decreased the BNP immediately and at follow-up (p=0.01). Farmakis et al. [25] found BNP was reduced only in CHF patients treated with levosimendan compared with furosemide, and that an obtained reduction of neurohormon >58% predicted a better 6-month prognosis. In contrast to that report [25], our echocardiographic results did not point out significant differences in LVEF, left ventricular volumes, diastolic function, pulmonary pressure and right ventricular performance (TAPSE) at 1-week follow-up after the levosimendan administration.
Our experience confirmed the robust results obtained by Nieminen et al. [6] that described the favourable effects of levosimendan infusion on CO and SV at the dosage of 0.1 μg/kg/min using a Swan-Ganz catheter. Furthermore, Lilleberg et al. [26] demonstrated in 11 CHF patients that the positive inotropic effect reached the maximal effect in reducing pulmonary wedge pressure after 6 hours and increasing CO after 24 hours of levosimendan infusion. Nevertheless, these positive effects, estimated to last more than 1 week, were obtained with echocardiographic measurements. Using a validated non-invasive method, we documented in our patients treated with levosimendan an improvement in CO and SV during the infusion, losing this effect after the end of infusion and at 1-week follow-up. These results generate 2 main considerations: a) the long half-life of the active levosimendan metabolite (OR-1896) (80–90 hours) [27], that should have sustained the hemodynamic effect of the drug and might have justified the intermittent/repetitive levosimendan administration, needs to be investigated extensively; and b) if the functional capacity of our patients improved at short-term follow-up irrespective of cardiac function, an effect on skeletal muscles might be involved. In fact, the intermittent infusion of inotropic drugs partially reversed the impairment of peripheral muscle circulation in 30 end-stage CHF patients [28], and levosimendan, as a calcium-sensitizer, is considered an emerging class of agents to enhance the quality of life of patients suffering from skeletal muscle disorders [29].
Finally, the clinical improvement of CHF patients treated with levosimendan was obtained without an impairment of renal function (Tables 2, 3), in contrast to the results of the furosemide group. Serum creatinine and urea nitrogen were strong and independent prognostic parameters in CHF patients [30,31]. In the LIDO trial [8], the levosimendan infusion improved renal function over 24 hours (mean change in creatinine concentration −0.10 mg/dl), while dobutamine did not (p=0.03). In the experience of Zemljic et al. [32], based on 20 CHF patients awaiting cardiac transplantation, a single levosimendan administration determined a significant improvement of plasma creatinine and creatinine clearance (p=0.005) at 3-month follow-up. The renoprotective effect of the drug might be related to an increase of renal medullar blood flow in spite of a reduction of the cortical flow [33], or to a change of inflammatory status (reduction of interleukin-6) [13], such that this favourable effect may be considered as a resource for anti-inflammatory therapy. In the control group, an increase of plasma creatinine was observed, not at the end of treatment, but at 1-week follow-up (p<0.01) – that could be explained by the tendency of reduced fluid overload to create a risk of subclinical dehydration.
Conclusions
Treating ADHF in end-stage CHF patients with clinical hyperhydration using levosimendan improved BNP and LVEF, but this effect disappeared after 1 week. The amelioration of 6MWT and NYHA class lasted longer after levosimendan infusion, without causing an impairment of renal function. Patients treated with levosimendan in comparison with furosemide infusion manifested a better quality of life.
Limitations of the study
The major limitation of this study is the absence of randomization. Moreover, the small numbers of patients involved did not permit any analysis of mortality or arrhythmic risk correlated to levosimendan infusion – these questions need to be addressed by a larger, randomized trial. Nevertheless, our limited experience suggests caution using an infusion pump for levosimendan infusion in out-patients with chronic, refractory CHF [34], eventually limiting such therapy in subjects protected by an AICD. In fact, in our experience a ventricular arrhythmic disorder occurred in 10% of levosimendan treatment, while in the LIDO trial it was recorded in only 4% of patients treated [8].
Acknowledgement
We would like to acknowledge Ms Federica Cecchin for her constant and valuable support in the elaboration of the manuscript.
Footnotes
Source of support: This study was not supported by any grant
References
- 1.McBride B, White M. Levosimendan: implications for clinicians. J Clin Pharmacol. 2003;43:1071–81. doi: 10.1177/0091270003257217. [DOI] [PubMed] [Google Scholar]
- 2.Haikala H, Kaivola J, Nissinen E, et al. Cardiac troponin C as a target protein for a novel calcium sensitizing drug, levosimendan. J Mol Cell Cardiol. 1995;27:1859–66. doi: 10.1016/0022-2828(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 3.Pollesello P, Ovaska M, Kaivola J, et al. Binding of a new Ca2+ sensitizer, levosimendan, to recombinant human cardiac troponin C: a molecular modelling, fluorescent probe and proton nuclear magnetic resonance study. J Biol Chem. 1994;269:25584–90. [PubMed] [Google Scholar]
- 4.Yokoshiki H, Katsube Y, Sunagawa M, Sperelakis N. Levosimendan, a novel Ca2+ sensitizer, activates the glibenclamide-sensitive K+ channel in rat arterial myocites. Eur J Pharmacol. 1997;333:249–59. doi: 10.1016/s0014-2999(97)01108-4. [DOI] [PubMed] [Google Scholar]
- 5.Kersten JR, Montgomery MW, Pagel PS, Warltier DC. Levosimendan, a new positive inotropic drug, decrease infarct size via activation (ATP) channels. Anesth Analg. 2000;90:5–11. doi: 10.1097/00000539-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Nieminen MS, Akkila J, Hasenfuss G, et al. Hemodynamic and neurohumoral effects of continuous infusion of levosimendan in patients with congestive heart failure. J Am Coll Cardiol. 2000;36:1903–12. doi: 10.1016/s0735-1097(00)00961-x. [DOI] [PubMed] [Google Scholar]
- 7.Moiseyev VS, Poder P, Andrejevs N, et al. Safety and efficacy of a novel calcium sensitizer, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction: a randomized placebo-controlled, double-blind trial. Eur Heart J. 2002;23:1422–32. doi: 10.1053/euhj.2001.3158. [DOI] [PubMed] [Google Scholar]
- 8.Follath F, Cleland JGF, Just H, et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomized double-blind trial. Lancet. 2002;360:196–202. doi: 10.1016/s0140-6736(02)09455-2. [DOI] [PubMed] [Google Scholar]
- 9.Lehmann A, Lang J, Boldt J, et al. Levosimendan in patients with cardiogenic shock undergoing surgical revscularization: a cese series. Med Sci Monit. 2004;10(8):MT89–93. [PubMed] [Google Scholar]
- 10.Mebazaa A, Nieminen MS, Packer M, et al. M. Levosimendan vs dobutamine for patients with acute decompensate heart failure: the SURVIVE Randomized trail. JAMA. 2007;297:1883–91. doi: 10.1001/jama.297.17.1883. [DOI] [PubMed] [Google Scholar]
- 11.Spargias KS, Aniftantakis A, Papadakis M, et al. Preliminary clinical experience with the repetitive administration of levosimendan in patients with end-stage heart failure. Ital Heart J. 2003;(Suppl 2):45S–49S. [PubMed] [Google Scholar]
- 12.Nanas JN, Papazoglou P, Ntalianis A, et al. Efficacy and safety of intermittent, long-term, concomitant dobuatmine and levosimendan infusions in severe heart failure refractory to dobutamine alone. Am J Cardiol. 2005;95:768–71. doi: 10.1016/j.amjcard.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 13.Parissis JT, Adamoupoulos S, Farmakis D, et al. Effects of serial levosimendan infusions on left ventricular performance and plasma biomarkers of myocardial injury and neurohormonal and immune activation in patients with advanced heart failure. Heart. 2006;92(12):1768–72. doi: 10.1136/hrt.2006.079707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mavrogeni S, Giamouzis G, Papadopoulou E, et al. A 6-month follow-up of intermittent levosimendan administration effect on systolic function, specific activity questionnaire and arrhythmia in advanced heart failure. J Cardiac Fail. 2007;13:556–59. doi: 10.1016/j.cardfail.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Mckee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of CHF. The Framingham study. N Engl J Med. 1971;285:1442–46. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 16.ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Eur Heart J. 2008;29:2388–42. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 17.Rector TS, Kubo SH, Cohn JN. Patients’ self-assessment of their congestive heart failure. Part 2: Content, reliability and validity of a new measure, the Minnesota Living with Heart Failure Questionnaire. Heart Failure. 1987:198–209. [Google Scholar]
- 18.Sahn DJ, Demaria A, Kisslo J, Weyman A. The committee on M mode standardisation of the American Society of Echocardiography: recommendations regarding quantification in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–83. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 19.Garcia MJ, Thomas JD, Klein AL. New Doppler echocardiographic applications for the study of diastolic function. J Am Coll Cardiol. 1998;32:865–75. doi: 10.1016/s0735-1097(98)00345-3. [DOI] [PubMed] [Google Scholar]
- 20.Gottdiener JS, Bednarz J, Devereux R American Society of Echocardiography. American Society of Echocardiography Recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17:1086–119. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Lindquist P, Calcutteea A, Henein M. Echocardiography in the assessment of right heart function. Eur J Echocardiogr. 2008;9:225–34. doi: 10.1016/j.euje.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Agostoni PG, Cattadori G, Apostolo A, et al. Non-invasive measurement of cardiac output during exercise by inert gas rebreathing technique: a New tool for heart failure evolution. J Am Coll Cardiol. 2005;46:1779–83. doi: 10.1016/j.jacc.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Rapezzi C, Bracchetti G, Branzi A, Magnani B. The case against outpatient parenteral inotropic therapy for advanced heart failure. J Heart Lung Transplant. 2000;19(Suppl):S58–S63. [PubMed] [Google Scholar]
- 24.Garin O, Ferrer M, Pont A, et al. Disease- specific health-related quality of life questionnaires for heart failure: a systematic review with meta-analyses. Qual Life Res. 2009;18:71–85. doi: 10.1007/s11136-008-9416-4. [DOI] [PubMed] [Google Scholar]
- 25.Farmakis D, Parissis JT, Bistola V, et al. Plasma B-type natriuretic peptide reduction predicts long-term response to levosimendan therapy in acutely decompensated chronic heart failure. Int J Cardiol. 2010;139:75–79. doi: 10.1016/j.ijcard.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Lilleberg J, Laine M, Palkama T, et al. Duration of the haemodynamic action of a 24-h infusion of levosimendan in patients with congestive heart failure. Eur J Heart Fail. 2007;9:75–82. doi: 10.1016/j.ejheart.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Parissis JT, Andreadou I, Bistola V, et al. Novel biologic mechanisms of levosimendan and its effect on the failing heart. Expert Opin Investig Drugs. 2008;17:1143–50. doi: 10.1517/13543784.17.8.1143. [DOI] [PubMed] [Google Scholar]
- 28.Nanas S, Gerovasili V, Dimopoulos S, et al. Inotropic agents improve the peripheral microcirculation of patients with end-stage chronic heart failure. J Card Fail. 2008;14:400–6. doi: 10.1016/j.cardfail.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Ochala J. Ca2+sensitizers: an emerging class of agents for counterbalancing weakness in skeletal muscle diseases? Neuromuscul Disord. 2010;20:98–101. doi: 10.1016/j.nmd.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Altenberger J, Parissis JT, Ulmer H, Poelzl G. Rationale and design of the multicenter randomized trial investigating the efficacy and safety of pulsed infusions of levosimendan in outpatients with advanced heart failure (LevoRep study) Eur J Heart Fail. 2010;12:186–92. doi: 10.1093/eurjhf/hfp189. [DOI] [PubMed] [Google Scholar]
- 31.Feola M, Aspromonte N, Canali C, et al. Prognostic value of plasma brain natriuretic peptide, Urea nitrogen and creatinine in outpatients >70 years of age with heart failure. Am J Cardiol. 2005;96(5):705–9. doi: 10.1016/j.amjcard.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 32.Zemljic G, Bunc M, Yazdanbakhsh AP, Vrtovec B. Levosimendan improves renal function in patients with advanced chronic heart failure awaiting cardiac transplantation. J Cardiac Fail. 2007;13:417–21. doi: 10.1016/j.cardfail.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Pagel P, Hettrick D, Warltier D. Influence of levosimendan, pimobendan and milrinone on the regional distribution of cardiac output in anaesthetized dogs. Br J Pharmacol. 1996;119:609–15. doi: 10.1111/j.1476-5381.1996.tb15716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parle NM, Thomas MD, Dembo L, Driscoll GO. Repeated infusions of levosimendan. Well tolerated and improves functional capacity in decompensated heart failure-a single-centre experience. Heart, Lung and Circulation. 2008;3:206–10. doi: 10.1016/j.hlc.2007.10.014. [DOI] [PubMed] [Google Scholar]