Summary
Background
The effective screening of myocardial infarction (MI) patients threatened by ventricular tachycardia (VT) is an important issue in clinical practice, especially in the process of implantable cardioverter-defibrillator (ICD) therapy recommendation. This study proposes new parameters describing depolarization and repolarization inhomogeneity in high resolution body surface potential maps (HR BSPM) to identify MI patients threatened by VT.
Material/Methods
High resolution ECGs were recorded from 64 surface leads. Time-averaged HR BSPMs were used. Several parameters for arrhythmia risk assessment were calculated in 2 groups of MI patients: those with and without documented VT. Additionally, a control group of healthy subjects was studied. To assess the risk of VT, the following parameters were proposed: correlation coefficient between STT and QRST integral maps (STT_QRST_CORR), departure index of absolute value of STT integral map (STT_DI), and departure index of absolute value of T-wave shape index (TSI_DI). These new parameters were compared to known parameters: QRS width, QT interval, QT dispersion, Tpeak-Tend interval, total cosines between QRS complex and T wave, and non-dipolar content of QRST integral maps.
Results
STT_DI, TSI_DI, STT_QRST_CORR, QRS width, and QT interval parameters were statistically significant (p≤0.05) in arrhythmia risk assessment. The highest sensitivity was found for the STT_DI parameter (0.77) and the highest specificity for TSI_DI (0.79).
Conclusions
Arrhythmia risk is demonstrated by both abnormal spatial distribution of the repolarization phase and changed relationship between depolarization and repolarization phases, as well as their prolongation. The proposed new parameters might be applied for risk stratification of cardiac arrhythmia.
Keywords: body surface potential mapping, myocardial infarction, ventricular tachycardia, implantable cardioverter-defibrillator
Background
The non-invasive risk assessment of life-threatening ventricular arrhythmia is of great clinical importance, especially in the prevention of sudden cardiac death (SCD) in patients after myocardial infarction (MI). Although there currently exist many noninvasive parameters quantifying the depolarization and repolarization process in time and space domains, these parameters remain the subject of intensive study. The ECG-based parameters calculated from the 12 standard leads or 3 orthogonal leads (e.g., prolonged QRS interval, prolonged QT interval, large QT dispersion, microvolt T-wave alternans, total cosine of mean angle between QRS complex and T wave) are considered as predictors of SCD [1–6]; nonetheless, their effectiveness is still debated [6–12]. Beat-to-beat analysis of subsequent cardiac cycles has recently become a very promising tool for the identification of patients at risk for ventricular tachycardia (VT). There is a large body of evidence supporting the assumption that increased QT variability, low heart rate variability, and heart rate turbulence show good diagnostic value for ventricular tachycardia (VT) detection [13–16]. VT risk assessment in MI patients was also studied using advanced frequency and time-frequency analysis of high resolution ECG. It was reported that parameters obtained by using the fast Fourier transform and parametric modeling method with an autoregressive model, as well as wavelet transform, have good discriminative properties [17–20].
Body surface potential mapping (BSPM) offers a possibility for more precise study of temporal and spatial distributions of cardiac electrical activity in comparison to standard 12-lead electrocardiography [21]. One of the most often examined parameters calculated from BSPMs is the area under the QRST complex [22], which was also used to assess repolarization heterogeneity described by non-dipolar content of QRST integral maps [21–25]. However, the QRST integral map’s ability to reveal repolarization dispersion has been questioned [7]. The search for reliable risk indices for ventricular arrhythmias is still one of the major remaining tasks in high resolution ECG mapping. BSPM might be studied using time-average technique [26] or beat-to-beat analysis [24,27]. In this preliminary study we focused on the time-average technique.
The values of depolarization and repolarization parameters for ventricular arrhythmia risk stratification were assessed by studying patients with chronic myocardial scar areas, with and without documented ventricular arrhythmias, as well as by comparing these 2 patient groups with a control group of volunteers with fully intact myocardium. We proposed and preliminarily verified new, non-invasive markers of VT risk assessment based on analysis of high resolution body surface potential maps (HR BSPM). The developed parameters reflect the abnormality in spatial distribution of the repolarization phase and its relation to changed spatial distribution of the depolarization phase in the group of MI patients with risk of VT. The statistical values obtained for these new parameters were compared to already known parameters calculated in the same studied groups of MI patients and healthy volunteers. The effectiveness of implanted cardioverter-defibrillators (ICD) to prevent SCD in MI patients is already proven [6,28–31]. However, highly effective identification of patients for ICD therapy is still difficult, and the number of ICDs needed to achieve 1 year of patient survival is still un-satisfyingly high [31,32]. The proposed parameters could also assist in noninvasive identification of MI patients for ICD therapy.
Material and Methods
Studied groups
Two groups of patients with remote myocardial infarction and a control group of healthy volunteers were studied. The first group comprised 26 patients with documented risk of ventricular tachycardia, called the MI-VT patients group. This group consisted of mainly secondary prevention patients with implanted cardioverter-defibrillator or qualified for ICD therapy due to the risk of ventricular tachycardia. The eligibility criteria to implant ICD were: 1) documented episodes of ventricular tachycardia (21 pts), and 2) induced sustained monomorphic VT during programmed electrical stimulation (5 pts). The second group consisted of 14 patients after myocardial infarction in whom any sustained or not-sustained ventricular tachycardia was stated, and is called the MI-non-VT patients group. The control group consisted of 25 volunteers who had normal electrocardiograms, no history of cardiovascular disease, and to whom any medications were administered. Basic data from all 3 studied groups are presented in Table 1.
Table 1.
Basic data of studied groups.
| Study group description | Total number | Age (mean ±SD) [Years] | LVEF (mean ±SD) [%] |
|---|---|---|---|
| MI patients with VT | 26 | 65±12 | 38±11 |
| MI patients without VT | 14 | 62±11 | 44±15 |
| Healthy volunteers | 25 | 49±15 | N/A |
LVEF – left ventricular ejection fraction; MI – myocardial infarction; VT – ventricular tachycardia; N/A – not available.
The study was approved by an institutional ethical review committee and the subjects gave informed consent.
Measurements and data processing
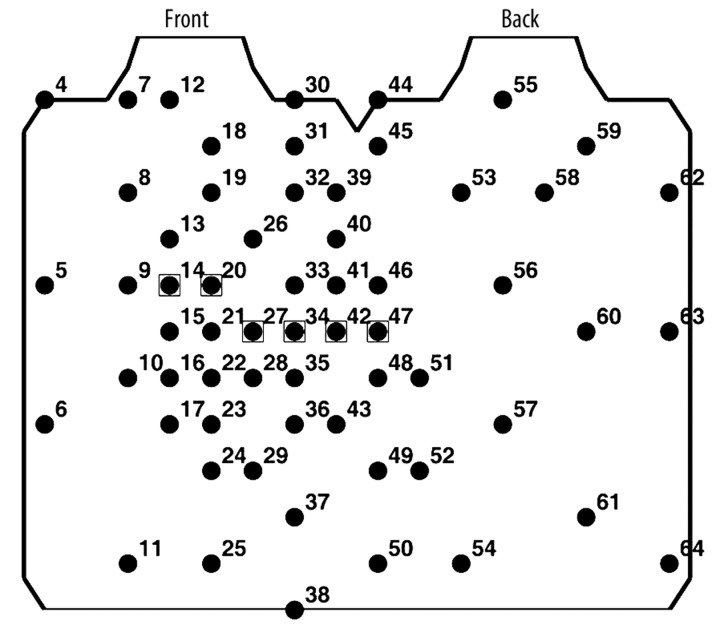
A high resolution multi-lead ECG system (Active Two, BioSemi B.V., The Netherlands) with 64 surface electrodes was used for data acquisition. Active electrodes (containing Ag2Cl contact sensors with preamplifiers) were located around the torso (Figure 1) according to the ECG lead system proposed by SippensGroenewegen et al. [33]. The 64 unipolar ECGs were simultaneously recorded for 15 minutes with 4096 Hz sampling frequency. Next, signals were amplified and converted into digital form with 24-bit amplitude resolution, sent through a fiber-optic transmitter to the computer, and stored on a hard disk for off-line processing.
Figure 1.
Lead arrangement around the torso for high resolution body surface potential mapping. Standard ECG leads are marked by squares.
The raw ECG data were filtered using low pass Butterworth filter limiting frequency to 300 Hz and decimate filter, decreasing sampling frequency to 1024 Hz. The reference ECG signal, known as Wilson’s central terminal (WCT), was subtracted at this preprocessing step. Then, ECG signals in each lead separately were averaged in time using a cross-correlation method. To receive a low noise level, both the number of averaged cycles (usually ca. 100 cycles) and the value of correlation coefficient (usually ≥0.98) were fitted. The level of noise was measured on the 20 ms isoelectric U-P interval, because, as we noticed, during PQ interval, atrial repolarization is still present, which has also been confirmed by Ihara et al [34]. The obtained root mean square (RMS) value of noise in averaged ECG signals was below 0.5 μV.
Data analysis
Detection of ECG characteristic time instances
To detect ECG characteristic time instances (onsets and offsets of both depolarization and repolarization waves), an algorithm proposed by Acar [1], based on singular value decomposition (SVD), was applied. For each subject, a rectangular matrix M (n,m), containing n averaged ECG leads signals, each of m samples length, was decomposed according to the following equation
| (1) |
where U(m,m),V(n,n) are the square, orthogonal matrices and ∑⌣ is the diagonal matrix (m,n) of singular values.
Next, the matrix S was obtained by projecting the matrix M down into the reduced space defined by only the first 3 left - singular vectors of the unitary matrix U. The RMS signal was calculated using 3 vectors of the matrix S (S1, S2, S3), as shown in Figure 2. The global (common for all leads) characteristic time instances of ECG curves were found for this RMS signal, and then were searched in each lead separately. Initially, R-peak was marked as the maximum of the RMS signal, and then T maximum was marked in relation to R-peak. The P-wave beginning was calculated by inspecting the stationarity of the time relation between the vectors S1 and S2 in respectively defined time windows. Next, the onset of Q-wave and the offset of S-wave were calculated from the difference of RMS signal using the thresholding method. The T-wave end was established as the minimum of RMS signal in time window related to T wave maximum. Then, in each lead individually, the waves’ ends were established in relation to the global waves’ ends using the thresholding method for Q onset, and P, S, T offsets and for P, R, T peaks. Finally, iso-amplitude maps were generated for various parameters described in the next section.
Figure 2.
ECG characteristic time instances detected on RMS signal obtained from three components S1, S2, S3 of singular value decomposition algorithm.
Arrhythmia risk parameters
To assess the risk of arrhythmia, 3 new BSPM parameters were proposed. The first parameter, called STT_QRST_CORR, was based on the spatial relationship between the maps of STT and QRST integrals. This parameter was defined as a Spearman rank correlation coefficient between STT area and QRST area maps (Eq. 2). Two sets of the data, STTi and QRSTi, were converted into the stti rankings qrsti and before calculating the Spearman correlation coefficient between ranks
| (2) |
where n is the subject number, i is the lead number, and M is the total number of leads.
The next 2 parameters, called STT_DI and TSI_DI, were proposed to quantify spatial changes in the repolarization phase. Departure index (DI) [35] was used to describe the changes between the group of healthy subjects and each individual subject of the entire studied population.
A TSI parameter (T wave Shape Index) was defined as the ratio of STT integral and T-wave length [26,36]:
| (3) |
where TSIn is the value of TSI parameter for subject n, V(t) is the ECG amplitude in the time instant t, Ton, Toff is the onset and offset of T wave, respectively, and L(V) is the length of T wave curve.
The TSI_DI parameter was defined as the sum of absolute values of departure indices of TSI parameter calculated in each lead. The formula describing the TSI_DI parameter is as follows:
| (4) |
where TSI_DIn is the value of TSI_DI parameter for subject n, is the mean value of TSI parameter calculated in the control group in the lead i, and σ(cSTTi ) is the standard deviation of TSI parameter calculated in control group in the lead i.
The STT_DI parameter was defined as the sum of absolute values of departure indices of STT integral calculated in each lead. The formula describing STT_DI parameter is as follows:
| (5) |
where STT_DI is the value of STT_DI parameter for subject n, , and σ (cSTTi ) are adequately the mean value and the standard deviation of STT integrals calculated in the control group in lead i.
The proposed parameters were compared to 6 well known arrhythmia indices: the QRS width, the QT interval, the dispersion of QT interval, the Tpeak-Tend interval, the averaged cosine of the angle between QRS complex, and the T-wave (TCRT) introduced by Acar [1], as well as the non-dipolar content (NDC) of QRST integral maps considered as the repolarization inhomogeneity index [23,37]. The values of the following parameters: the QRS width, the QT interval, the dispersion of QT interval, and the Tpeak-Tend interval were calculated for each subject and each lead separately, then were averaged over all leads. The TCRT gives information about the relation between propagation of depolarization and repolarization waves in myocardium. First, 12-lead ECG signals are decomposed to minimum dimensional space by SVD (singular value decomposition). TCRT is then computed as an averaged cosine between threshold vectors of QRS (vectors above threshold value around R peak) and the unit vector with the maximum T-wave energy. More details can be found in the original article [1]. The non-dipolar content was calculated using principal component analysis and Karhunen-Loève transform [38], providing information about the multipolarity of QRST integral maps.
Statistics
For studied groups, the mean values and standard deviations, as well as the 25th, the 50th (median), and the 75th percentiles of all parameters, were calculated. The statistical significance was assessed by means of non-parametric Mann-Whitney test, since the normal distribution of the values could not have been assumed. The level of statistical significance was set at p≤0.05.
To assess the effectiveness of new parameters and to define the risk criteria of ventricular tachycardia for MI patients, the specificity and the sensitivity of proposed parameters were calculated. The diagnostic criterion assessing VT risk in the MI patient group was calculated as a maximal value of the product of the specificity and the sensitivity.
Results
Basic statistical data (the mean and the standard deviation values of all examined parameters in the 3 studied groups, as well as statistical differences (p value) between the groups calculated by Mann-Whitney tests) are summarized in Table 2. Statistical differences were calculated between MI-VT and MI-non-VT patients group, as well as between the MI–non-VT patients group and the control group.
Table 2.
Values of examined parameters for studied groups (2nd, 4th, 6th columns) and statistical differences (p-value) obtained by Mann-Whitney test between MI patients with and without VT (3rd column), as well as between non VT MI patients group and control group (5th column).
| Parameter | MI-VT patients (mean ±SD) | Statistical differences MI-VT vs. MI-nonVT (p value) | MI–nonVT patients (mean ±SD) | Statistical differences MI-nonVT vs. control (p value) | Control group (mean ±SD) |
|---|---|---|---|---|---|
| STT_QRST_CORR | 0.35±0.54 | 0.05 | 0.63±0.47 | 0.004 | 0.91±0.09 |
| STT_DI | 1.81±0.61 | 0.01 | 1.32±0.41 | 0.0001 | 0.80±0.27 |
| TSI_DI | 2.53±1.15 | 0.03 | 1.71±1.07 | 0.003 | 0.77±0.27 |
| QRS INT [ms] | 137±30 | 0.03 | 120±13 | 0.005 | 107±13 |
| QT INT [ms] | 482±54 | 0.02 | 441±36 | 0.005 | 405±31 |
| QT INT DISP [ms] | 78±32 | ns (0.68) | 77±25 | ns (0.16) | 65±25 |
| Tpeak–Tend INT [ms] | 123±29 | ns (0.09) | 109±17 | ns (0.54) | 105±16 |
| TCRT | −0.37±0.50 | ns (0.06) | 0.03±0.65 | ns (0.48) | 0.23±0.44 |
| NDC [%] | 9.7±9.8 | ns (0.33) | 13±15 | 0.02 | 6±4 |
NDC – non dipolar content of QRST integral maps, STT_DI – departure index of absolute value of STT integral map, STT_QRST_CORR – correlation coefficient between STT and QRST integral maps, TCRT – total cosine between QRS complex and T wave, Tpeak-Tend INT – T wave peak to T wave end interval, TSI_DI – departure index of absolute value of T-wave shape index, QRS INT – QRS interval, QT INT – QT interval, QT INT DISP – dispersion of QT interval, SD – standard deviation, and ns – not significant.
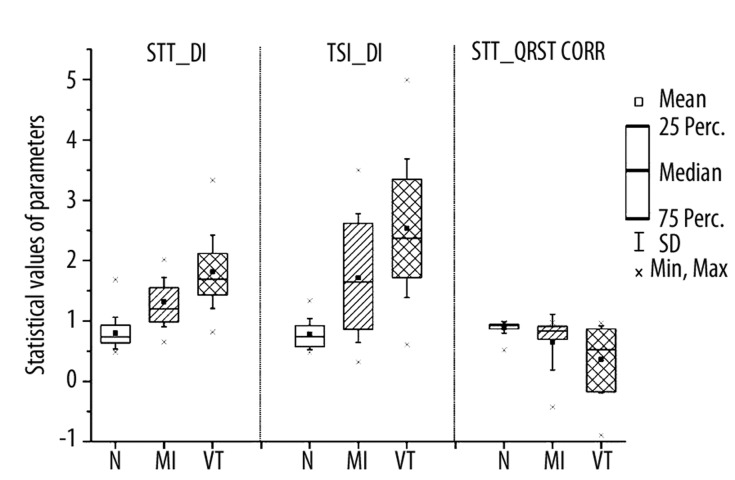
The statistical data of 3 proposed parameters: STT_DI, TSI_DI and STT_QRST_CORR are also presented graphically in Figure 3. Besides the mean and the standard deviation, values of the 25th, the 50th (median) and the 75th percentiles are shown.
Figure 3.
Mean ±SD values and 25th, 50th (median) and 75th percentiles of proposed new parameters: STT_DI, TSI_DI and STT_QRST_CORR.
The best results of the Mann-Whitney test in a discrimination between MI patients groups with and without the ventricular tachycardia were obtained using STT_DI parameter (p<0.01), QT interval (p<0.02), TSI_DI parameter (p<0.0.3), QRS width (p<0.03) and STT_QRST_CORR parameter (p=0.05). The differences were not statistically significant for: TCRT parameter (p<0.06), Tpeak-Tend interval (p<0.09), QT interval dispersion (p<0.33) and for NDC of QRST integral maps (p<0.68). The obtained results indicate, however, that for TCRT parameter and Tpeak-Tend interval, differences are very close to the limit of significance level (p=0.05) and might be statistically significant in a larger database of MI patients.
The differences between the control group and MI-non-VT patients group were not statistically significant for the following parameters: Tpeak-Tend interval, TCRT parameter and QT dispersion. The differences between the control group and MI-non-VT patients group were statistically significant for the remaining parameters (Table 2).
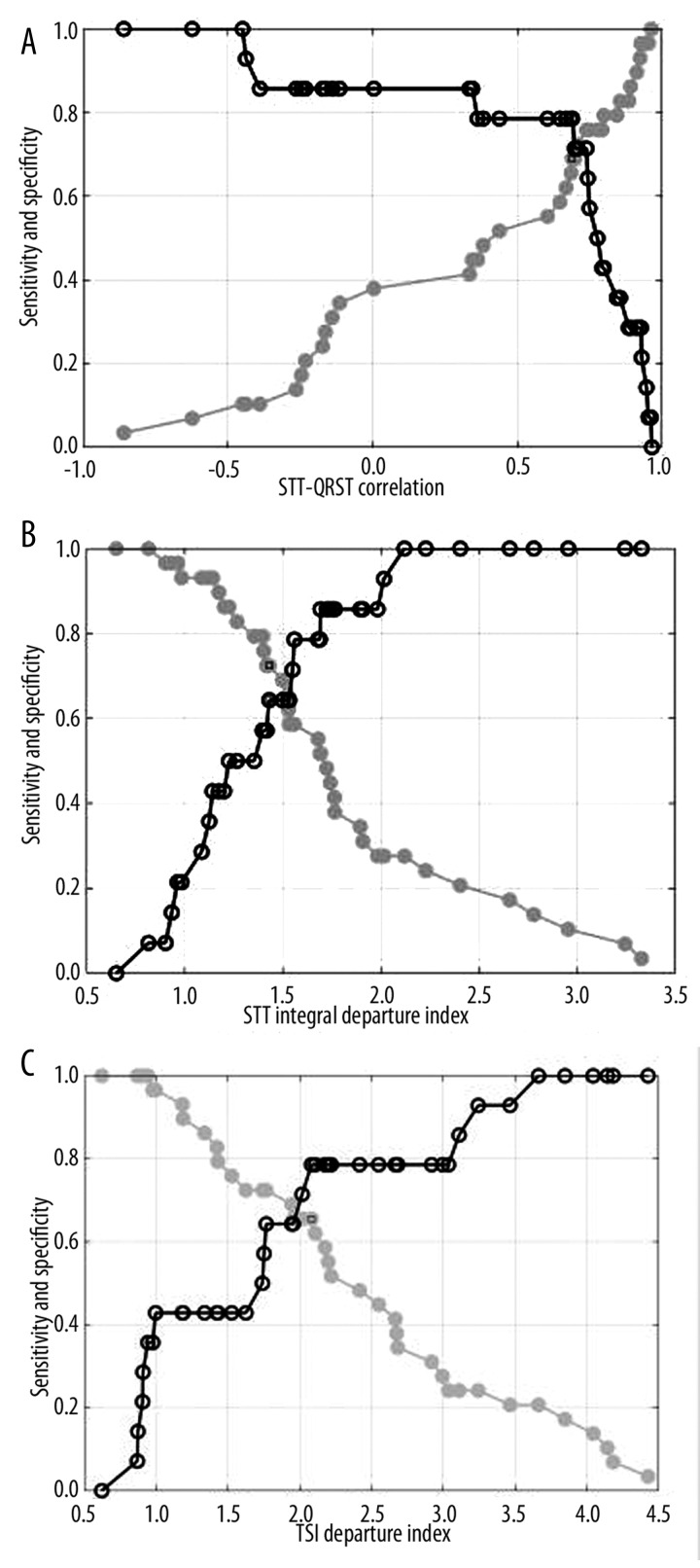
For statistically significant parameters in discrimination between MI patients groups with and without VT, sensitivity and specificity were calculated, and diagnostic criteria were proposed. The obtained results are presented in Table 3. For 3 proposed parameters, sensitivity, specificity and diagnostic criteria are also shown graphically in Figure 4.
Table 3.
Values of sensitivity, specificity and diagnostic criteria for selected parameters.
| Parameter | Sensitivity | Specificity | Criterion |
|---|---|---|---|
| STT_QRST_CORR | 0.73 | 0.71 | 0.7 |
| STT_DI | 0.77 | 0.64 | 1.4 |
| TSI_DI | 0.69 | 0.79 | 2.1 |
| QRS INT [ms] | 0.73 | 0.71 | 122 |
| QT INT [ms] | 0.73 | 0.71 | 459 |
Abbreviations as in Table 2.
Figure 4.
Sensitivity ● and specificity ○ of proposed parameters: (A) STT_QRST CORR, (B) STT_DI, (C) TSI_DI.
The highest value of sensitivity was found for STT_DI parameter (0.77), and the highest value of specificity for TSI_DI (0.79). STT_QRST_CORR parameter, QRS width and QT interval had the same values of sensitivity (0.73) and specificity (0.71).
The calculated diagnostic criteria concerning the risk of VT amounted to: 1.4 for STT_DI parameter, 2.1 for TSI_DI parameter, 0.7 for STT_QRST_CORR, 122 ms for QRS width and 459 ms for QT interval.
Additionally, the sensitivities of the above mentioned parameters were also calculated for 14 patients from the MI-VT group, in whom the episodes of VT occurred during follow-up study (Table 4). The highest sensitivity showed (0.79) for STT_QRST_CORR parameter and (0.71) for TCRT and STT_DI parameters. The ejection fraction value in those patients ranged from 15% to 65%. In 11 out of 14 MI-VT patients with episodes of VT during follow- up study, the value of EF was above 30%.
Table 4.
Best values of sensitivity of parameters in MI-VT patients who experienced ICD intervention during follow up study.
| Parameter | ICD Follow up (14 pts) |
|---|---|
| STT_DI | 0.71 |
| STT-QRST CORR | 0.79 |
| TCRT | 0.71 |
| QRS INT | 0.64 |
| QT INT | 0.64 |
Abbreviations as in Table 2.
Discussion
To identify MI patients at risk for ventricular tachycardia, 3 new parameters calculated from averaged HR BSPM were proposed: STT_QRST_CORR, STT_DI, and TSI_DI. For comparison, 6 parameters already used in VT risk assessment (QRS interval, QT interval, QT interval dispersion, Tmax-Tend interval, TCRT parameter, and NDC of QRST integral maps) were calculated.
Two newly proposed parameters, TSI_DI and STT_DI, are directly connected to repolarization phase, and represent a measure of departure from the mean distribution of TSI parameter and STT integral in the control group. Departure maps were developed for easy recognition of abnormalities in the body surface potential maps and for representation of the location and extent of abnormal increase or decrease. These maps are useful for assessment of myocardial infarction, ventricular hypertrophy, and myocardial ischemia [39]. In our study, departure indices were used to show an aggregated increase in abnormal changes of re-polarization parameters distributions accompanying pathological changes of the cardiac muscle affected by infarction and at risk of VT. Both parameters were statistically significant in discrimination between MI patients with and without documented risk of VT. Thus, the STT_DI and TSI_DI parameters could be sensitive markers based on repolarization spatial abnormality.
The third parameter, STT_QRST_CORR, was also statistically significant in discrimination between MI patients with and without VT. The STT_QRST_CORR parameter might be connected with the changes in shape and duration of the action potential of ischemic cells. The QRST integrals are considered to reveal local repolarization process independent of activation sequence. In the healthy cardiac muscle, the recovery process, which is influenced by both activation sequence and local repolarization, is dominated by local repolarization due to fast spread of activation, where the Purkinje system plays the key role [40]. Therefore, in the control group, the STT integral and QRST integral maps are highly correlated. On the other hand, Geselowitz [41] has shown that spatial variations of the QRST integral (ventricular gradient) are related to the local spatial variation of the action potential area. In ischemic cells, an increase in resting potential, a decrease in peak amplitude, an increase in the rise time of the upstroke, and a change in its duration, cause the change in the action potential area that influences the QRST area [42]. This is probably because the correlation coefficient between STT and QRST integral maps decreases in pathological changes of the myocardium. The depolarization process, which constitutes the shape of action potential as well as the repolarization process, is no longer as fast, and activation sequence is no longer as ordered due to the presence of ischemic, structurally impaired, or dead cells within cardiac muscle. We concluded that for diseased cardiac muscle, the QRST maps reveal not only local repolarization, but also pathological depolarization variations in cardiac muscle affected by ischemia, the structurally impaired transition area from normal to scar tissue, and, especially, by scars from myocardial infarction itself.
Statistically significant results were obtained with 2 commonly used temporal indices: QRS duration and QT interval (influenced distinctly by QRS interval, except for long QT syndrome). The third temporal parameter, Tpeak-Tend interval, was not statistically significant in the studied groups’ separation, but its values increased in both MI patient groups in comparison to the control group. The TCRT parameter has shown similar properties. These 2 parameters might have been statistically significant if studied in a larger database.
In our study, dispersion of repolarization phase expressed by dispersion of QT interval and non-dipolar content of QRST integral maps (NDC) were not statistically significant in discrimination between MI patients groups with and without VT. The dispersion of QT interval is still the subject of debate regarding its ability to supply reliable information on the repolarization heterogeneity of cardiac muscle [7]. However, the discrimination between the healthy group and MI patients without VT was statistically significant. The results obtained using NDC parameter were also statistically significant in discrimination between the control group and the MI patients group without VT risk, which reveals its ability to quantify multipolarity in QRST maps in cardiac muscles affected by pathological changes.
This study had some limitations. First, due to the limited number of studied MI patients, the results should be considered as preliminary, requiring further analysis in a larger number of more precisely selected MI patients groups, especially those who are recommended for primary prevention by ICD implementation (i.e., with the EF below or equal to 30% [29]. Secondly, averaging ECG time-series prolonged the analysis process, which had to be done offline. However, in this study a precise assessment of the ends of ECG waves was crucial for QT dispersion calculation as well as for all temporal parameters such as QT interval, QRS width and Tpeak-Tend interval. The new parameters proposed in this work are less sensitive to time interval identification and might be used in on-line analysis and even calculated from a single selected heart beat with a low signal-to-noise ratio. Many studies have shown that pathological changes in beat-to-beat variability of subsequent cardiac cycles could be connected with increased risk of arrhythmia. Vulnerability to arrhythmia can be linked with dynamic changes in QT interval [9,13], RR interval variability (HRV) [6,14], heart rate turbulence (HRT) [15], or T-wave alternans (TWA) [2,8]. The analysis of beat-to-beat variability of proposed BSPM parameters is an important issue needing future research.
Conclusions
The obtained results indicate that the risk of arrhythmia increases with abnormal disturbances of both depolarization and repolarization processes. The best indices of the threat of ventricular tachycardia were: 1) the depolarization and repolarization prolongation expressed by QRS interval and QT interval, 2) the changed relation between depolarization and repolarization phases described by STT_QRST_CORR, and 3) spatial changes in repolarization phase expressed by STT_DI and TSI_DI parameters. Thus, the proposed new parameters might be applied for risk stratification of cardiac arrhythmia. These parameters might additionally support noninvasive identification of MI patients for ICD therapy. However, this preliminary study requires further analysis in a larger number of more precisely selected MI patients groups, especially patients who are recommended for primary prevention by ICD therapy.
Acknowledgements
The authors thank Ms Anna Zbiec for her technical assistance during data acquisition.
Footnotes
Source of support: This work was partly supported by the Polish Ministry of Science and Higher Education (research projects 3 T11E 005 30 and NN 518 504 339) and by the Austrian Ministry of Education, Science and Culture (Ernst Mach scholarship)
References
- 1.Acar B, Yi G, Hnatkova K, Malik M. Spatial, temporal and wavefront direction characteristics of 12-lead T-wave morphology. Med Biol Eng Comput. 1999;37(1):574–84. doi: 10.1007/BF02513351. [DOI] [PubMed] [Google Scholar]
- 2.Buxton AE. Risk stratification for sudden death in patients with coronary artery disease. Heart Rhythm. 2009;6(6):836–47. doi: 10.1016/j.hrthm.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 3.Janusek D, Pawlowski Z, Kania M, et al. Vectocardiographic representation of Concordant and Discordant T-wave Alternans. In: Murray A, editor. Proceedings of the Computers in Cardiology 2009; Park City, Utah, USA. [Google Scholar]
- 4.Kania M, Fereniec M, Janusek D, et al. Optimal ECG lead system for arrhythmia assessment with use of TCRT parameter. Biocybernetics and Biomedical Engineering. 2009;29(2):75–82. [Google Scholar]
- 5.Piorecka-Makula A, Werner B. Prolonged QT dispersion in children with congenital valvular aortic stenosis. Med Sci Monit. 2009;15(10):CR534–38. [PubMed] [Google Scholar]
- 6.Zipes D, Faha F, Camm A, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. J Am Coll Cardiol. 2006;48(5):e247–46. doi: 10.1016/j.jacc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Burnes JE, Ghanem RN, Waldo AL, Rudy Y. Imaging dispersion of myocardial repolarization, I: comparison of body-surface and epicardial measures. Circulation. 2001;104(11):1299–305. doi: 10.1161/hc3601.094276. [DOI] [PubMed] [Google Scholar]
- 8.Gold MR, Bloomfield DM, Anderson KP, et al. A comparison of T-wave alternans, signal averaged electrocardiography and programmed ventricular stimulation for arrhythmia risk stratification. J Am Coll Cardiol. 2000;36(7):2247–53. doi: 10.1016/s0735-1097(00)01017-2. [DOI] [PubMed] [Google Scholar]
- 9.Goldberger JJ, Cain ME, Hohnloser SH, et al. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death: a scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. Circulation. 2008;118(14):1497–518. [PubMed] [Google Scholar]
- 10.Kardys I, Kors JA, van der Meer IM, et al. Spatial QRS-T angle predicts cardiac death in a general population. Eur Heart J. 2003;24(14):1357–64. doi: 10.1016/s0195-668x(03)00203-3. [DOI] [PubMed] [Google Scholar]
- 11.Kors JA, van Herpen G, van Bemmel JH. QT dispersion as an attribute of T-loop morphology. Circulation. 1999;99(11):1458–63. doi: 10.1161/01.cir.99.11.1458. [DOI] [PubMed] [Google Scholar]
- 12.Straus SM, Kors JA, De Bruin ML, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47(2):362–67. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 13.Berger RD, Kasper EK, Baughman KL, et al. Beat-to-beat QT interval variability: novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation. 1997;96(5):1557–65. doi: 10.1161/01.cir.96.5.1557. [DOI] [PubMed] [Google Scholar]
- 14.Camm AJ, Pratt CM, Schwartz PJ, et al. Mortality in patients after a recent myocardial infarction: a randomized, placebo-controlled trial of azimilide using heart rate variability for risk stratification. Circulation. 2004;109(8):990–96. doi: 10.1161/01.CIR.0000117090.01718.2A. [DOI] [PubMed] [Google Scholar]
- 15.Francis J, Watanabe MA, Schmidt G. Heart rate turbulence: a new predictor for risk of sudden cardiac death. Ann Noninvasive Electrocardiol. 2005;10(1):102–9. doi: 10.1111/j.1542-474X.2005.10102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuch M, Janiszewski M, Mamcarz A, et al. Major adverse cardiac event predictors in survivors of myocardial infarction with asymptomatic left ventricular dysfunction or chronic heart failure. Med Sci Monit. 2009;15(6):PH40–48. [PubMed] [Google Scholar]
- 17.Jones DL, Touvannas JS, Lander P, Albert DE. Advanced time-frequency methods for signal-averaged ECG analysis. J Electrocardiol. 1992;25( Suppl):188–94. doi: 10.1016/0022-0736(92)90099-l. [DOI] [PubMed] [Google Scholar]
- 18.Lewandowski P, Meste O, Maniewski R, et al. Risk evaluation of ventricular tachycardia using wavelet transform irregularity of the high-resolution electrocardiogram. Med Biol Eng Comput. 2000;38(6):666–73. doi: 10.1007/BF02344873. [DOI] [PubMed] [Google Scholar]
- 19.Mroczka T, Lewandowski P, Maniewski R, et al. Effectiveness of high resolution ECG spectral analysis in discrimination of patients prone to ventricular tachycardia and fibrillation. Med Sci Monit. 2000;6(5):1018–26. [PubMed] [Google Scholar]
- 20.Rix H, Meste O. Wavelet theory and harmonic analysis in applied sciences. In: d’Attellis C, Fernández-Berdaguer E, editors. Wavelet theory and harmonic analysis in applied sciences. Boston: Birkhauser; 1997. pp. 143–51. [Google Scholar]
- 21.De Ambroggi L, Corlan A. Clinical use of body surface potential mapping in cardiac arrhythmias. Anadolu Kardiyol Derg. 2007;7:8–10. [PubMed] [Google Scholar]
- 22.Gardner MJ, Montague TJ, Armstrong CS, et al. Vulnerability to ventricular arrhythmia: assessment by mapping of body surface potential. Circulation. 1986;73(4):684–92. doi: 10.1161/01.cir.73.4.684. [DOI] [PubMed] [Google Scholar]
- 23.Hren R, Steinhoff U, Gessner C, et al. Value of magnetocardiographic QRST integral maps in the identification of patients at risk of ventricular arrhythmias. Pacing Clin Electrophysiol. 1999;22(9):1292–304. doi: 10.1111/j.1540-8159.1999.tb00622.x. [DOI] [PubMed] [Google Scholar]
- 24.Kozmann G, Haraszti K, Preda I. Beat-to-beat interplay of heart rate, ventricular depolarization, and repolarization. J Electrocardiol. 2010;43(1):15–24. doi: 10.1016/j.jelectrocard.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Peeters HAP, SippensGroenewegen A, Schoonderwoerd BA, et al. Body-Surface QRST Integral Mapping: Arrhythmogenic Right Ventricular Dysplasia Versus Idiopathic Right Ventricular Tachycardia. Circulation. 1997;95(12):2668–76. doi: 10.1161/01.cir.95.12.2668. [DOI] [PubMed] [Google Scholar]
- 26.Fereniec M, Maniewski R, Karpinski G, et al. High-resolution multichannel measurement and analysis of cardiac repolarization. Biocybernetics and Biomedical Engineering. 2008;28(3):61–69. [Google Scholar]
- 27.Janusek D, Fereniec M, Kania M, et al. Spatial Distribution of T-Wave Alternans. In: Murray A, editor. Proceedings of the Computers in Cardiology 2007; 2007; Durham, North Carolina, USA. [Google Scholar]
- 28.Connolly SJ, Hallstrom AP, Cappato R, et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. Eur Heart J. 2000;21(24):2071–78. doi: 10.1053/euhj.2000.2476. [DOI] [PubMed] [Google Scholar]
- 29.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117(21):e350–408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 30.Kuck KH, Cappato R, Siebels J, Ruppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH) Circulation. 2000;102(7):748–54. doi: 10.1161/01.cir.102.7.748. [DOI] [PubMed] [Google Scholar]
- 31.Moss AJ, et al. Prophylactic Implantation of a Defibrillator in Patients with Myocardial Infarction and Reduced Ejection Fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 32.Haigney MC, Zareba W, Gentlesk PJ, et al. QT interval variability and spontaneous ventricular tachycardia or fibrillation in the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II patients. J Am Coll Cardiol. 2004;44(7):1481–87. doi: 10.1016/j.jacc.2004.06.063. [DOI] [PubMed] [Google Scholar]
- 33.SippensGroenewegen A, Spekhorst H, van Hemel NM, et al. Body surface mapping of ectopic left and right ventricular activation. QRS spectrum in patients without structural heart disease. Circulation. 1990;82(3):879–96. doi: 10.1161/01.cir.82.3.879. [DOI] [PubMed] [Google Scholar]
- 34.Ihara Z, van Oosterom A, Hoekema R. Atrial repolarization as observable during the PQ interval. J Electrocardiol. 2006;39(3):290–97. doi: 10.1016/j.jelectrocard.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Ikeda K, Tomoike H. ST-T and U wave changes in Myocardial Ischemia assessed by body surface mapping. In: Yasui S, Abildskov JA, Yamada K, Harumi K, editors. Advances in Body Surface Mapping and High Resolution ECG. 1994. pp. 68–75. [Google Scholar]
- 36.Fereniec M, Karpinski G, Maniewski R, Opolski G. Evaluation of T-wave morphology in high-resolution ECG mapping. Int J Bioelectromagn. 2002;2(2):101–2. [Google Scholar]
- 37.De Ambroggi L, Aime E, Ceriotti C, et al. Mapping of ventricular repolarization potentials in patients with arrhythmogenic right ventricular dysplasia: principal component analysis of the ST-T waves. Circulation. 1997;96(12):4314–18. doi: 10.1161/01.cir.96.12.4314. [DOI] [PubMed] [Google Scholar]
- 38.De Ambroggi L, Bertoni T, Locati E, et al. Mapping of body surface potentials in patients with the idiopathic long QT syndrome. Circulation. 1986;74(6):1334–45. doi: 10.1161/01.cir.74.6.1334. [DOI] [PubMed] [Google Scholar]
- 39.Fereniec M, Kepski R, Karpinski G, et al. Assessment of Spatial Disparity of Repolarization Phase by HRECG Maps in Patients Qualified to ICD Therapy. Folia Cardiol. 2005;12(Supp D) [Google Scholar]
- 40.Ramanathan C, Jia P, Ghanem R, et al. Activation and repolarization of the normal human heart under complete physiological conditions. Proc Natl Acad Sci USA. 2006;103(16):6309–14. doi: 10.1073/pnas.0601533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geselowitz DB. The ventricular gradient revised: relation to the area under the action potential. IEEE Trans Biomed Eng. 1983;BME-30:76–78. doi: 10.1109/tbme.1983.325172. [DOI] [PubMed] [Google Scholar]
- 42.Mirvis D. Electrocardiography: A physiologic approach. Mosby Inc.; 1993. [Google Scholar]