Summary
Background
Isolated sphenoid sinus pathologies are relatively rare. In the majority of cases, symptoms do not arise in the early stages of the disease or are non-specific, therefore making diagnosis difficult. The aim of this study was to investigate the diagnostic process and the reasons for development of complications in patients with isolated sphenoid sinus pathology.
Material/Methods
The clinical data and observation charts of 32 patients were investigated to determine how long the main symptoms of sphenoid pathology had been present before the patients were referred for medical treatment, and the time that elapsed from the first ambulatory medical assessment to the initial diagnosis.
Results
Complaints and symptoms of sphenoid sinus pathology had been present for 10.2 months before the diagnosis was established. Although the duration of complaints in “ORL” (diagnosed by otorhinolaryngologist) and “non-ORL” (diagnosed by other specialists) group of patients was similar (10.8 and 9.5 months on average, respectively), unexpectedly, in the “non-ORL” group of patients, the time necessary for making the initial diagnosis was actually shorter than in the “ORL” group (1.8 vs 4.1 months). At the time of hospital admission, endoscopic examination revealed no abnormalities in 31.2% of patients. In 28.1% of patients the pathological process in the sphenoid sinus was diagnosed only after the onset of complications.
Conclusions
The occult character of the disease and the lack of severe and specific symptoms, rather than the delay in getting extensive diagnostic tests, are responsible for the delayed diagnosis and treatment.
Keywords: sphenoiditis, isolated lesion, complications
Background
Isolated sphenoid sinus pathologies are relatively rare. Among patients with rhinosinusitis, isolated sphenoiditis is diagnosed in 2.7–3% of cases [1,2]. Neoplastic lesions of this region are even less common and account for 15–16% of isolated sphenoid sinus disease cases [3,4].
In the majority of cases of sphenoid sinus pathology, symptoms do not arise in the early stages of the disease or are non-specific. Therefore, making the diagnosis becomes difficult and the term dumb sinus is found in the literature. Nonetheless, timely and accurate diagnosis is of utmost importance due to the contiguity of the sinus with vital neuro-vascular structures. Unnecessary delay in proper initiation of treatment may result in catastrophic consequences. It is a commonly believed that due to scarce and vague symptoms of sphenoid sinus pathology, patients are usually not referred to the otolaryngologist immediately, but instead are initially treated by other specialists, thus adding further delay to the diagnosis.
According to Friedman et al. [3], in over 1/3 of patients the symptoms related to sphenoid sinus disease had been present for more than 1 year before the diagnosis was established.
The present study attempted to evaluate diagnostic delay in a series of patients with isolated sphenoid sinus disease. The reasons for complications of sphenoid sinus disease are also assessed.
Material and Methods
A retrospective review of the medical records, including clinical data, observation charts and radiological findings, of 32 (19 M, 13 F) consecutive patients aged 21–63 years (average age 44.3) years with sphenoid sinus pathology, who were referred to our clinic between 2005 and 2009, was performed. The initial diagnosis, based on symptomatology and CT and/or MRI findings, was made in ambulatory settings. Only patients with isolated pathological process of the sphenoid sinus were qualified for the study. Presence of other sinus pathologies was an exclusion criterion.
All patients’ complaints were thoroughly analyzed in order to define the leading symptoms of the disease, determine how long the main symptoms of sphenoid pathology had been present before the patients were referred for medical treatment, assess the time from the first ambulatory medical assessment to the establishment of the initial diagnosis, and the type of specialist who established the initial diagnosis on the basis of imaging studies.
Thorough otorhinolaryngological examination, including transnasal endoscopy, was performed in all patients. Patients underwent endoscopic surgery using direct trans-nasal (through natural ostium) or transethmoidal approach to the sphenoid sinus.
Results
The study group consisted of 22 (68.7%) patients with isolated inflammatory lesions of the sphenoid sinus, and 10 (31.3%) patients with tumors and non-inflammatory sphenoid disease.
Although differential diagnosis of sphenoid sinus lesions based on imaging studies is very complex, initial diagnosis could be made in the ambulatory settings. CT scanning in bacterial sphenoiditis revealed mucosal thickening, and partial or total opacification of the sinus. Bony wall sclerosis was present in some cases with chronic bacterial inflammatory process. In patients with fungal sphenoiditis, CT demonstrated opacification of the sphenoid, often with associated hyperdense foci, bony wall thickening or expansion. However, in some cases with fungal infection of this region, the results of imaging studies may be ambiguous [5]. Mucoceles appeared as a mass or complete opacification causing remodeling of bone. In some cases bony destruction was also present. Administration of gadolinum did not result in enhancement of the mass. MRI signal was dependent on the protein content and fluid consistency within the mucocele. In patients with malignancies, contrast enhancement was the rule. They present as expansile masses with aggressive radiological features leading to bone destruction with irregular shape and poorly-defined margins. MRI helped to distinguish the extent of the mass from its surrounding inflammatory effect. Diagnosis of internal carotid aneurysm was established by angiography. In 2 patients with spontaneous CSF leak, the air-fluid level was observed and small bony dehiscences were found in the posterior and lateral walls of the sphenoid on high resolution CT. The site of the leak was subsequently detected by intrathecal contrast medium administration.
The final diagnosis was made on the basis of intraoperative findings and pathoanatomical results that are summarized in Table 1.
Table 1.
Groups of isolated pathologic lesions of the sphenoid sinus, complications, and results of endoscopic examination of sphenoethmoidal recess.
| Pathology/number of patients | Complications/number of patients | Absence of abnormalities during endoscopic examination/number of patients |
|---|---|---|
| Malignant tumors/4 (undifferentiated carcinoma plasmocytoma, squamous cell carcinoma, chordoma) | Ocular motility disturbances, blurred vision/4 | |
| Benign tumors/2 (osteoma, fibrous dysplasia) | Visual field deficits/1 | 2 |
| Pseudotumors/2 (meningoencephalocele internal carotid aneurysm) | Recurrent meningitis/1 | 1 |
| Spontaneous cerebrospinal fluid leak/2 | ||
| Bacterial sinusitis/11 | Blurred vision, visual field deficits/1 | 3 |
| Fungal sinusitis/2 | Sudden visual loss/1 | |
| Mucocele and cystic lesions/6 | Blurred vision, visual field deficits/1 | 2 |
| Mucosal hypertrophy/3 | 2 | |
| Total 32 | 9 | 10 |
The most common symptoms related to sphenoid sinus pathology were headaches of various intensity and locations, present in 75.8% of patients, sometimes accompanied by nasal discharge/postnasal drip (44.8%), nasal congestion (31%) and olfactory deterioration (3.4%). The rhinological examination on admission revealed no abnormalities in 10 out of 32 (31.2%) patients. In the remaining 22 (68.8%) patients, various types of pathology, including edematous or polypoid tissue, pathological mass, mucopurulent discharge in the region of the posterior part of the nasal meatus, in the nasopharynx and in the sphenoethmoidal recess, were found.
Complaints and symptoms of sphenoid sinus pathology had been present for 10.2 months on average (range: 3 days – 24 months) before the diagnosis was established.
The mean time interval between the first medical assessment and the diagnosis based on imaging studies was 3.1 months on average (range: 1 day – 10 months).
In 9 (28.1%) cases the pathological process in the sphenoid sinus was diagnosed only after the onset of complications such as visual acuity impairment and/or ocular motility disturbances, visual field deficits or recurrent meningitis (Table 1).
In 2 patients (with undifferentiated carcinoma and chordoma) the complications occurred after their initial referral to the physician, while the remaining 7 patients were referred for their first medical assessment when the complications were already fully developed.
In 15 (46.8%) cases the initial diagnosis was made by other specialists (“non-otorhinolaryngologist”), including neurologist, neurosurgeon, ophthalmologist, hematologist, and infectious diseases specialist, and the patients were subsequently referred to our attention. The symptoms of sphenoid sinus pathology in the “non-otorhinolaryngological” (non-ORL) group of patients were present for 9.5 months on average (range 3 days – 24 months). The mean time interval between the first medical assessment and the initial diagnosis based on imaging studies was 1.8 months on average (range 1 day – 5 months).
Seventeen patients (53.2%) of our series were referred from other ENT departments or otorhinolaryngological outpatient clinics (ORL – group). The symptoms of sphenoid sinus pathology in this group of patients were present for 10.8 months on average (range: 1–24 months) and the time that elapsed from first medical assessment to established initial diagnosis (based on CT and/or MRI) was 4.1 months on average (range 4 days – 10 months) (Table 2).
Table 2.
Comparison of the mean duration of symptoms and the mean time necessary for making initial diagnosis based on imaging studies in patients diagnosed by otorhinolaryngologist (otorhinolaryngological group) and other specialists (non- otorhinolaryngological group).
| Number of patients (%) | The mean duration of symptoms (months) | The mean time from the first medical assessment to the imaging studies (months) | |
|---|---|---|---|
| Non-otorhinolaryngological group | 15 (46.8%) | 9.5 | 1.8 |
| otorhinolaryngological group | 17 (53.2%) | 10.8 | 4.1 |
| Average | 10.2 | 3.1 |
Discussion
Today, isolated sphenoid sinus disease is diagnosed more frequently than in the past, due to increased availability and accuracy of radiological studies. One of the first large series of patient was reported by Wyllie et al. [6] in 1973, who described 45 patients treated at the Mayo Clinic during 37 years. Our 32 patients were selected from patients referred to our clinic between 2005 and 2009.
The symptoms and signs of sphenoid sinus disease are usually rare and non-specific; therefore, there is always a particular risk that they may be ignored either by the patient or by the doctor. As reported by many authors, the most frequent complaint in these patients is headache of various intensities and locations [7–10]. Although headache was also the most common symptom in the study group, sometimes serious pathology of the sphenoid sinus remained totally asymptomatic until complications emerged (Figure 1).
Figure 1.
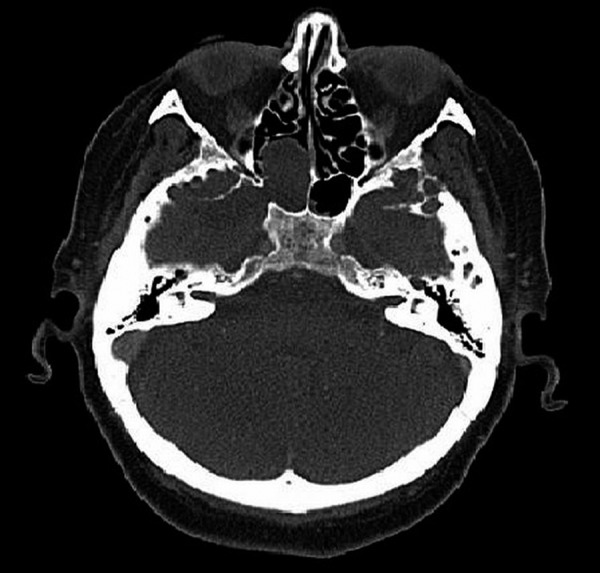
Meningocele – the cause of recurrent meningitis – CT, axial plane.
Endoscopy is a very useful tool for detecting paranasal sinuses pathologies; nonetheless, the diagnosis in patients with isolated sphenoid sinus disease is not that obvious. According to Sethi [11], normal appearance of the sphenoethmoidal recess does not exclude sphenoid pathology. In our series the endoscopic examination of the sphenoethmoidal recess did not show any abnormality in 10 patients (31.2%), despite the presence of subjective symptoms. All these patients had benign tumors, pseudotumors, cystic lesions or chronic inflammatory processes. In all cases endoscopic examination of patients with malignant lesions revealed pathological mass or discharge in the vicinity of the natural ostium of the sphenoid (Figure 2).
Figure 2.
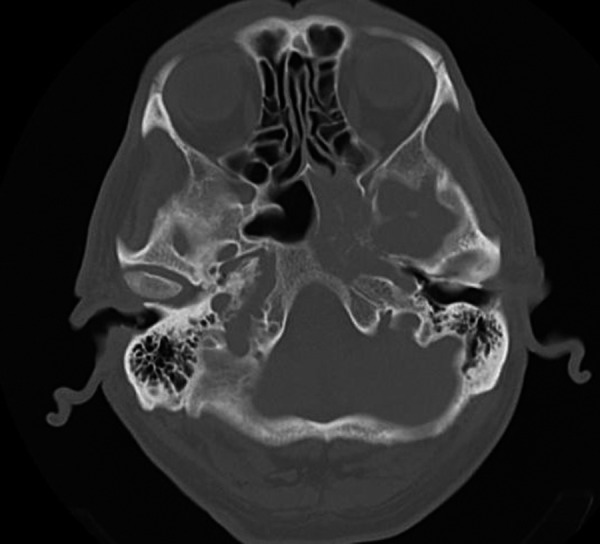
Undifferentiated carcinoma – edema and polypoid tissue in the sphenoethmoidal recess destruction of the bony walls of sphenoid – CT, axial plane.
Friedman [3], Martin [7] and Sethi [11] reported a similar rate of negative endoscopic findings in symptomatic patients (34%, 38% and 28%, respectively). In other reports endoscopy failed to demonstrate any pathological abnormalities around the sphenoidal ostium in as many as 50% of cases [8,12]; hence, the initial diagnosis of sphenoid sinus pathology is still based on imaging studies. The difficulties repeatedly observed in making an appropriate diagnosis of sphenoid sinus pathology can be ascribed to the anatomic location of the sphenoid sinus itself (i.e., deep inside in the nasal cavity) while its vicinity to vital neuro-vascular structures could be related to the onset of serious complications if diagnosis is inappropriately delayed. Lew et al. [13] describe a series of 30 hospitalized patients in which delay in diagnosis resulted in irreversible blindness or cavernous sinus thrombosis in 2 cases, and death of 7 patients, despite the administration of intravenous antibiotics and surgical management.
In our series, 9 out of 32 (28.1%) patients were diagnosed after the complication had occurred. The complication rate in our series is comparable to that reported in the literature [7,10,14]. Pearlman [14] and Friedman [3] showed that cranial nerve deficits are more likely to be found in patients with neoplastic lesions rather than in those with inflammatory pathologies of the sphenoid. In our series the complications also tended to develop more frequently in patients affected with neoplastic diseases. They were observed in all 4 patients with malignancies, and in only 3 out of 22 patients with inflammatory process in the sphenoid sinus (Figure 3). In the remaining 2 patients, complications were caused by pseudotumors (meningogence-pholocele) and benign space-occupying lesions (displasia fibrosa). Seven of these patients did not seek medical advice earlier, presumably due to limited clinically relevant symptoms. The remaining 2 patients (with undifferentiated carcinoma and chordoma) developed the complications several weeks after their first medical assessment; however, the mild and intermittent character of the symptoms prevented them from attending the planned follow-up appointment.
Figure 3.
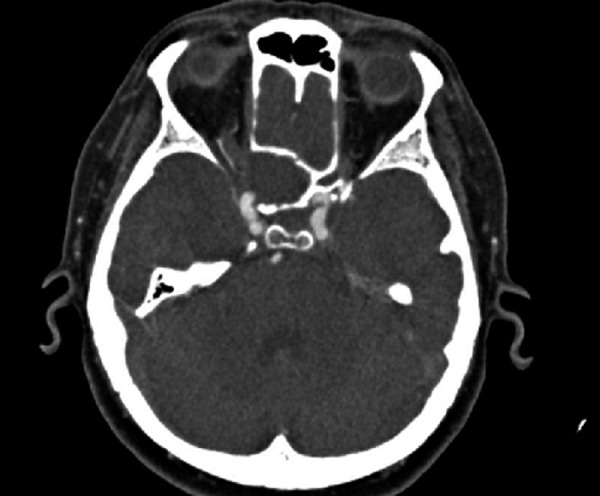
Mucocele causing sphenoid lateral bony wall erosion and optic nerve compression – contrast enhanced CT, axial plane.
The duration of symptoms before the onset of complications is unpredictable, and may vary from several hours to several months or even years. Such observations have been documented in previous reports [8,15] and were also observed in our study. In one of our patients with chronic fungal rhinosinusitis, the symptoms had been present for about 8 months before the onset of complications, and in another patient with bacterial rhinosinusitis, just 3 days before the complications emerged.
Symptoms related to sphenoid sinus pathology had been present in our patients for an average of 10.2 months before the diagnosis was established. This is comparable with other reports [3,8]. Batra et al. [16] documented much longer delay, up to an average of 2.9 years, in the diagnosis of sphenoiditis in patients who underwent transsphenoidal hypophysectomy. This may suggest that the symptoms were intermittent or not severe enough to cause patients seek medical advice earlier. The mean time elapsed from the first medical assessment to the CT/MRI examination in our series was only 3.1 months, which also suggests that the delay was not caused by an inadvertently prolonged diagnostic process.
Complaints and symptoms of isolated sphenoid sinus pathology are often vague and non-characteristic. For this reason, patients are usually not referred immediately to the laryngologist, but are instead initially treated by other specialists, which may further delay diagnosis. In our, 46.8% patients were initially assessed, treated and eventually diagnosed in “non-otorhinolaryngological” departments; however, without causing any delay in establishing the appropriate diagnosis. Although the duration of complaints in “ORL” and “non-ORL” groups of patients was similar (10.8 and 9.5 months on average, respectively), unexpectedly, in the “non-ORL” group of patients, the time necessary to make the initial diagnosis and/or to perform imaging studies was actually shorter than in the “ORL” group (1.8 vs 4.1 months).
There are at least 3 reasons possibly explaining the aforementioned situation. Firstly, doctors are more likely to perform urgent imaging studies in cases with headaches of unknown origin. If there are no evident signs of rhinosinusitis, which may be difficult to verify for the non-otorhinolaryngologist, patients are usually treated symptomatically, thus yielding only temporary relief, and they therefore tend to seek further medical review and are eventually sent for imaging studies.
Secondly, when subjective and/or objective symptoms suggestive of rhinosinusitis are present, an intensive medical treatment is continued for several weeks before obtaining radiological investigations.
Finally, the initial treatment with anti-inflammatory drugs frequently relieves the symptoms quickly, often leading to an inappropriate discontinuation of the therapy by some patients, and consequently worsening the same symptoms. These patients are referred for medical assessment again, when their symptoms are aggravated, very often after weeks or even months, and that is the real cause of delayed diagnosis.
In addition, the overuse of antibiotics in chronic rhinosinusitis may lead to a decrease in normal intranasal flora and overgrowth of much more dangerous methicillin-resistant Staphylococcus aureus (MRSA), especially in patients who had a history of prior infection with this type of pathogen [17].
Even though imaging studies will probably remain the cornerstone of the process of diagnosing sphenoid sinus pathology, other noninvasive and simple tests (e.g., nasal nitric oxide assessment) that already have proven useful in the diagnosis of various rhinologic pathologies [18], are worth investigation.
Conclusions
Although isolated sphenoid lesions are currently diagnosed much more frequently than in the past, there are still cases in which the proper diagnosis is made too late. The latent character of the disease and the lack of severe and specific symptoms, rather than the delay in getting extensive diagnostic tests, are responsible for the delayed diagnosis and treatment.
Because isolated sphenoid pathology may manifest itself only with a very poor symptomatology, and the endoscopic examination may fail to demonstrate any pathological abnormalities around the sphenoidal ostium even in symptomatic patients, imaging studies in cases of high clinical suspicion are recommended.
Footnotes
Source of support: Departmental sources
References
- 1.Cheung DK, Martin GF, Rees J. Surgical approaches to the sphenoid sinus. J Otolaryngol. 1992;21:1–8. [PubMed] [Google Scholar]
- 2.Lew D, Southwick FS, Montgomery WW, et al. Sphenoid sinusitis – a review of 30 cases. N Engl J Med. 1983;309:1149–54. doi: 10.1056/NEJM198311103091904. [DOI] [PubMed] [Google Scholar]
- 3.Friedman A, Batra PS, Fakhri S, et al. Isolated sphenoid sinus disease: Etiology and management. Otolaryngol Head Neck Surg. 2005;133:544–50. doi: 10.1016/j.otohns.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Lawson W, Reino AJ. Isolated sphenoid sinus disease: an analysis of 132 cases. Laryngoscope. 1997;107:1590–95. doi: 10.1097/00005537-199712000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Sinha NR, Szmigielski W, Ismail M. Hypophyseal fossa aspergillosis mimicking a pituitary macroadenoma with bleed: A case report. Pol Przegl Radiol. 2009;74(3):73–77. [Google Scholar]
- 6.Wyllie JW, Kern EB, Dyalilian M. Isolated sphenoid sinus lesions. Laryngoscope. 1973;83:1252–65. doi: 10.1288/00005537-197308000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Martin TJ, Smith TL, Smith MM, Loehrl TA. Evaluation and surgical management of isolated sphenoid sinus disease. Arch Otolaryngol Head Neck Surg. 2002;128:1413–19. doi: 10.1001/archotol.128.12.1413. [DOI] [PubMed] [Google Scholar]
- 8.Nour YA, Al-Madani A, El-Daly A, Gaafar A. Isolated sphenoid sinus pathology: spectrum of diagnostic and treatment modalities. Auris Nasus Larynx. 2008;35:500–8. doi: 10.1016/j.anl.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Wang ZM, Kanoh N, Dai CF, et al. Isolated sphenoid sinus disease: an analysis of 122 cases. Ann Otol Rhinol Laryngol. 2002;111:323–27. doi: 10.1177/000348940211100407. [DOI] [PubMed] [Google Scholar]
- 10.Castelnuovo P, Pagella F, Semino L, et al. Endoscopic treatment of the isolated sphenoid sinus lesions. Eur Arch Otorhinolaryngol. 2005;262:142–47. doi: 10.1007/s00405-004-0764-6. [DOI] [PubMed] [Google Scholar]
- 11.Sethi DS. Isolated sphenoid lesions: diagnosis and management. Otolaryngol Head Neck Surg. 1999;120:730–36. doi: 10.1053/hn.1999.v120.a89436. [DOI] [PubMed] [Google Scholar]
- 12.Socher JA, Cassano M, Filheiro CA, et al. Diagnosis and treatment of isolated sphenoid sinus disease: a review of 109 cases. Acta Otolaryngol. 2008;128:1004–10. doi: 10.1080/00016480701793735. [DOI] [PubMed] [Google Scholar]
- 13.Lew D, Southwick FS, Montgomery WW, et al. Sphenoid sinusitis. A review of 30 cases. N Engl J Med. 1983;309:1149–54. doi: 10.1056/NEJM198311103091904. [DOI] [PubMed] [Google Scholar]
- 14.Pearlman SJ, Lawson W, Biller HF, et al. Isolated sphenoid sinus disease. Laryngoscope. 1989;99:716–20. doi: 10.1288/00005537-198907000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Güvenç MG, Kaytaz A, Ozbilen Acar G, Ada M. Current management of isolated sphenoiditis. Eur Arch Otorhinolaryngol. 2009;266:987–92. doi: 10.1007/s00405-008-0873-8. [DOI] [PubMed] [Google Scholar]
- 16.Batra PS, Citardi MJ, Lanza DC. Isolated sphenoid sinusitis after transsphenoidal hypophysectomy. Am J Rhinol. 2005;19:185–89. [PubMed] [Google Scholar]
- 17.Nicholas BD, Bhargave G, Hatipoglu A, et al. Preoperative prevalence of methicillin-resistant Staphylococcus aureus (MRSA) colonization in patients undergoing intranasal surgery. Med Sci Monit. 2010;16(8):CR365–68. [PubMed] [Google Scholar]
- 18.Santamaria F, De Stefano S, Montella S, et al. Nasal nitric oxide assessment in primary ciliary dyskinesia using aspiration, exhalation, and humming. Med Sci Monit. 2008;14(2):CR80–85. [PubMed] [Google Scholar]


