Summary
Background
Recent studies suggest that adipose tissue hormones are involved in the pathogenesis of obstructive sleep apnoea syndrome (OSAS). The role of leptin, obestatin and apelin still needs to be established.
Material/Methods
Ten patients with newly diagnosed OSAS (AHI >10/h and ESS >10 points) were enrolled in the study as well as ten healthy volunteers as controls. All underwent measurements for Leptin, Obestatin and Apelin in four hour intervals during diagnostic polysomnography for 24 h and the patients also three months after onset of CPAP treatment. Furthermore the HOMA-index and body composition were quantified.
Results
Plasma apelin levels in the patients decreased under CPAP therapy, but showed no significant difference in patients and volunteers. We found a positive correlation to AHI, BMI in the therapy group at all observation points.
Leptin plasma levels were higher in the patient group and decreased after onset of CPAP therapy. Leptin plasma levels were positively correlated to the BMI, min. 02 and AHI in the patient group before therapy.
Plasma obestatin levels did not differ significantly in these three observation groups, but were partly correlated to AHI and weight in the newly diagnosed OSAS group.
Conclusions
In agreement with previous investigations, we could demonstrate a difference in leptin plasma levels between healthy volunteers and patients with newly diagnosed OSAS. Apelin decreases under CPAP therapy, but not significantly.
Obestatin remains unchanged after onset of CPAP. We further found a linkage between leptin plasma levels and BMI, AHI and weight in the untreated patient group.
Keywords: OSAS, adipose tissue hormones, CPAP therapy, apelin, obestatin, leptin
Background
The obstructive sleep apnoea syndrome (OSAS) is a disorder with a high prevalence characterized by an increased cardiovascular risk. Obesity and insulin resistance are typical metabolical features of OSAS. Since obesity has reached epidemic proportions globally [1] numerous efforts have been made to understand the complex mechanisms involved in the regulation of food intake, hunger, satiety, energy storage and consumption.
In the last two decades, several novel regulatory peptides such as leptin, ghrelin or adiponectin have been discovered and studied emphasizing their role in energy homeostasis. The demonstration of a possible role of some of these peptides in sleep regulation as independent effects apart from those in energy regulation was a fascinating additional finding [2,3].
Apelin is a peptide that was identified in 1998 [4]. The effect best characterized of this hormone is within the cardiovascular system. A hypotensive effect of apelin results from the activation of receptors expressed at the surface of endothelial cells [5], but the hormone has also been intrigued in angiogenesis [6]. Apelin receptors have been found within the lung [7] and Eyries et al. recently demonstrated that apelin expression was induced by hypoxia in cell cultures as well as in mice exposed to hypoxia [8].
With this in mind, we investigated, whether there might be differences in Apelin secretion in patients with OSAS compared to healthy people due to chronic nocturnal hypoxia in the first group and potential effects of CPAP therapy after reversal of these events. Since patients with OSAS are insulin-resistant [9], and insulin-induced apelin expression was recently demonstrated in adipocytes [10] the groups might have different apelin secretion in general and not only during some periods.
Another novel aminopeptide is obestatin. To date, effects on food intake, weight gain and intestinal motility have been investigated due to its encodement on the same gene as ghrelin [11]. We were particularly interested in its role in sleep regulation that has been investigated in rats [12,13], but not humans yet. To our knowledge, serial measurements of obestatin with regard to its putative role in sleep regulation were not performed. Patients with OSAS thus, served as a “biomodel” of disturbed sleep behaviour were compared to healthy controls and furthermore, we investigated possible alterations of obestatin secretion after restoration of normal sleep in OSAS patients.
An interaction of apelin and obestatin with insulin resistance as a well characterized feature of OSAS patients has already been described; and we also determined their state of insulin (in)sensitivity by use of the HOMA model.
The role of leptin in OSAS has already been more precisely characterized. Fasting leptin levels in patients with OSAS decrease after initiation of CPAP (continuous positive airway pressure) treatment without changes in weight and are discussed as a respiratory stimulus [14]. With interactions between insulin resistance and leptin [15,16] as well as between leptin and apelin [7] and obestatin [12], we chose to determine serial leptin measurements as well to characterize possible interactions between these three hormones.
Material and Methods
Subjects
Ten patients (n=10, male) with newly diagnosed symptomatic obstructive sleep apnoea syndrome (apnoea-hypopnea-index, AHI >10 per hour and Epworth-sleepiness-scale, ESS >10 points) were enrolled in the study. Obstructive apnoeas were defined as the absence of oronasal flow for at least 10s. Hypopnoeas were defined as reduction in airflow to ≤50% of the preceding stable baseline for 10s or longer together with a dip in oxygen saturation ≥4%. The mean number of apnoeas and hypopnoeas per hour of sleep was calculated as the apnoea/hypopnoea index (AHI). The Epworth-sleepiness-scale is a questionnaire to evaluate especially daytime sleepiness.
As a first step diagnostic polysomnography was performed. In this setting the degree of the OSAS and the sleep disturbance was quantified. The AHI as well as the sleep quality were assessed. Before polysomnographic measurements subjects underwent a complete medical history, clinical chemistry and physical examination. This was done to rule out serious other diseases (such as diabetes, hepatitis, cancer) and medication with an impact on insulin sensitivity, which might influence the analyses of adipocyte-derived hormones.
Three months later and having used the nCPAP therapy reliably all examinations were repeated (polysomnography under nCPAP therapy, questionnaire, clinical chemistry and DXA) und compared with the initial results.
The hormones leptin, obestatin and apelin were measured in four hour intervals (2, 6, 10 a.m. and 2, 6, 10 p.m.) including the night of diagnostic and therapeutic polysomnography. Normal sleep wake rhythms were retained, the average sleep time duration lay between six and nine hours. The light was turned off at 10 to 11 p.m., the patients were waked up at about 6 to 7 a.m.
Blood drawings were performed throughout an indwelling superficial forearm catheter to minimalize the patients disturbance. In the second night a titration to find out the minimal sufficient therapeutic pressure was made. The goal was to reduce the pathologic AHI to a normal rage (<5/h). Additionally as a potential marker of influence the exact body composition was measured by using DXA (Lunar Prodigy™). Before demission each patient was instructed to use the nCPAP therapy regularly each night for at least six hours to reach a therapeutic effect. To clarify CPAP adherence, the built-in data stores of the CPAP devices were read out. This allows to establish the number of days of use within the last three months and to calculate the mean duration of use per night of treatment.
Ten healthy volunteers (n=9 male and n=1 female) with no sleep disorder were recruited to serve as a control group. As the ten patients with OSAS they underwent the same examinations (DXA, clinical chemistry, questionnaire etc.). To rule out subjects with OSAS the volunteers were measured under study conditions with Apnoe Screen (ApnoeScreen Pro, VIASYS Healthcare GmbH, Leibnizstrasse 7, 97204 Höchberg, Germany). Finally a comparison of the data of the volunteers and the patients regarding circadian rhythm of the adipocyte-derived hormones were made.
All persons studied gave written informed consent to participate in the study, the study protocol was approved by the local ethics committee.
The samples were collected in ethylendiamine tetraacetate-coated polypropylene tubes, centrifuged immediately at 3.000 rpm for 20 min at 0°C, and the clear plasma supernatant was then stored until plasma leptin, obestatin and apelin levels were measured as follows:
Measurement of serum apelin
Serum apelin was measured directly by a specific radioimmunoassay kit with a measurement range from 10–1280 pg/ml (Human Apelin-36 RIA kit, Phoenix Pharmaceuticals, Inc., 330 Beach Road, Burlingame, CA 94010, U.S.A.). All samples and standards were assayed in duplicate within the same assay.
Measurement of serum obestatin
Peripheral obestatin levels were measured using a commercial radioimmunoassay kit with a measurement range from 50–6400 pg/ml (Human Obestatin RIA kit, Phoenix Pharmaceuticals, Inc., 330 Beach Road, Burlingame, CA 94010, U.S.A.). All samples and standards were assayed in duplicate within the same assay.
Measurement of serum leptin
Serum leptin levels were measured by a commercially available radioimmunoassay kit (Human Leptin RIA kit, Millipore Corporation, 290 Concord Road, Billerica, MA 01821). The sensitivity limit was 0,5 ng/mL. All samples and standards were assayed in duplicate within the same assay.
Sleep studies
The polysomnographies were performed according to the recommendations of the American Thoracic Society [17] and the German Sleep Society [18,19]. Sleep parameters were determined using the criteria of Rechtschaffen and Kahles [20] and microarousals were defined in accordance with the definitions of the American Sleep Disorders Association (ASDA) [21]. All patients were monitored for at least 6h in our sleep laboratory. The measured parameters included submental electromyography, snoring detected by a microphone, electrocardiography, thoracic and abdominal movements, bilateral electrooculography, electroencephalography, nasal airflow measured by oronasal thermistors during diagnostic polysomnographics and by an pneumotachograph during CPAP studies and oxyhaemoglobin saturation using a finger oxymeter (Microspan 3040G™, Jaeger amd Toennies, Würzburg, Germany). The measured data were evaluated by the same qualified doctor.
Results
Patients and the healthy controls were approximatly the same age (58.9±10.2 vs. 53.6±7.7, p>0.05). As to be expected for caucasian subjects the two groups differ significantly regarding weight (BMI 31.7±3.2 vs. 26.7±2.3 kg/m2, p<0.05). Corresponding to this fact the patients had a significant higher percental body fat (26.6±7.5 vs. 33.7±3.7, p<0.05). The patients before therapy had a significant higher AHI (40.4±18.9 vs. 2.7±3.1 /h, p<0.05) and a lower sleep quality (ESS 11.7±1.7 vs. 5.1±2.1 points, p<0.05) compared to the healthy volunteers. In total 10 patients and 10 healthy controls were studied. From the 10 patients at the beginning 3 refused further CPAP therapy in the first 4–6 weeks due to discomfort. The remaining 7 patients applied the therapy according to the initial instructions for use (at least 6 hours per night). The patient group was measured twice: Before therapy and after three months of application of CPAP. The CPAP treatment significantly eliminated the previously observed obstructions. In consequence the initial AHI was reduced to a normal range (42.1±16.2 vs. 4.7±6.0 /h, p<0.05) and the mean SaO2 was ensured (91.2±4.1 vs. 94.6±1.7%, p<0.05). The subjective sleepiness and sleep quality were improved (ESS 11.8±1.8 vs. 5.0±2.0, p<0.05) (Table 1).
Table 1.
Characteristics of patients and controls.
| Controls | Patients t=0* | Patients t=3** | p-value | |
|---|---|---|---|---|
| Age yrs | 53.6±7.7 | 58.9±10.2 | 58.9±10.2 | ns |
| BMI kg/m² | 26.7±2.3 | 31.7±3.2 | 32.1±3.1 | <0.05/ns |
| ESS/h | 5.1±2.1 | 11.7±1.7 | 5.0±2.0 | <0.05/<0.05 |
| Male/female n | 9 and 1 | 10 | 7 | |
| AHI/h | 2.7±3.1 | 40.4±18.9 | 4.7±6.0 | <0.05/<0.05 |
| HOMA-Index | 2.73±3.37 | 19.15±29.83 | 11.62±16.36 | <0.05/ns |
| Body fat % | 26.61±7.53 | 33.72±3.77 | 33.33±3.04 | <0.05/ns |
Characteristics of the 10 patients before therapy;
characteristics of the 7 patients 3 months under therapy.
HOMA-index between patients before and after therapy showed no significant differences (22.35±42.64 vs. 11.62±16,36, p>0.05). In contrast the HOMA-index comparing patients before therapy and healthy controls differ in a significant manner (19.15±29,83 vs. 2.73±3.37, p<0.05).
Other parameters such as pCO2, pO2, pH and lung function parameters (FEV1 and VC) were also analysed. None of them could line out significancies between patients before or under therapy and volunteers, except pO2. The healthy volunteers showed a significant higher mean pO2 in comparison to the patients with newly diagnosed OSAS (82.1±8.01 vs. 72.5±11.3, p<0.05).
Furthermore we examined any possible correlation of the parameters (pO2, pCO2, VC, FEV1, pH, age, height, weight, HOMA-Index, body fat, therapeutical pressure of CPAP, min. O2, basal O2, glucose, insulin, BMI, AHI and ESS points) to the peptide hormones (apelin, leptin, obestatin):
Leptin plasma levels of the patient group before therapy had at each time of the observation a significant correlation to the body mass index (pmin. 0.55; pmax. 0.69), min. O2 (pmin. –0.76; pmax. −0.58) and AHI (pmin. 0.55; pmax. 0.62). A significant correlation was found between Leptin measurements of newly diagnosed OSAS patients and weight at 2 a.m., 2, 6, 10 p.m. as well as the body fat percentage at 6, 10 a.m., 2 p.m.. In the therapy group positive significant correlations could be observed between leptin plasma levels and pH (at 2, 6, 10 a.m., 2, 6, 10 p.m.), BMC in g (at 2, 6, 10 a.m., 2, 6, 10 p.m.), body fat in% (at 6 a.m., 2 p.m.), FEV1 and VC (at 2, 6, 10 a.m., 2, 10 p.m.) and age (6, 10 a.m., 2, 10 p.m.).
Between Apelin plasma levels and the mentioned parameters we could not find any significancies in the newly diagnosed OSAS group. In contrast the plasma apelin levels showed a significant correlation to the AHI (pmin. 0.82; pmax. 0.91), min. O2 (pmin. −0.75; pmax. −0.86) and BMI (pmin. 0.78; pmax. 0.85) in the therapy group at all observation points. Furthermore there was a significant correlation to the basal O2 (at 2, 6 p.m.), pressure and pO2 (at 2, 6, 10 a.m., 6, 10 p.m.).
Obestatin plasma levels were significantly correlated at time of OSAS diagnosis to AHI and weight (at 6, 10 p.m.), to body fat in% (at 2, 6 a.m., 2 p.m.), BMC in g (at 10 a.m., 2 p.m.) and pCO2 (at 6 a.m., 2 p.m.). After three months of therapy there was only a significant correlation to weight and height (at 10 a.m.).
Further we analysed the plasma levels of the three adipose tissue hormones in the three different observation groups:
Leptin
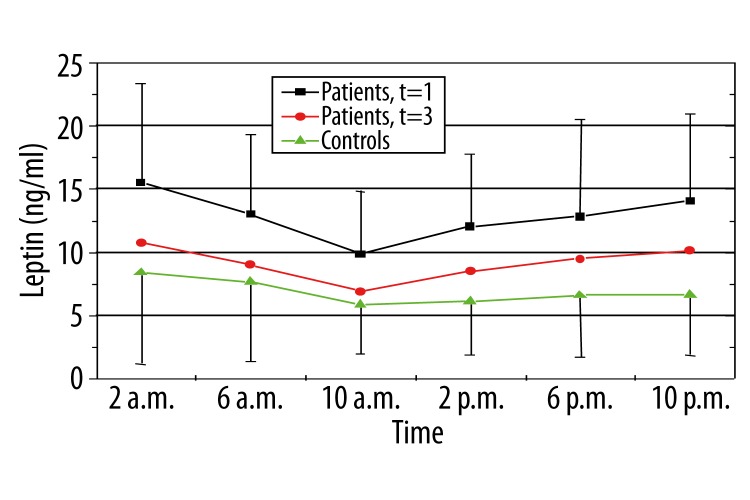
Controls had significant lower plasma leptin levels as the patients at time of diagnosis of OSAS at all observation points. Leptin levels decrease after 3 therapeutic months, in a significant manner at 6.00 and 10.00 a.m. (Figure 1).
Figure 1.
Levels of plasma leptin in controls and patients before and under therapy (green: controls, black: patients at time of diagnosis of OSAS, red: patients three months after nCPAP therapy).
Apelin
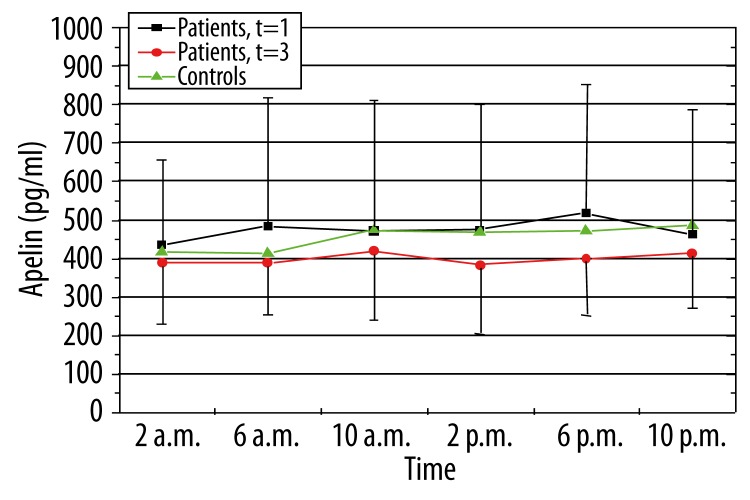
The mean plasma apelin levels of patients with newly diagnosed OSAS are higher than those of the patients under therapy, but failed to reach significance (Figure 2).
Figure 2.
Levels of plasma apelin in controls and patients before and under therapy (green: controls, black: patients at time of diagnosis of OSAS, red: patients three months after nCPAP therapy).
Obestatin
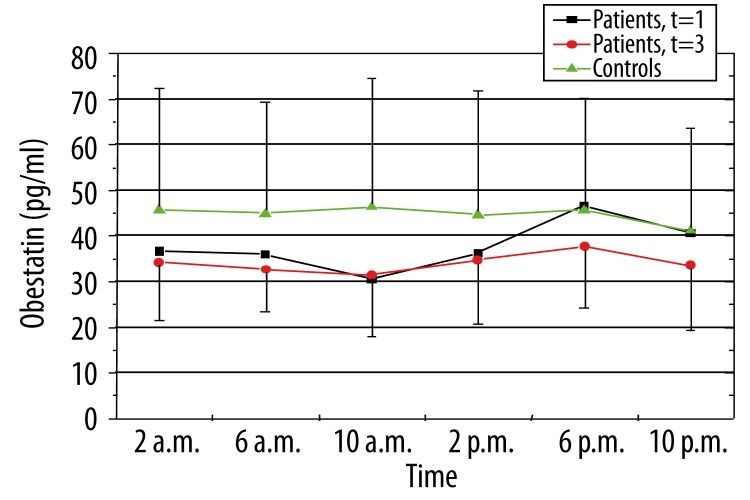
Plasma obestatin levels of the volunteers were higher in comparison to the patients before or after three months of therapy except at 6 and 10 p.m. (Figure 3).
Figure 3.
Levels of plasma obestatin in controls and patients before and under therapy (green: controls, black: patients at time of diagnosis of OSAS, red: patients three months after nCPAP therapy).
We also analysed correlations between the three adipose tissue hormones. We found a significant correlation between leptin and obestatin in the patient group before and after onset of CPAP therapy (except at 2 a.m. in the CPAP group).
Discussion
The obstructive sleep apnoea syndrome is a common disorder in the adult population, especially in men. It is characterised by repetitive episodes of hypoxaemia caused by an obstruction of the upper airways. In consequence the patients suffer from sleep disturbance at night due to “arousals” [22]. Furthermore other diseases such as diabetes and hypertension are associated with OSAS [14,23]. Treatment with positive continous airway pressure (CPAP therapy) can eliminate the obstructions and therefore the states of hypoxaemia [24]. Sleep quality and daytime vigilance restores and the risk of suffering from associated other diseases like hypertension, insulin resistance or cardiovascular complications, etc. is reduced. Unfortunately, besides these positive effects CPAP does not always impact on the weight of the patients, as demonstrated in our study group.
With the recent discovery of novel peptides like apelin and obestatin [25], data from animal models suggested, that some of these hormones play a role in OSAS independently of obesity. This has also been hypothesized in the case of leptin. Leptin regulates body fat mass by decreasing food intake and increasing resting energy expenditure [26]. Its production declines during starvation [27]. It is established, that leptin levels are elevated in an obese population as well as in patients with OSAS. Leptin levels decrease under CPAP therapy independently from the obesity of the patients, which can be confirmed in our study. In previous studies, leptin was already discussed as a respiratory stimulus, which has to date only been confirmed in animal models [14].
Apelin is a hormone with cardiovascular properties and impact on glucose homoeostasis [28,29]. Apelin is secreted and expressed by human adipocytes and up-regulated by insulin and obesity [30]. The expression is strongly inhibited by fasting [31] and recovered after refeeding, in a similar way to insulin [30]. Several studies have demonstrated that Apelin is a potent angiogenic factor. And like other angiogenic factors the apelin gene is upregulated under hypoxia conditions [32]. In addition, inhibition of apelin signaling during frog embryonic development resulted in severe reduction in the formation of vascular structures [33]. A most recent study on the pathophysiologic role played by apelin showed that intraperitoneal administration in normal and obese mice for 14 days reduced body fat without affecting food intake, reduced insulin, leptin and triglycerides level and respiratory quotient [29]. It is mentioned that Apelin might have different effects, depending on whether it is acting in brain or peripherally [32]. As OSAS is strongly correlated with obesity, hyperinsulinaemia and hypoxic states we analysed this hormone before and after onset of CPAP therapy. Our measurements during a 24h period in patients with newly diagnosed OSAS could show higher apelin plasma levels than in the same observation group three months after sufficient nCPAP therapy. Apelin levels had a trend to decrease in CPAP treated subjects as previously demonstrated in leptin, too. This could be related to changes in the body fat distribution, that we could not evaluate in our study. However, the weight of our patients remained unchanged. Thus, respiratory effects of apelin (e.g. maintaining respiratory drive in the chronically hypoxic state of OSAS) can be discussed and warrant further studies. Elevated apelin levels in patients with OSAS have also recently been reported by Henley et al. after glucose challenge. They did also not find a salient variability of apelin levels during a 24h period. They reported lower apelin levels after CPAP therapy overnight, a trend that we can confirm in our patient group. As in previous observations [31] apelin plasma levels in our study group (therapied OSAS patients) were positively correlated to the body mass index. Like Henley et al. we found no correlation between plasma apelin and BMI in untreated OSAS patients [25].
Obestatin, as well as Ghrelin, is derived from a 117-residue prepro-peptide by posttranslational cleavage (Prepro Ghrelin) [11]. From animal models, Obestatin was first discussed as an antagonist of ghrelin due to suppression of food intake, inhibition of insulin secretion and suppression of gastric emptying [34]. Data about effects on sleep behaviour are rare. A significant increase in non REM sleep was reported after intraperitoneal or intracerebroventricular injection in rats [13]. To our knowledge, data about humans with OSAS are not available. With our approach, we could not demonstrate differences in the obestatin levels of our obese patient group with OSAS before and 3 months after onset of CPAP therapy. Apart from the conclusion, that there seem to be no significant direct effects of obestatin on respiratory function, these data fit well with the study of Anderwald-Stadler et al. [35], who observed almost no effects of insulin on obestatin during a clamp study in insulin-resistant humans. Patients with OSAS are typically insulin-resistant [36–38]. The small amount of an improvement of insulin sensitivity by CPAP in OSAS subjects demonstrated by our study group [36] does obviously not impact on obestatin levels.
Conclusions
Previous studies raised suspicion that regulatory peptides such as leptin, apelin and obestatin might play a role in the pathogenesis of OSAS. In our study we could demonstrate different plasma levels of leptin and apelin regarding OSAS patients before and after onset of CPAP therapy in comparison to healthy volunteers, but not in obestatin. Further investigations and different approaches will have to investigate, if these hormones might have direct effects on OSAS and through which signaling pathways they might exhibit their effects, or if these hormonal changes are rather associated to concomitant metabolical changes in treated OSAS patients such as a modulation of insulin sensitivity or changes in body fat distribution.
Footnotes
Source of support: Self financing
References
- 1.http://www.who.int/dietphysicalactivity/publications/facts/obesity/en/
- 2.Chin K, Shimizu K, Nakamura T, et al. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation. 1999;100(7):706–12. doi: 10.1161/01.cir.100.7.706. [DOI] [PubMed] [Google Scholar]
- 3.Harsch IA, Konturek PC, Koebnick C, et al. Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment. Eur Respir J. 2003;22(2):251–57. doi: 10.1183/09031936.03.00010103. [DOI] [PubMed] [Google Scholar]
- 4.Tatemoto K, Hosoya M, Habata Y, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251(2):471–76. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 5.Lee DK, Cheng R, Nguyen T, et al. Characterization of apelin, the ligand for the APJ receptor. J Neurochem. 2000;74(1):34–41. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- 6.Cox CM, DÁgostino S, Miller MK, et al. Apelin, the ligand for the endothelial G-protein-coupled receptor, APJ, is a potent angiogenic factor required for normal vascular development of the frog embryo. Dev Biol. 2006;296(1):177–89. doi: 10.1016/j.ydbio.2006.04.452. [DOI] [PubMed] [Google Scholar]
- 7.O’Carroll AM, Selby T, Palkovits M, Lolait SJ. Distribution of mRNA encoding B78/apj, the rat homologue of the human APJ receptor, and its endogenous ligand apelin in brain and peripheral tissues. Biochim Biophys Acta. 2000;1492(1):72–80. doi: 10.1016/s0167-4781(00)00072-5. [DOI] [PubMed] [Google Scholar]
- 8.Eyries M, Siegfried G, Ciumas M, et al. Hypoxia-induced apelin expression regulates endothelial cell proliferation and regenerative angiogenesis. Circ Res. 2008;103(4):432–40. doi: 10.1161/CIRCRESAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- 9.Harsch IA, Hahn EG, Konturek PC. Insulin resistance and other metabolic aspects of the obstructive sleep apnea syndrome. Med Sci Monit. 2005;11(3):RA70–75. [PubMed] [Google Scholar]
- 10.Glassford AJ, Yue P, Sheikh AY, et al. HIF-1 regulates hypoxia- and insulin-induced expression of apelin in adipocytes. Am J Physiol Endocrinol Metab. 2007;293(6):E1590–96. doi: 10.1152/ajpendo.00490.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang SQ, Jiang Q, Zhang YL, et al. Obestatin: its physicochemical characteristics and physiological functions. Peptides. 2008;29(4):639–45. doi: 10.1016/j.peptides.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Bodosi B, Gardi J, Hajdu I, et al. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2004;287(5):R1071–79. doi: 10.1152/ajpregu.00294.2004. [DOI] [PubMed] [Google Scholar]
- 13.Szentirmai E, Krueger J. Obestatin alters sleep in rats. Neurosci Lett. 2006;404(1–2):222–26. doi: 10.1016/j.neulet.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 14.Ip MS, Lam K, Ho C, et al. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest. 2000;118(3):580–86. doi: 10.1378/chest.118.3.580. [DOI] [PubMed] [Google Scholar]
- 15.Boden G, Chen X, Kolaczynski JW, Polansky M. Effects of prolonged hyperinsulinemia on serum leptin in normal human subjects. J Clin Invest. 1997;100(5):1107–13. doi: 10.1172/JCI119621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vgontzas AN, Papanicolaou D, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85(3):1151–58. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 17.American Thoracic Society. Indications and standards for use of nasal continuous positive airway pressure (CPAP) in sleep apnea syndromes. American Thoracic Society. Official statement adopted March 1944. Am J Respir Crit Care Med. 1994;150(6 Pt 1):1738–45. doi: 10.1164/ajrccm.150.6.7952642. [DOI] [PubMed] [Google Scholar]
- 18.Penzel T, Hajak G, Hoffmann RM. Empfehlungen zur Dürchführung und Auswertung polygraphischer Ableitungen im diagnostischen Schlaflabor. EEG-EMG. 1993;24:65–70. [in German] [Google Scholar]
- 19.Ficker JH, Wiest G, Lehnert G, et al. Evaluation of an auto-CPAP device for treatment of obstructive sleep apnoea. Thorax. 1998;53(8):643–48. doi: 10.1136/thx.53.8.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rechtschaffen A, Kahles A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Public Health Service, US Government Printing Office; 1968. [Google Scholar]
- 21.Bonnet M, Charley D, Carskadon MA. EEG arousals: Scoring rules and examples. Sleep. 1992;15:174–84. [PubMed] [Google Scholar]
- 22.Hernandez C, Abreu J, Abreu P, et al. Nocturnal melatonin plasma levels in patients with OSAS: the effect of CPAP. Eur Respir J. 2007;30:496–500. doi: 10.1183/09031936.00051906. [DOI] [PubMed] [Google Scholar]
- 23.Tokuda F, Sando Y, Matsui H, et al. Serum Levels of Adipocytokines, Adiponectin and Leptin, in Patients with Obstructive Sleep Apnea Syndrome. Inter Med. 2008;47:1843–49. doi: 10.2169/internalmedicine.47.1035. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro GK, Shapiro GM. Factors that influence CPAP adherence: an overview. Sleep Breath. 2010;14(4):323–35. doi: 10.1007/s11325-010-0391-y. [DOI] [PubMed] [Google Scholar]
- 25.Henley DE, Buchanan F, Gibson R, et al. Plasma apelin levels in obstructive sleep apnea and the effect of continuous positive airway pressure therapy. J Endocrinol. 2009;203:181–88. doi: 10.1677/JOE-09-0245. [DOI] [PubMed] [Google Scholar]
- 26.Ciftci TU, Kokturk O, Bukan N, Bilgihan A. Leptin and Ghrelin Levels in Patients with Obstructive Sleep Apnea Syndrome. Respiration. 2005;72:395–401. doi: 10.1159/000086254. [DOI] [PubMed] [Google Scholar]
- 27.Aleman MR, Santolaria F, Batista N, et al. Leptin role in advanced lung cancer. A mediator of the acute phase response or marker of the status of nutrition? Cytokine. 2002;19:21–26. doi: 10.1006/cyto.2002.1051. [DOI] [PubMed] [Google Scholar]
- 28.Beltowski J. Apelin and visfatin: Unique „beneficial“ adipokines upregulated in obesity? Med Sci Monit. 2006;12(6):RA112–19. [PubMed] [Google Scholar]
- 29.Ladeiras-Lopes R, Ferreira-Martins J, Leite-Moreira A. The Apelinergic System: The Role Played in Human Physiology and Pathology and Potential Therapeutic Applications. Arq Bras Cardiol. 2008;90(5):343–49. doi: 10.1590/s0066-782x2008000500012. [DOI] [PubMed] [Google Scholar]
- 30.Boucher J, Masri B, Daviaud D, et al. Apelin, a Newly Identified Adipokine Up-Regulated by Insulin and Obesity. Endocrinology. 2005;146:1764–71. doi: 10.1210/en.2004-1427. [DOI] [PubMed] [Google Scholar]
- 31.Castan-Laurell I, Vitkova M, Daviaud D, et al. Effect of hypocaloric diet-induced weight loss in obese women on plasma apelin and adipose tissue expression of apelin and APJ. European Journal of Endocrinology. 2008;158:905–10. doi: 10.1530/EJE-08-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rayalam S, Della-Fera MA, Krieg PA, et al. A putative role for apelin in the etiology of obesity. Biochem Biophys Res Commun. 2008;368:815–19. doi: 10.1016/j.bbrc.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Cox CM, D’Agostino SL, Miller MK, et al. Apelin, the ligand for the endothelial G-protein-coupled receptor, APJ, is a potent angiogenic factor required for normal vascular developement of the frog embryo. Dev Biol. 2006;296:177–89. doi: 10.1016/j.ydbio.2006.04.452. [DOI] [PubMed] [Google Scholar]
- 34.Reimer MK, Pacini G, Ahrén B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology. 2003;144(3):916–21. doi: 10.1210/en.2002-220819. [DOI] [PubMed] [Google Scholar]
- 35.Anderwald-Stadler M, Krebs M, Promintzer M, et al. Plasma obestatin is lower at fasting and not suppressed by insulin in insulin-resistant humans. Am J Physiol Endocrinol Metab. 2007;293(5):E1393–98. doi: 10.1152/ajpendo.00330.2007. [DOI] [PubMed] [Google Scholar]
- 36.Harsch IA, Pour Schahin S, Radespiel-Tröger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156–62. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 37.Ip MS, Lam B, Ng MMT, et al. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670–76. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 38.Harsch IA, Koebnick C, Wallaschofski H, et al. Resistin levels in patients with obstructive sleep apnoea syndrome – the link to subclinical inflammation? Med Sci Monit. 2004;10(9):CR510–15. [PubMed] [Google Scholar]