Summary
Background
This study sought to assess the vestibulocochlear organ in patients meeting radiologic criteria of vascular compression syndrome (VCS) of the eighth cranial nerve.
Material/Methods
The authors performed a retrospective analysis of 34 patients (18 women, 16 men; mean age, 49 years) treated in between 2000 and 2007, with VCS of the eighth cranial nerve by MRI. Contrasted magnetic resonance imaging identified an anterior inferior cerebellar artery vascular loop adhering to the vestibule-cochlear nerve in all 34 cases. All patients were given pure tone audiometry, distortion product otoacoustic emissions, auditory brainstem response, and electroneurographic examinations.
Results
Most-common symptoms were unilateral hearing loss (82%), unilateral tinnitus (80%), and dizziness (74%). Most-frequent abnormalities in performed examinations were specific auditory brainstem response changes (interpreted according to Möller’s criteria) in 86% of cases and sensorineural hearing loss in pure tone audiometry (82%). Abnormal changes in electronystagmography were found in the absence (12%) or weakness (35%) of a caloric response. No patients were surgically treated.
Conclusions
Significantly, there is no more weakness or absence of the caloric response of a vestibular organ in a patient with vascular compression of the vestibulocochlear nerve. Despite an absence of electrophysiologic testing of vestibular organ dysfunction, most examined patients (meeting the radiologic criteria of VCS of the eighth cranial nerve) had subjective symptoms like vertigo and dizziness. Disabling positional vertigo should be considered in the differential diagnosis of vertigo when accompanied by tinnitus or deafness.
Keywords: vertigo, hearing loss, tinnitus, nerve compression syndromes
Background
The term vascular compression syndrome was introduced to professional literature by McKenzie in 1936 [1] and popularized by Jannetta in 1975, to refer to a group of diseases caused by direct contact of a blood vessel with a cranial nerve stem [2]. Most authors believe that the anterior inferior cerebellar artery is the vessel responsible for the vascular compression syndrome of the eighth cranial nerve [3,4].
Numerous theories explain the pathogenesis of the vascular compression syndrome in the vestibulocochlear nerve and facial nerve. Sabarbati and associates state that the place of nerve lesion is situated strictly in the transition zone of the cranial nerve, where the myelin of the central nervous system of the neurolemma turns into peripheral myelin [3]. This transition zone is also labeled the Obersteiner-Redlich zone [4].
The blood vessel adhering to the nerve axons in the transition zone may initially cause, as Rasminsky claims, a topical demyelinization of the neurolemma, followed by nerve lesion. In may lead to ectopic nerve stimulations for dromic as well as antidromic conduction [5]. Consequences of that are disturbances in the vestibular and cochlear nuclei appearing as hearing loss, tinnitus, and vertigo.
Jannetta in 1984 introduced a new term, disabling positional vertigo [6]. Disabling positional vertigo refers to a group of patients with symptoms of the vascular compression of eighth nerve, and it is used to describe a cochleovestibular organ impairment in these cases. This syndrome was distinct from other established vertigo syndromes based on clinical and electrophysiologic criteria. Existence of disabling positional vertigo syndrome is still not universally accepted, and no specific findings have been credible enough to detect this syndrome [7,8]. Only Möller proposed specific auditory brainstem response abnormalities as criteria in disabling positional vertigo diagnosis [9]. According to some researchers, the diagnosis of disabling positional vertigo could be based on a combination of clinical symptoms and electrophysiologic findings. Disabling positional vertigo comprises constant positional vertigo or dysequilibrium, constant nausea, sensation of the floor constantly moving “as if one was on a boat,” vertigo with any head movement (positional vertigo), and commonly associated symptoms include hearing loss and tinnitus [6,9–11]. This study sought to assess the vestibulocochlear organ in patients meeting radiologic criteria of the vascular compression syndrome of eighth cranial nerve.
Material and Methods
Material consisted of 34 patients (18 women, 16 men; mean age, 49 years; age range, 36–74 years), with from vascular compression syndrome of eighth cranial nerve recognized by means of angio-MRI. Contrasted magnetic resonance imaging identified a vascular loop of the anterior inferior cerebellar artery near to cochleovestibular nerve in all 34 cases. After taking a history and doing an otolaryngologic examination, all 34 patients underwent pure tone audiometry, impendence audiometry, distortion product otoacoustic emissions, auditory brainstem response, electroneurographic, radiographs of temporal bones in Stenvers projection (evaluation of eighth nerve tumors), as well as neurologic and ophthalmologic consultations. These were followed by an MRI targeting the areas of the cerebellopontine angle in sequences SE/T1, T2, an PD in transverse planes and frontal planes (where SE and PD means technical parameters of the MRI examination).
Results
The most-common symptoms were unilateral tinnitus in 27 patients (79% cases), unilateral hearing loss in 28 patients (82%), and dizziness in 25 patients (74%). The symptoms lasted for 2 to 20 years before the diagnosis (mean, 8 years). As regarding the otorhinolaryngology organs, no pathogenic lesions have been noted. Pure tone audiometry revealed sensorineural high-frequency deafness (over 80 dB) in 28 patients (82%). Impedance audiometry measurements (middle ear examination) showed tympanogram A type in 32 patients (94%) and C type in 2 patients (6%). Auditory brainstem response examination revealed retrocochlear impairment in 29 patients (86%) (meeting Möller’s criteria of disabling positional vertigo-prolongation of wave I–III), co-chlear impairment in 3 patients (9%), and conductive impairment in 2 patients (6%). Electroneurographic examination revealed spontaneous and positional nystagmus present in 6 patients (18%) and it was absent in 28 (82%); optokinetic nystagmus was normal in 14 (41%) and disturbed in 20 patients (59%). Bicaloric testing revealed a normal response in 17 patients (50%), caloric weakness in 14 patients (41%), and absence of a caloric response in 3 cases (9%). Evoked otoacoustic emission (distortion product otoacoustic emissions, DP-gram 1–4 kHz frequencies in patients with ipsilateral, retrocochlear impairment in auditory brainstem response showed distorted product otoacoustic emissions in 9 patients (26%). Radiographs of ears, according to Stenvers revealed no changes. In all cases, MR imagining showed that the anterior inferior cerebellar artery was adjacent to the vestibulocochlear nerve. All patients were referred for neurosurgical consultations, yet so far, none has given consent for surgery (Figures 1, 2).
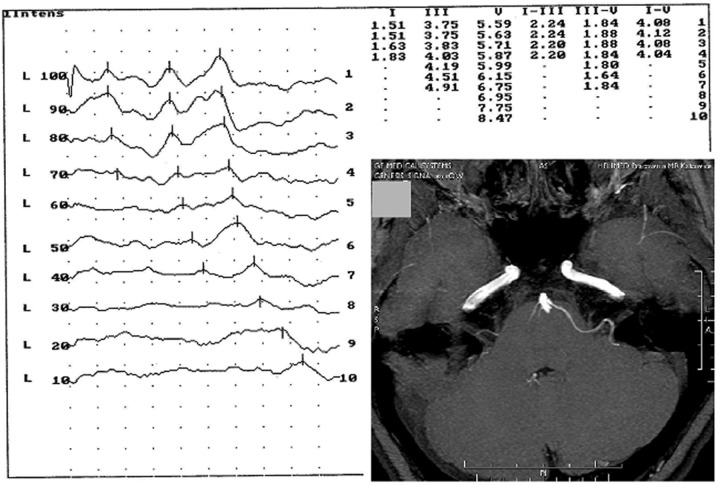
Figure 1.
Case 1: T1-weighted MRI after gadolinium administration scan showing the nerve adjacent to the eighth nerve by the anterior inferior cerebellar artery and auditory brainstem response record showing the retrocochlear hearing loss in the same patient.
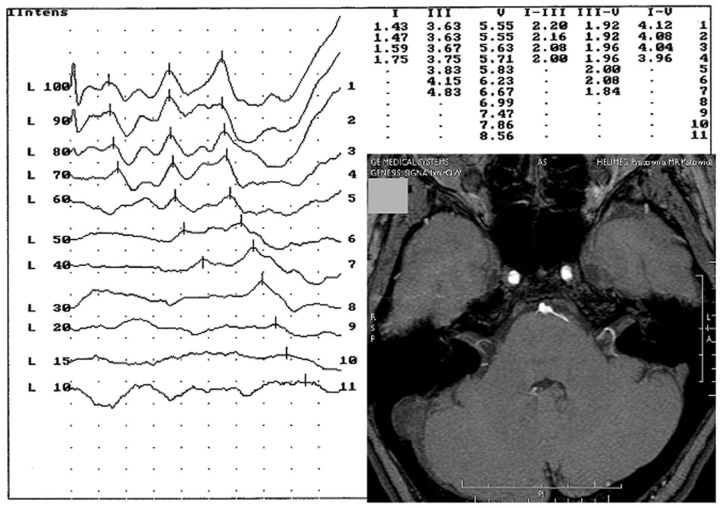
Figure 2.
Case 2: Angio MRI scan showing the nerve adjacent to the eighth nerve by the anterior inferior cerebellar artery and auditory brainstem response record showing the retrocochlear hearing loss in the same patient.
Discussion
In 2000–2007, among all the patients of our department with sensorineural hearing loss, tinnitus, and dizziness, we found 34 meeting the radiologic criteria of vascular compression syndrome of the eighth cranial nerve. In 25 cases, typical subjective symptoms accompanying disabling positional vertigo could be recognized. Vascular compression syndrome, as claimed by Schwaber, is found in both men and women between the ages of 20 and 70 years (2:1 in incidence in women and men) [12]. Other authors report the following spectrum of symptoms of disabling positional vertigo: Schwaber mentions 77% of patients with unilateral hypoacusia and 57% with tinnitus, 84% of patients with periodic rotatory vertigo, and in 6.9% of patients with vertigo, nausea, and vomiting [12]. Makins found hypoacusia in 85% of the cases and tinnitus in 41% [1]. Electroneurographic examinations revealed changes in nearly 90% of patients; they comprise, according to Möller and Ryu, the incidence of spontaneous nystagmus and positional nystagmus, as well as a reduction of, or lack of, labyrinthian excitability in caloric tests [13,14]. In 10% of cases, Schwaber revealed hypersensitivity of the labyrinth in caloric tests, which was not confirmed in our studies [12].
Auditory brainstem response examination is an important element in the diagnostics of vascular compression syndrome, as it allows us to locate the site of nerve lesion, thus being indicative for MRI (Magnetic Resonance Imaging). All authors confirm the invaluable character of imaging, in particular MRI, and MRI angiography in diagnosing vascular compression syndrome of the eighth nerve and qualifying its cases for microsurgery. The presence of an adjacent loop of a vessel (usually the anterior inferior cerebellar artery) touching the vestibulocochlear nerve, coupled with manifestations characteristic for vascular compression syndrome can be found in 25% (according to Makins) or 35% of cases (according to Schwaber) [1,12]. Much controversy in diagnosing vascular compression syndrome arises from frequent lack of congruence between MRI (showing the adjacency of vessel and nerve) and lack of otoneurologic manifestations. There are many theories explaining that phenomenon. The most credible one comes from Sabarbati according to whom, nerve lesions may occur only when the vessel is adjacent to the transition zone of the nerve [3].
Schwaber and Hall selected a group of 63 patients with diagnosed vascular compression syndrome of the eighth. Hearing loss was recognized in 51 patients (81%), 33 of whom had high-frequency loss, and 14 patients had mid-frequency loss. In auditory brainstem response examination, neuritic hearing loss was diagnosed in 75% patients, whereas a decrease in cochlear excitability was observed in 93% of patients [12].
Noguchi and Ohgaki suggest that vessel compression of the eighth nerve as a cause of vertigo is still a debatable issue [15]. They examined 5 patients with VSC (Vascular Compression Syndrome eighth, diagnosed with an angio-MRI scan, who also complained of vertigo. Audiometric tests showed normal results in 2 cases, bilateral midfrequency hypoacusia in 1 case, and fluctuating hypoacusia as in Meniere disease in 2 cases. Prolonged wave I–III interval in an auditory brainstem response examination, suggested by Möller as a criterion for diagnosing eighth nerve damage caused by vessel compression, was observed only in 1 case. Lack of cochlear excitability occurred in 2 cases, whereas spontaneous nystagmus, and optokinetic nystagmus were absent in all cases. The authors conclude that they found no specific symptoms of cochleovestibular apparatus that might be caused by eighth nerve compression suggested by an MRI examination. Adamczyk said, that contact between a trigeminal nerve root and an artery in the prepontine cistern is a frequently seen anatomical variant. Therefore, detection of such a variant is not equivalent to finding the cause of a patient’s complaints [16].
Ryu and Yamamoto examined 10 patients with vascular compression syndrome of the eighth nerve. Only 2 patients showed abnormal auditory brainstem response pattern, and electroneurographic examination revealed a slight decrease in cochlear excitability in 3 cases. They also mention “still continuing skepticism about the existence of vascular compression syndrome of the eighth nerve” [13,14]. All the results obtained may be treated as an unquestionable evidence that the examination with the use of MRI should be the basic investigation for the visualisation of neurovascular suggested in the cases of nerves V, VII, VIII, IX, X, XI compression. [17–19].
Conclusions
We conclude the following:
Significantly, there is no more weakness or absence of a caloric response of the vestibular organ in a patient with vascular compression syndrome of vestibulocochlear nerve.
Despite of absence of electrophysiologic signs of vestibular organ dysfunction, most of the examined patients (meeting the radiologic criteria of vascular compression syndrome of eighth cranial nerve) had subjective symptoms, such as vertigo and dizziness. However, 86% of patients had abnormal auditory brainstem responses meeting Möller’s criteria.
Disabling positional vertigo is the syndrome that should be considered in the differential diagnosis in cases of vertigo, especially when accompanied by tinnitus or deafness.
Footnotes
Source of support: Departmental sources
References
- 1.Makins A, Nikolopoulos T, Ludman C, O’Donoghue G. Is there a correlation between vascular loops and unilateral auditory symptoms ? Laryngoscope. 1998;108( 11):1739–42. doi: 10.1097/00005537-199811000-00027. [DOI] [PubMed] [Google Scholar]
- 2.Jannetta PJ. Neurovascular cross – compression in patients with hyper-active dysfunction symptoms of the eight cranial nerve. Surg Forum. 1975;26:467–68. [PubMed] [Google Scholar]
- 3.Sabarbati A, Carner M, Colletti V, Osculati F. Myelin – containing corpora amylacea in vestibular root entry zone. Ultrastruct – Path. 1995;20(5):437. doi: 10.3109/01913129609016346. [DOI] [PubMed] [Google Scholar]
- 4.Schwaber MK. Neurootology. Mosby; 1993. Vascular Compression Syndromes; pp. 880–85. [Google Scholar]
- 5.Rasminsky M. Peripherial Neuropathy. WB Saunders; 1984. Ectopic impulse generation in pathologic nerve fibres. [Google Scholar]
- 6.Jannetta PJ, Moller MB, Moller AR. Disabling positional vertigo. N Engl J Med. 1984;310:1700–5. doi: 10.1056/NEJM198406283102604. [DOI] [PubMed] [Google Scholar]
- 7.Bergsneider M, Becker D. Vascular compression syndrome of the vestibular nerve: a critical analysis. Otolaryngol Head Neck Surg. 1995;112:118–24. doi: 10.1016/S0194-59989570310-1. [DOI] [PubMed] [Google Scholar]
- 8.Jannetta PJ, Moller MB, Moller AR, Sekhar LN. Neurosurgical treatment of vertigo by microvascular decompression of the eight cranial nerve. Clin Neurosurg. 1996;33:645–65. [PubMed] [Google Scholar]
- 9.Möller MB, Möller AR. Vascular compression of the eight cranial nerve: clinical correlations and surgical findings. Neurol Clin. 1990;8:421–39. [PubMed] [Google Scholar]
- 10.Möller MB, Moller AR, Jannetta PJ. Diagnosis and surgical treatment of disabling positional vertigo. J Neurosurg. 1996;64:21–28. doi: 10.3171/jns.1986.64.1.0021. [DOI] [PubMed] [Google Scholar]
- 11.Möller MB. Results of microvascular decompression (MVD) of the eight nerve as treatment for disabling positional vertigo (DPV) Ann Otol Rhinol Laryngol. 1990;99(9):724–29. doi: 10.1177/000348949009900911. [DOI] [PubMed] [Google Scholar]
- 12.Schwaber MK, Hall JW. Cochleovestibular nerve compression syndrome. I. Clinical features and audiovestibular findings. Laryngoscope. 1992;102(9):1020–29. doi: 10.1288/00005537-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Ryu H, Yamamoto S, Sugiyama K, et al. Neurovascular decompression of the eight cranial nerve in patients with hemifacial spasm and incidental tinnitus: an alternative way to study tinnnitus. J Neurosurg. 1998;88:232–36. doi: 10.3171/jns.1998.88.2.0232. [DOI] [PubMed] [Google Scholar]
- 14.Ryu H, Yamamoto S, Sugiama K, et al. Neurovascular compression syndrome of the eight cranial nerve. Can the site of compression explain the symptoms? Acta Neurochir. 1999;141:495–501. doi: 10.1007/s007010050330. [DOI] [PubMed] [Google Scholar]
- 15.Noguchi Y, Ohgaki T, Tsunoda A, et al. Clinical study in vertiginuous patients suspected of having neurovascular compression of the eight cranial nerve. Nippon Jibiinkoka Gakkai Kaiho. 1997;100(5):492–98. doi: 10.3950/jibiinkoka.100.492. [DOI] [PubMed] [Google Scholar]
- 16.Adamczyk M, Bulski T, Sowińska J, et al. Trigeminal nerve-artery contact in people without trigeminal neuralgia: MR study. Med Sci Monit. 2007;13(Suppl 1):38–43. [PubMed] [Google Scholar]
- 17.Hermann M, Słoniewski P, Zieliński P. An attempt at objective evaluation of neurovascular compression with the use of magnetic resonance imaging. Med Sci Monit. 1998;4(3):532–37. [Google Scholar]
- 18.Polensek S, Tusa R. Unnecessary diagnostic tests often obtained for benign paroxysmal positional vertigo. Med Sci Monit. 2009;15(7):MT89–94. [PubMed] [Google Scholar]
- 19.Manzari L, Modugno GC. Nystagmus induced by bone (mastoid) vibration in otosclerosis: A new perspective in the study of vestibular function in otosclerosis. Med Sci Monit. 2008;14(10):CR505–10. [PubMed] [Google Scholar]