Summary
Background
Root canal disinfection needs to be improved because actual techniques are not able to eliminate all microorganisms present in the root canal system. The aim of the present study was to investigate the in vitro cytotoxicity of FotoSan (CMS Dental APS, Copenhagen Denmark), 17% EDTA and 2% chlorhexidine.
Material/Methods
Fibroblasts of periodontal ligament from healthy patients were cultured. FotoSan (with and without light activation for 30 sec.), 17% EDTA and 2% chlorexidine were used for the cell viability tests. Untreated cells were used as control. The cellular vitality was evaluated by MTT test. The production of reactive oxygen species (ROS) was measured using an oxidation-sensitive fluorescent probe. Results were statistically analyzed by ANOVA, followed by a multiple comparison of means by Student-Newman-Keuls, and the statistical significance was set at p<0.05.
Results
MTT tests showed that cytotoxic effects of FotoSan (both photocured and uncured) were statistically lower (p<0.05) than that observed using 2% Chlorhexidine, while no significant differences were found in comparison with 17% EDTA. No alterations in ROS production were detectable in any of the tested materials.
Conclusions
Since the toxicity of the FotoSan photosensitizer, both light-activated and not light-activated, is similar to common endodontic irrigants, it can be clinically used with precautions of use similar to those usually recommended for the above-mentioned irrigating solutions.
Keywords: cytotoxicity, light-activated disinfection, root canal
Background
Endodontic failure may occur in cases of persistent bacteria in the root canal system as a consequence of poor disinfection and debridement of the endodontic space, untreated canals, inadequate filling or coronal leakage [1]. It is well established that the elimination of pathogens from root canals during endodontic treatment is difficult [2,3] and current endodontic techniques are unable to consistently disinfect the canal [4,5]. Mechanical instrumentation alone is not able to obtain a complete cleaning of the root canal system.6 To assist in the cleaning and debridement of the canal, a wide range of irrigating and disinfecting solutions have been used [7].
Recently, a novel method of disinfection for use in both caries and endodontics has become available. This is photo-activated disinfection (PAD). The principle on which it operates is that photo-sensitizer molecules attach to the membrane of the bacteria. Irradiation with light at a specific wavelength matched to the peak absorption of the photo-sensitizer leads to the production of singlet oxygen, which causes the bacterial cell wall to rupture, killing the bacteria [8]. Extensive laboratory studies have shown that an important aspect of this system is that the 2 components when used independently produce no effect on bacteria or on normal tissue. It is only the combination of photo-sensitizer and light that produces the effect on the bacteria [8–10]. Using the principles described above, a system has been developed for endodontic use consisting of a lamp (FotoSan, CMS Dental APS, Copenhagen, Denmark). This antibacterial treatment has been called light-activated disinfection (LAD). LAD, also called PACT (Photodynamic Antimicrobial Chemo Therapy), is a treatment based on the combination of a photo-sensitizer and a powerful red light. The photo-sensitizer attaches to the membranes of microorganisms and binds itself to their surface, absorbs energy from the light and then releases this energy to oxygen (O2), which is transformed into highly reactive oxygen species (ROS), such as oxygen ions and radicals. The ROS reacts strongly and destroys the microorganisms instantly and effectively. The results of a study by Bouillaguet et al. [11] support the use of blue- or red-light-absorbing photo-sensitizers as candidates to produce ROS for clinical applications. The photo-sensitizer is available in low, medium and high viscosities. All solutions have the same concentration of active ingredients. The LAD principle is not only effective against bacteria, but also against other micro-organisms including viruses, fungi and protozoa [12–14]. The applied photo-sensitizers have far less affinity to mammalian cells; thus, no negative side effects in the treatment have been reported by toxicological tests [15].
Clinically, after completion of canal preparation, the canal is inoculated with the photo-sensitizer solution, which is left in situ for a fixed period of time (60 seconds) to permit the solution to come into contact with the bacteria and diffuse through any biofilm structures. The emitter is then placed in the root canal and irradiation carried out for 30 seconds in each canal. This has been demonstrated in the laboratory to kill high concentrations of bacteria generally found in root canals [16].
Care must be taken to ensure maximum wetting, as it is important that the PAD solution contacts the bacteria, otherwise the photosensitization process will not occur [17]. The results of a previous study showed that the PAD technique was successful in eliminating all the cultivable bacteria when the photo-sensitizer reached the bacteria [17]. Furthermore, it highlighted the need for caution in the use of the emitter to ensure that it is not bent too tightly or trapped in the canal [17].
However, since the photo-sensitizer molecules in aqueous solution are injected by a syringe, they can be inadvertently forced beyond the apex and come into contact with the periapical tissues. In such cases the apically extruded solution would probably be inert, being not activated by the light (uncured), but this cannot be scientifically proven. Therefore, in the present study both the activated and the non-activated solution were evaluated. Since the effectiveness and the mode of delivery and removal are very similar to traditional endodontic irrigants, the biological properties of different irrigating solutions were tested and compared.
Therefore, the aim of the present study was to investigate the in vitro cytotoxicity of FotoSan (CMS Dental APS, Copenhagen Denmark) (photocured or uncured) and to compare this with 17% EDTA and 2% chlorhexidine.
Material and Methods
Unless otherwise specified, all chemicals and reagents used in this study (cell culture grade) were obtained from Sigma Chemical Co., Milan, Italy.
Isolation and cell culture
Fibroblasts of periodontal ligament were obtained from premolar teeth of patients undergoing tooth extractions for orthodontic reasons; the authorization for the use of the biological material was obtained from each patient.
The extracted premolars were rinsed twice with ‘explant’ medium (Dulbecco’s Modified Eagle Medium, DMEM) containing FCS (10%), gentamicin sulphate, fungizone (2.5 μg/ml), penicillin (100 units/ml), streptomycin (100 μg/ml) and non-essential amino acids. To avoid the contamination of the periodontal ligament cell cultures with gingival and apical tissues, the middle third of the periodontal ligament was gently curetted and removed from the root surface of the extracted tooth. Periodontal ligament tissues were rinsed in DMEM, cut into small pieces, enzymatically digested for 1 h at 37°C in a solution of type I collagenase (3 mg/ml) and dispase (4 mg/ml), and then dispersed in tissue-culture dishes to allow the pegging of explant cultures. The latter were subsequently cultured in DMEM containing FCS (10%), penicillin (50 units/ml), streptomycin (50 μg/ml) and non-essential amino acids. Cells were used between the fourth and the eighth in culture transfer.
Products to be tested
FotoSan (with and without light activation, and both high and medium viscosity), 17% EDTA (Vista Dental, Racine, WI, US) and 2% chlorhexidine (Vista Dental, Racine, WI, US).
In clinical practice FotoSan is used for light-activated disinfection in combination with a photosensitizer (FotoSan Agent) containing toluidine blue O as an active ingredient, used to catalyze the photochemical process. In this study we used the medium and high viscosity material preparations containing the same concentration of the active principle.
Cells treatment
In order to evaluate the cytotoxic effects of the analyzed products, fibroblasts (1×104) in DMEM (200 μL) were seeded into each well of a 96-well tissue culture plate (Costar, Cambridge, MA) and cultured to subconfluent monolayer for 24 hours. The medium was removed and the products were then added to monolayers (20 μL during 30 sec). When necessary, the FotoSan specimens were light-activated with the FotoSan lamp for 30 seconds; after the treatments the cells were washed 2 times with DMEM (200 μL), and the cellular vitality was evaluated by MTT test. Untreated cells were used as control.
Cytotoxicity evaluation
MTT test was performed according to Wataha et al. [18] MTT solution (20 μL) in PBS (phosphate buffer, 5 mg/mL) was added to the medium (200 μL) and, after incubation (4 h, 37°C), the intracellular formazan crystals produced were solubilized with a solution of HCl in isopropanol (4×10−2 N, 200 μL). The optical density (OD) of the solution contained in each well was determined using an automatic microplate photometer (Packard Spectracount™, Packard BioScience Company, Meriden, U.S.A.) at a wavelength of 570 nm.
Each experiment was performed 6 times and the cell cytotoxicity was calculated according to the following equation [19]:
Measurement of the reactive oxygen species
The production of reactive oxygen species (ROS) was measured using an oxidation-sensitive fluorescent probe 2′,7′-dichlorodihydrofluorescin diacetate (H2DCF-DA) [20,21]. The non-polar H2DCF-DA readily diffuses into the cells, where it is enzymatically deacetylated, by intracellular esterases, to a polar non-fluorescent derivate (probe) trapped inside. In the presence of ROS, the probe is oxidized to 2′,7′-dichlorodihydrofluorescin (DCF); fluorescence levels depend on the intracellular ROS concentration [22]. Human normal fibroblasts (2×105) were seeded into each well of a 6-well plate, pre-incubated with DCFH-DA (2.5 μl, 10 mm) for 30 min at 37°C, and incubated for 24 h at 37°C. The cells were subsequently exposed to FotoSan, EDTA or chlorhexidine for 30 seconds. DCF fluorescence was measured using a Glomax Multi detection system fluorimeter (Promega, Milan, Italy) (490 nm excitation and 526 nm emission wavelengths). Results of DCF fluorescence intensity were expressed as arbitrary units (a.u.).
Statistical analysis
Each value represents the mean of 4 experiments, each repeated 6 times. All results are expressed as mean ± standard error of the mean (SEM). The group means were compared by analysis of variance (ANOVA), followed by a multiple comparison of means by Student-Newman-Keuls; if necessary, comparison of means by t test was used. p<0.05 was considered significant.
Results
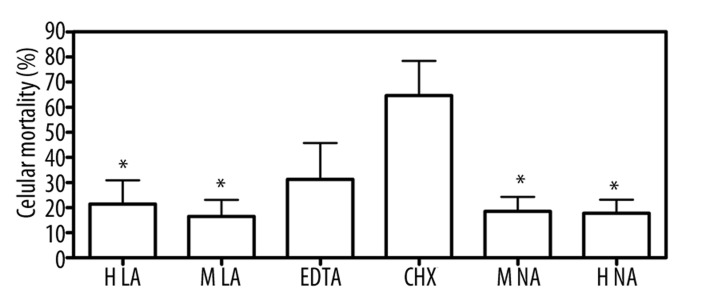
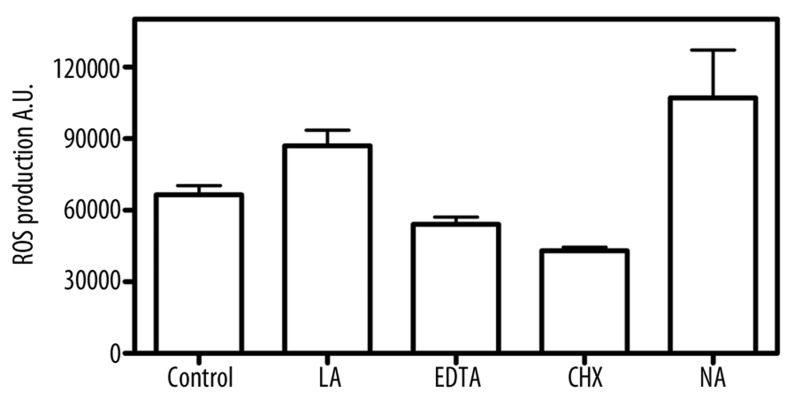
MTT tests showed that cytotoxic effects of FotoSan (both photocured and uncured) were statistically lower (p<0.05) than that observed using chlorhexidine, while no significant differences were found in comparison with EDTA (Figure 1). No statistically significant alterations in ROS production were detectable in any of the tested materials (Figure 2).
Figure 1.
Cytotoxic effect of materials on fibroblasts: fibroblasts were seeded in a 96-well tissue culture plate and cultured to subconfluent monolayer for 24 hours. The medium was then removed and the products were added to monolayers. After the cellular vitality was evaluated by MTT test. Untreated cells were used as control. Each value represents the mean ±SEM for 4 independent experiments. * (p<0.05) indicates significant statistical differences between FotoSan treated cells and Chlorexidine treated cells. (H LA: High light-activated; H NA: high non activated; M LA: medium light-activated; M NA: medium non activated; CHX: Chlorexidine).
Figure 2.
Induction of ROS levels in fibroblasts exposed to materials. ROS levels were determined by the DCFH-DA as described in materials and methods. (LA: light-activated; NA: non activated; CHX: Chlorexidine).
Discussion
The biocompatibility of endodontic materials has been of concern to dentistry for many decades because they can come into contact with the connective periapical tissue. Molecules present in these materials could produce irritation or even degeneration in the surrounding tissues [23]. An ideal endodontic material, in addition to having suitable chemical and physical properties, should be biologically compatible and well tolerated by the periapical tissues, avoiding any possible alteration and delay of the healing process [24]. A careful evaluation of the interactions between the components of these materials and the host is therefore important. In vitro tests are especially suitable for this purpose, allowing separate analysis of the different metabolic aspects, whereas the same results could not be obtained by in vivo trials [25]. In vitro tests, characterized by quickness, inexpensiveness, sensitivity and reproducibility, can be performed both directly or through eluate analysis [26,27]. Unfortunately, the results obtained by this type of tests are not sufficient for a conclusive clinical evaluation. Permanent cell lines (i.e., 3T3 cells) and primary cells (oral fibroblasts) are frequently used for in vitro tests with cell culture [25,28]. Human fibroblasts are considered a suitable model for preliminary studies of the possible cytotoxic effects of root filling materials [27,29] because this type of cell better reproduces the in vivo behavior of oral mucosa [25,27,30–32].
The LAD principle appears to be not only effective against bacteria, but also against biofilms [33]. Advanced non-invasive LAD using a photosensitizer formulation containing oxidizer and oxygen carrier has been demonstrated to disrupt the biofilm matrix and to facilitate comprehensive inactivation and disinfection of matured endodontic biofilm [34].
The results of MTT tests in the present study showed that FotoSan produced a slight cytotoxic effect, similar to that produced by 17% EDTA and significantly less than that produced by 2% chlorhexidine. The cytotoxic effect of irradiated FotoSan was similar to that produced by non-irradiated material. This means that inadvertent extrusion of the material beyond the apex can lead to some reactions in the periapical tissues, independently from the photoactivation of the material. Such a non-significant difference can be explained by the overall low toxic effect induced by FotoSan in both experimental conditions (photocured and uncured) and by the sensitivity of the experimental methodology. Since the values are low, if there were slight differences in the toxicity, MTT assay would not be able to detect them. These results supported those from a previous study that reported excellent biocompatibility of LAD, and it becomes obvious that the slightly toxic effect caused by FotoSan is not due to an increase in ROS production.
Conclusions
Preliminary results are encouraging, but further studies are needed to more precisely evaluate any differences, if any, between the activated and non-activated solutions, and possibly to identify the components responsible for the slightly cytotoxic reactions. Data from the present study showed that FotoSan photo-sensitizer, both activated and non-activated, can be routinely used in endodontic therapy, with precautions of use similar to those usually recommended for common endodontic irrigants.
Footnotes
Source of support: Departmental sources
References
- 1.Siqueira JF., Jr Aetiology of root canal treatment failure: why well-treated teeth can fail. Int Endod J. 2001;34:1–10. doi: 10.1046/j.1365-2591.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- 2.Bystrom A, Sundquist G. Bacteriological evaluation of the effect of 0.5% sodium hypochlorite in endodontic therapy. Oral Surg Oral Med Oral Pathol. 1983;55:307–12. doi: 10.1016/0030-4220(83)90333-x. [DOI] [PubMed] [Google Scholar]
- 3.Bystrom A, Claesson R, Sundquist G. The antimicrobial effects of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endod Dent Traumatol. 1985;1:170–75. doi: 10.1111/j.1600-9657.1985.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 4.Engstrom B, Frostell G. Bacteriological studies of the non-vital pulp in cases with intact pulp cavities. Acta Odontol Scandanav. 1961;19:23–39. doi: 10.3109/00016356108993941. [DOI] [PubMed] [Google Scholar]
- 5.Sjogren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997;30:297–306. doi: 10.1046/j.1365-2591.1997.00092.x. [DOI] [PubMed] [Google Scholar]
- 6.Peters OA, Schönenberger K, Laib A. Effects of four Ni-Ti preparation techniques on root canal geometry assessed by micro computed tomography. Int Endod J. 2001;34:221–30. doi: 10.1046/j.1365-2591.2001.00373.x. [DOI] [PubMed] [Google Scholar]
- 7.Zehnder M. Root canal irrigants. J Endod. 2006;32:389–98. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Burns T, Wilson M, Pearson GJ. Sensitisation of cariogenic bacteria to killing by light from a helium neon laser. J Med Microbiol. 1993;38:401–5. doi: 10.1099/00222615-38-6-401. [DOI] [PubMed] [Google Scholar]
- 9.Williams J, Pearson GJ, Wilson M, Colles J. Use of a novel light source as a means of killing bacteria using PAD. Caries Res. 2003;37:190–93. doi: 10.1159/000070443. [DOI] [PubMed] [Google Scholar]
- 10.Williams J, Pearson GJ, Colles J, Wilson M. The antibacterial effect of TBO on bacterial colonies in a collagen matrix and carious dentine. Caries Res. 2004;38:530–36. doi: 10.1159/000080582. [DOI] [PubMed] [Google Scholar]
- 11.Bouillaguet S, Wataha JC, Zapata O, et al. Production of reactive oxygen species from photosensitizers activated with visible light sources available in dental offices. Photomed Laser Surg. 2010;28(4):519–25. doi: 10.1089/pho.2009.2505. [DOI] [PubMed] [Google Scholar]
- 12.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;2:436–50. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jori G. Photodynamic therapy of microbial infections: state of the art and perspectives. J Envir Pathol Toxicol Oncol. 2006;25:505–19. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.320. [DOI] [PubMed] [Google Scholar]
- 14.Konopka K, Goslinski T. Photodynamic therapy in dentistry. J Dent Res. 2007;86:694–707. doi: 10.1177/154405910708600803. [DOI] [PubMed] [Google Scholar]
- 15.Kömerik N, Curnow A, MacRobert AJ, et al. Fluorescence biodistribution and photosensitising activity of toluidine blue o on rat buccal mucosa. Lasers Med Sci. 2002;17:86–92. doi: 10.1007/s101030200015. [DOI] [PubMed] [Google Scholar]
- 16.Williams JA, Pearson GJ, Wilson M, Colles MJ. Antibacterial action of photo-activated disinfection (PAD) used on endodontic bacteria in planktonic suspension and in artificial and human root canals. J Dent. 2006;34:363–71. doi: 10.1016/j.jdent.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Bonsor SJ, Nichol R, Reid TMS, Pearson GJ. Microbiological evaluation of photo-activated disinfection in endodontics (An in vivo study) Br Dent J. 2006;200:337–41. doi: 10.1038/sj.bdj.4813371. [DOI] [PubMed] [Google Scholar]
- 18.Wataha JC, Hanks CT, Craig RG. In vitro synergistic, antagonistic, and duration of exposure effects of metal cations on eukaryotic cells. J Biomed Mat Res. 1992;26:1297–309. doi: 10.1002/jbm.820261004. [DOI] [PubMed] [Google Scholar]
- 19.Hashie IA, Cosset A, Franquin JC, Camps J. In vitro cytotoxicity of one-step dentin bonding systems. J Endod. 1999;25:89–92. doi: 10.1016/S0099-2399(99)80003-X. [DOI] [PubMed] [Google Scholar]
- 20.Robinson JP, Bruner LH, Bassoe CF, et al. Measurement of intracellular fluorescence of human monocytes relative to oxidative metabolism. Leukoc Biol. 1988;43:304–10. doi: 10.1002/jlb.43.4.304. [DOI] [PubMed] [Google Scholar]
- 21.Brubacher JL, Bols NC. Chemically de-acetylated 2′,7′ dichlorodihydrofluorescein diacetate as a probe of respiratory burst activity in mononuclear phagocytes. J Immunol Methods. 2001;251:81–91. doi: 10.1016/s0022-1759(01)00308-8. [DOI] [PubMed] [Google Scholar]
- 22.Sletten G, Dahl JE. Cytotoxic effects of extracts of compomers. Acta Odontol Scand. 1999;57:316–21. doi: 10.1080/000163599428544. [DOI] [PubMed] [Google Scholar]
- 23.Tepel J, Darwisch el Sawaf M, Hoppe W. Reaction of inflamed periapical tissue to intracanal medicaments and root canal sealers. Endod Dent Traumatol. 1994;10:233–38. doi: 10.1111/j.1600-9657.1994.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 24.Gambarini G, Romeo U, Tucci E, et al. Cytotoxicity of epiphany SE endodontic sealer: a comparative in vitro study. Med Sci Monit. 2009;15(4):PI15–18. [PubMed] [Google Scholar]
- 25.Schmalz G. Use of cell cultures for toxicity testing of dental materials-advantages and limitations. J Dent. 1994;22:S6–11. doi: 10.1016/0300-5712(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 26.Granchi D, Stea S, Ciapetti G, et al. Endodontic cements induce alterations in the cell cycle of in vitro cultured osteoblasts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:359–66. doi: 10.1016/s1079-2104(05)80230-6. [DOI] [PubMed] [Google Scholar]
- 27.Willerhausen B, Marroquin BB, Schaefer D, Schulze R. Cytotoxicity of root canal filling materials to three different human cell lines. J Endod. 2000;26:703–7. doi: 10.1097/00004770-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Hauman CHJ, Love RM. Biocompatibility of dental material used in contemporary endodontic therapy: a review. Part 1. Intracanal drugs and substances. Int Endod J. 2003;36:75–85. doi: 10.1046/j.1365-2591.2003.00631.x. [DOI] [PubMed] [Google Scholar]
- 29.Al-Nazhan S, Spangberg L. Morphological cell changes due to chemical toxicity of a dental material: an electron microscopy study on human periontal ligament fibroblasts and L929 cells. J Endod. 1990;16:129–34. doi: 10.1016/S0099-2399(06)81589-X. [DOI] [PubMed] [Google Scholar]
- 30.Weller RN, Kimbrough WF, Anderson RW. A comparison of thermoplastic obturation techiniques: adaptation to the canal walls. J Endod. 1997;23:703–6. doi: 10.1016/S0099-2399(97)80406-2. [DOI] [PubMed] [Google Scholar]
- 31.Chang YC, Chou MY. Cytotoxicity of halothane on human gingival fibroblast cultures in vitro. J Endod. 2001;27:82–84. doi: 10.1097/00004770-200102000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Szep S, Grumann L, Ronge K, et al. In vivo cytoyoxicity of medicated and non medicated gutta-percha points in cultures of gingival fibroblasts. J Endod. 2003;29:36–40. doi: 10.1097/00004770-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 33.George S, Kishen A. Photophysical, photochemical, and photobiological characterization of methylene blue formulations for light-activated root canal disinfection. J Biomed Opt. 2007;12:29–34. doi: 10.1117/1.2745982. [DOI] [PubMed] [Google Scholar]
- 34.George S, Kishen A. Augmenting the antibiofilm efficacy of advanced noninvasive light activated disinfection with emulsified oxidizer and oxygen carrier. J Endod. 2008;34:1119–23. doi: 10.1016/j.joen.2008.06.019. [DOI] [PubMed] [Google Scholar]