Abstract
MicroRNAs (miRNAs) regulate many biological processes including muscle development. However, little is known regarding miRNA regulation of muscle regeneration. Murine tibialis anterior muscle was evaluated after cardiotoxin-induced injury and used for global miRNA expression analysis. From day 1 through day 21 following injury, 298 miRNAs were significantly changed at least at one time point, including 86 miRNAs that were altered >10-fold compared with uninjured skeletal muscle. Temporal miRNA expression patterns included inflammation-related miRNAs (miR-223 and -147) that increased immediately after injury; this pattern contrasted to that of mature muscle-specific miRNAs (miR-1, -133a, and -499) that abruptly decreased following injury followed by upregulation in later regenerative events. Another cluster of miRNAs were transiently increased in the early days of muscle regeneration including miR-351, a miRNA that was also transiently expressed during myogenic progenitor cell (MPC) differentiation in vitro. Based on computational predictions, further studies demonstrated that E2f3 was a target of miR-351 in myoblasts. Moreover, knockdown of miR-351 expression inhibited MPC proliferation and promoted apoptosis during MPC differentiation, whereas miR-351 overexpression protected MPC from apoptosis during differentiation. Collectively, these observations suggest that miR-351 is involved in both the maintenance of MPC proliferation and the transition into differentiated myotubes. Thus, a novel, time-dependent sequence of molecular events during muscle regeneration has been identified; miR-351 inhibits E2f3 expression, a key regulator of cell cycle progression and proliferation, and promotes MPC proliferation and protects early differentiating MPC from apoptosis, important events in the hostile tissue environment after acute muscle injury.
Keywords: microRNA, myogenic progenitor cell, muscle regeneration, muscle differentiation
skeletal muscles are damaged and repaired repeatedly throughout life. The capacity for skeletal muscle growth and regeneration is conferred by satellite cells located between the basal lamina and the sarcolemma of mature myofibers (21). Upon injury, satellite cells reenter the cell cycle, proliferate, and then exit the cell cycle either to renew the quiescent satellite cell pool or to differentiate into mature myofibers (1). Despite recent advances, genes involved in these processes are still largely unknown. Understanding the molecular mechanisms that regulate satellite cell activities could promote development of novel countermeasures to enhance muscle regeneration that is compromised by diseases or aging.
Both cell proliferation and differentiation programs are essential for muscle regeneration. The decision to differentiate or proliferate represents a critical point in the development of multicellular organisms. Mammalian cells must cease proliferating to enter the G0 phase of the cell cycle either right before or during differentiation; terminal cell cycle exit is regulated by a complex network of extra- and intracellular signaling pathways and checkpoints. For example, the retinoblastoma tumor suppressor family (pRb, p107, and p130) acts as transcriptional regulators in concert with E2f to control cell cycle entry and progression (4, 46, 53). On the other hand, myogenic differentiation is controlled by interactions of a network of myogenic transcription factors. Paired box proteins (Pax3 and Pax7) are involved in myogenic cell lineage determination and specification (47, 50, 63), whereas primary basic helix-loop-helix myogenic regulatory factors (MRFs), MyoD and Myf5, and secondary MRFs, myogenin and MRF4, function downstream in terminal differentiation (31). Thus, a dynamic transcription factor network regulates myogenic cell proliferation and differentiation.
Recent evidence suggests that small noncoding RNAs, such as microRNAs (miRNAs), are involved in muscle proliferation and differentiation (5, 17). MiRNAs silence gene expression by binding to the 3′-untranslated region (UTR) of target mRNAs, inhibiting initiation of translation and/or promoting transcript cleavage (16). Muscle-specific miRNAs (miR-1, -133, -206, -208, and -499) have been identified and are involved in a range of processes including muscle development and disease (6, 38, 41, 58, 68). MiR-133a increases myoblast proliferation via repression of serum response factor (SRF) (6), while miR-1 stimulates myoblast differentiation via inhibition of histone deacetylase 4 (HDAC4) (6). Similarly, miR-206 influences the differentiation program via an indirect downregulation of the helix-loop-helix protein Id, a repressor of MyoD (30). In addition, nonmuscle-specific miRNAs, such as miR-26a and miR-181, also regulate skeletal muscle differentiation (40, 64) and regeneration after injury (40). Using primary cultures of myogenic progenitor cells (MPC), we have identified multiple miRNAs that are differentially expressed during proliferation and/or differentiation (7). These miRNAs may represent new regulatory elements in muscle regeneration.
Given the growing importance of miRNAs in regulating gene expression in skeletal muscle, the objectives of the present study were to identify miRNAs involved in muscle regeneration. Using a cardiotoxin (CTX)-induced mouse muscle injury/regeneration model, comprehensive array analyses revealed distinct miRNA expression patterns following muscle injury and regeneration. Functional analysis of one of the miRNAs, miR-351, indicated regulatory roles in MPC proliferation and survival during differentiation. Further evidence has been provided that E2f3 is a direct target of miR-351, thus revealing a novel role of this miRNA in the regulation of muscle regeneration.
MATERIALS AND METHODS
CTX muscle injury mouse model.
All procedures complied with the National Institutes of Health (NIH) Animal Care and Use Guidelines and were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio and at the South Texas Veterans Health Care Systems (San Antonio, TX). C57Bl/6J male mice were obtained from The Jackson Laboratory and used at 4–6 mo of age. Mice were anesthetized with inhalation of 1–2% isoflurane (Vedco) and placed on a warming pad to maintain body temperature during the procedure. CTX (Calbiochem) (2.5 μM in pyrogen-free saline) was injected intramuscularly below the knee into the anterior (100 μl) compartments of both hind limbs. Tibialis anterior (TA) muscles were collected at baseline (no injections) and at days 1, 3, 4, 7, and 21 after CTX injection (n = 4 mice/time point). The tissues were weighed; half of the muscle was homogenized in QIAzolLysis Reagent (QIAGEN) using TissueRuptor (QIAGEN) and stored at −80°C until use; the other half was placed in 10% neutral buffered formalin (NBF) for histological examination to confirm the extent and time course of muscle injury and regeneration.
Histochemistry.
TA muscle tissues were collected en bloc from the anterior compartment and placed in 10% NBF for routine paraffin embedding. All paraffin-embedded specimens were sectioned (6 μm) and stained with hematoxylin and eosin prior to light microscopic examination.
RNA isolation.
Total RNA was isolated using miRNeasy Minikit (QIAGEN) according to manufacturer's instructions. RNA integrity was assessed by Agilent 2100 Bioanalyzer (Agilent Technologies) and RNA samples with RNA integrity number >9 were used in the array studies.
Quantitative real-time reverse transcriptase polymerase chain reaction.
The mRNA expression of E2f3 during in vivo muscle regeneration was determined by quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR) using the Taqman Universal PCR Master Mix and Taqman microRNA Assays (Applied Biosystems, Foster City, CA). Specific primers were designed based on published gene sequences (NCBI Entrez search system). The comparative cycle threshold (Ct) method was used to calculate the relative E2f3 expression compared with baseline (uninjured) muscle. The housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an endogenous control after confirming the % coefficient of variation of the mRNA expression was <5% over the time course of the experiment.
TaqMan qRT-PCR microRNA array.
The stem-loop qRT-PCR based TaqMan Rodent MicroRNA Array A (Applied Biosystems) containing primers and probes of 335 murine miRNAs was used. qRT-PCR reactions were performed as previously described (8). All reagents were obtained from Applied Biosystems. Briefly, 500 ng of total RNA was reverse-transcribed using Megaplex RT Primers and the TaqMan miRNA reverse transcription kit. cDNA templates were amplified using Megaplex PreAmp Primers and TaqMan PreAmp Master Mix. Quantitative real-time RT-PCR was performed using an Applied Biosystems 7900HT system and a TaqMan Universal PCR Master Mix. Ct values were calculated with SDS software v.2.3 (Applied Biosystems) using automatic baseline settings and a threshold of 0.2. The comparative Ct method was used to calculate the relative miRNA expression. The Ct value of an endogenous control gene (MammU6, snRNA) was subtracted from the corresponding Ct value for the target gene resulting in the ΔCt value.
MPC isolation and cell culture.
MPC isolations were performed as previously described (7). Cells were isolated from the hind limb muscles of 8–16 wk old male C57BL/6J mice (Jackson Labs); all muscles from the thigh and below the knee from two mice were used for each isolation. Primary MPC were cultured in growth medium (GM) containing Ham's F-10 (Invitrogen), 20% FBS, 10 ng/ml fibroblast growth factor-2 (Promega), 100 U/ml penicillin G, and 100 μg/ml streptomycin and grown on type I collagen (0.1 mg/ml, Sigma)-coated tissue culture plates. All analyses were performed with primary MPC cultures within five passages. For MPC proliferation, cells were cultured in GM and plated on type I collagen-coated flasks at a density of 5 × 105 cells/T25 flask. Cell proliferation was evaluated by total cell counts using a hemocytometer and cell cycle analysis as previously described (7). For MPC differentiation, cells were seeded on entactin-collagen IV-laminin (ECL, 5 μg/cm2, Millipore)-coated plates at a density of 4 × 105 cells/60 mm dish, allowed to adhere for 24 h in GM (day 0), and switched to differentiation medium (DM) composed of 2% horse serum (Invitrogen), 100 U/ml penicillin and 100 μg/ml streptomycin in DMEM.
Mouse C2C12 myoblasts [American Type Culture Collection (ATCC)] were cultured in growth media containing Dulbecco's Modified Eagle Medium (DMEM) (ATCC), supplemented with 10% fetal bovine serum (FBS) (Hyclone) and 1% penicillin-streptomycin (Mediatech) at 37°C in a humidified atmosphere of 5% CO2 in air.
Transfection and luciferase assays.
MiR-351 overexpression and inhibition were achieved by transfection of miRIDIAN miRNA mimic (Thermo Scientific) and LNA antisense oligonucleotides (Exiqon), respectively as previously described (7). Transfection was performed in growth medium at a final concentration of 25 nM using Lipofectamine RNAiMAX reagent (Invitrogen); reverse transfection procedure was performed according to the manufacturer's instructions.
Full-length 3′-UTR of E2f3 gene was amplified by PCR and inserted into the firefly luciferase reporter immediately downstream from the stop codon (22). Mutation in the miR-351 target site was introduced by PCR amplification using appropriate oligonucleotides. For luciferase assays, C2C12 cells were transfected in six-well plates, using Lipofectamine LTX, with firefly luciferase reporter vectors (0.7 μg), together with a Renilla luciferase control vector (0.07 μg) (Promega). For each well, 25 nM miR-351 mimic or a scramble control miRNA was cotransfected with the reporter constructs. Luciferase activity was measured 24 h after transfection using the Dual Luciferase Reporter Assay System (Promega). Firefly luciferase activity was normalized to Renilla luciferase activity.
Apoptosis assay.
MPC were transfected with miR-351 mimic or antisense and switched to serum-free medium (DMEM + 1% bovine serum albumin) or differentiation medium 1 day after transfection (day 0). Caspase activity was determined at indicated time points using the Image-iT LIVE Green Poly Caspases Detection Kit (Invitrogen). The cell cultures were washed with PBS and treated with the caspase reagent as specified in the manufacturer's protocol. The cells were digitally photographed using a fluorescence microscope with a ×20 objective lens (Eclipse TE2000-U microscope equipped with a high resolution DXM 1200F digital camera; Nikon, Melville, NY). For a given field, caspase-positive nuclei were determined and expressed as a percentage of the total number of nuclei (identified with Hoechst 33342).
Western blot.
Nuclear extracts were made using the NE-PER kit (Pierce). E2f3 and TATA binding protein (TBP, nuclear protein loading control) were detected using the anti-E2f3 (N-20, Santa Cruz) and anti-TBP (1TBP18, Abcam) antibodies. For immunoblotting, cell lysates (20 μg) were resolved on 15% SDS-polyacrylamide gels and transferred onto polyvinylidene difluoride membranes (Hybond-P, Amersham Biosciences). Membranes were blocked with 2% nonfat milk for 1 h and then incubated with the primary antibody followed by a horseradish peroxidase-conjugated secondary antibody, and ECL-PLUS Detection System (Amersham Biosciences) was used as substrate for chemiluminescent detection. The band intensity was quantitated using Image J software. Data were normalized to the intensity of TBP bands.
Data analysis.
MiRNAs were included in analyses when at least 50% of the measurements (≥12 measurements) were detectable (Ct ≤30). The LIMMA software package was used to estimate the log fold-change and false discovery rate (FDR) (52), a corrected P value for multiple comparisons in multiple hypothesis testing. A miRNA was identified as differentially expressed when FDR <0.05 and fold change >2. The clustering was performed using the hierarchical method with average linkage and Euclidean distance metric (12). The Log2-transformed intensity values were centered by subtracting the median Log2 values across all the samples for individual miRNAs and used for cluster analysis to generate the heat map (Java Treeview) (48). Comparisons of miRNA expression and MPC proliferation were performed by paired t-test; data are expressed as means ± SD. Comparisons of E2f3 expression during muscle regeneration were made relative to baseline and significance determined by a one-way ANOVA test.
RESULTS
Distinct miRNA expression patterns during skeletal muscle injury and regeneration.
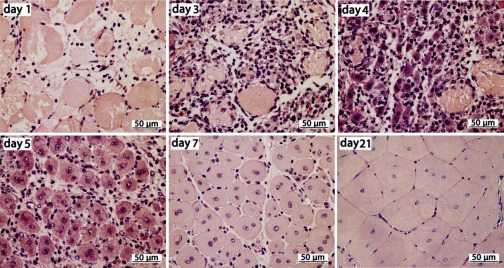
CTX injected into TA muscles of the hind limb created a consistent histological pattern of skeletal muscle injury and regeneration (Fig. 1). Within 1 day post-CTX, extensive myocyte necrosis was associated with profound edema and inflammatory cell infiltrates consisting mainly of neutrophils (Fig. 1). Mononuclear cells largely replaced neutrophils by days 2–3. Regenerated myofibers were readily identified by day 4 as mono- or binucleated, large cells with prominent nuclei and an intensely basophilic cytoplasm. Myofibers progressively increased in size through day 21. Muscle morphology at day 21 after injury was not different from that of the uninjected, control muscles except for the presence of centrally located nuclei in myofibers, a histological hallmark of recent muscle regeneration (69). These results confirmed the time course of early events in CTX-induced injury and subsequent regeneration of TA muscle, i.e., the regeneration process morphologically shifts from myocyte necrosis and mononuclear cell proliferation to myocyte differentiation/myofiber formation at days 3–4 after injury (67).
Fig. 1.
Time course of cardiotoxin (CTX)-induced tibialis anterior muscle injury and regeneration. Representative images; paraffin sections (4 μm) with hematoxylin and eosin stain. Day after CTX injection indicated on each image.
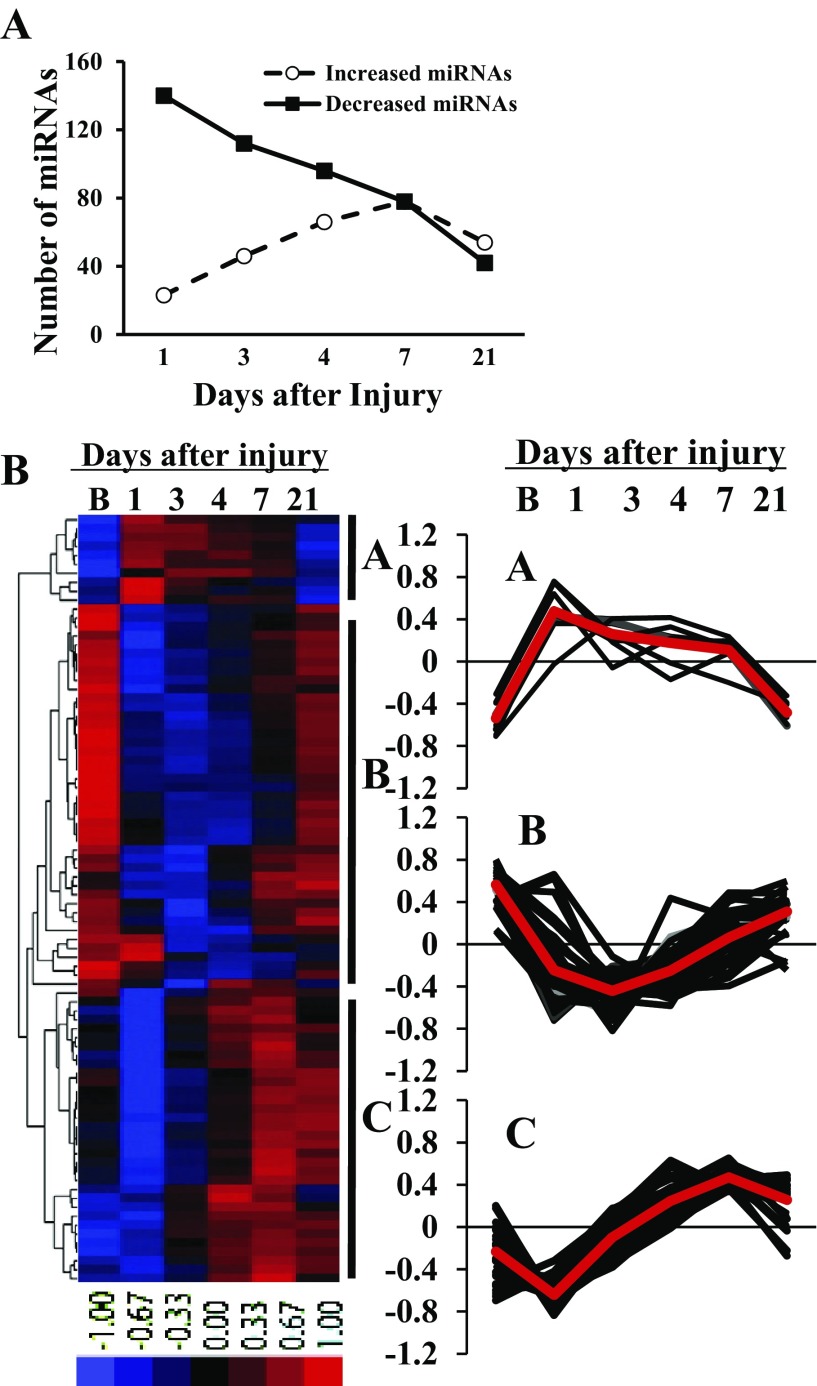
MiRNA profiling in baseline noninjured muscle (day 0) was compared with miRNA expression at days 1, 3, 4, 7, and 21 post-CTX. Of the 335 murine miRNAs examined, 212 (63%) or 86 (26%) were differentially expressed with more than 2- or 10-fold change compared with baseline muscle, respectively (FDR < 0.05). Additionally, five miRNAs (miRs-17, 20b, 92a, 221, and 342) exhibited significant (FDR < 0.05) but lower fold change between 1.5 and 2. The number of differentially expressed miRNAs varied throughout the time course of muscle regeneration (Fig. 2A). Compared with baseline, 140 miRNAs were downregulated with >2-fold change at day 1. Although individual miRNA subsequently proceeded through unique expression patterns of up- and downregulation, the net number of miRNAs that were initially decreased was reduced over time. Conversely, the number of upregulated miRNAs progressively increased and was maximum at day 7 postinjury. Data for all miRNA expression were submitted to the Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo) with the series accession number GSE37479.
Fig. 2.
Distinct miRNA expression patterns during skeletal muscle injury and regeneration. A: number of miRNAs differentially expressed with >2-fold change during muscle regeneration compared with baseline, noninjured muscle. Data derived from 4 mice at each time point. B: hierarchical cluster analysis of differentially expressed miRNAs with >10-fold change during the time course of muscle injury and regeneration. Cluster analysis was performed for 86 differentially expressed miRNAs with >10-fold change after data adjustment (log transformation, median center and normalization). The color codes of red, black, and blue represent expression levels of high, average, and low, respectively. A–C, temporal expression of individual miRNA in cluster A (n = 10), cluster B (n = 43), and cluster C (n = 33) during muscle regeneration. The average value of miRNA expression in each cluster is presented as a red line; B, baseline, noninjured muscle. Data derived from 4 mice at each time point.
Patterns of miRNA expression during muscle regeneration were assessed by a hierarchical cluster analysis of the 86 differentially expressed miRNAs with more than a 10-fold change. Thus, miRNAs were clustered into one of three distinct expression patterns (Fig. 2B): cluster A, upregulation between days 1 and 7 postinjury followed by a decrease to baseline expression levels (n = 10); cluster B, downregulation between days 1 and 7 postinjury followed by an increase to baseline expression levels (n = 43); and cluster C, transient (day 1) downregulation followed by progressive upregulation through days 4–7 postinjury and a return toward baseline expression levels thereafter (n = 33). Multiple miRNAs have been confirmed their expression patterns by individual real-time PCR (data not shown). MiRNAs within each cluster are provided in Supplemental Table S1.1
The temporal patterns of miRNA expression identified above suggested distinct regulatory functions of clustered miRNAs relative to key biological events following injury and during muscle regeneration. In keeping with this possibility, inflammation-associated miRNAs, miR-223 and -147 (29, 35), in cluster A were transiently upregulated immediately after injury, a likely result of participation in essential inflammatory cell events. Similarly, cluster B included miRNAs associated with mature muscle, i.e., miR-1, -133, and -499, which were downregulated immediately after injury and returned toward baseline at day 21; this expression pattern is consistent with the role of these miRNAs in the maintenance of muscle differentiation and/or fiber type specification (6, 58). To explore the general roles of microRNAs in cluster A in inflammation and cluster B in myogenesis, predicted gene targets of various miRNAs in both clusters were explored using the TargetScan Mouse 5.1 algorithm (http://www.targetscan.org/) and their functional annotation determined by the DAVID analysis (http://david.abcc.ncifcrf.gov/)(Supplemental Tables S2 and S3). Many of the miRNAs explored in cluster A regulate genes that would promote inflammation, which indicates an important role of these miRNA in controlling the inflammatory response. While miRNAs explored in cluster B clearly regulate genes involved in myogenesis, particularly key myogenic transcription factors (i.e., Pax3 and Pax7). Given the temporal relationships of these well-studied miRNAs relative to morphologically confirmed events in our highly reproducible in vivo model of muscle injury and regeneration, further efforts were undertaken to identify novel miRNAs involved in muscle regeneration.
MiR-351 exhibited peak expression during the early phase of muscle differentiation.
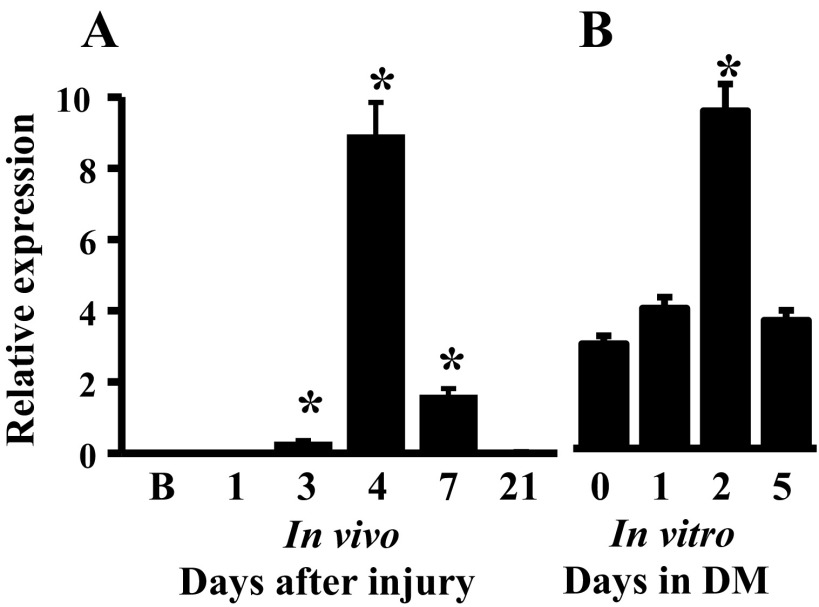
Since miRNAs in cluster C have peak expression at day 4 or day 7, concurrent with morphological evidence of myogenic differentiation, i.e., regenerated myofiber formation, we focused on genes with peak expression patterns in this cluster as being the most likely to serve a role in the coordinated induction of muscle differentiation. Not surprisingly, several cell cycle-related miRNAs, miR-31, -34c, and -292-3p (2, 28, 34), were present in cluster C. Also within this cluster, miR-351 exhibited a significant change with peak expression at day 4 postinjury (Fig. 3A).
Fig. 3.
miR-351 expression patterns during in vivo muscle regeneration (A) and in vitro myogenic progenitor cell (MPC) differentiation (B). MiRNA expression was determined by qRT-PCR-array. Data represent the mean ± SD of 4 mice/time point in vivo and 4 different MPC cultures, *P ≤ 0.01 compared with day 0 in vitro or baseline muscle in vivo; B, baseline noninjured muscle; DM, differentiation medium.
To explore the muscle specificity of miR-351 in muscle regeneration, the expression of miR-351 was assessed during the time course of MPC differentiation in vitro. Cultured MPC rapidly differentiate in low serum (7). Herein, 30 ± 2% of murine MPC were differentiated within 1 day as evidenced by myosin heavy chain (MHC) protein expression. Subsequently, the percentage of differentiated cells progressively increased, reaching 73 ± 5% and 94 ± 1% at days 2 and 5, respectively. In parallel, miR-351 was upregulated during MPC differentiation with peak expression at day 2 followed by a decline thereafter (Fig. 3B). Thus, both in vivo and in vitro, miR-351 expression patterns were temporally similar and correlated with muscle cell differentiation.
MiR-351 antisense inhibited MPC proliferation.
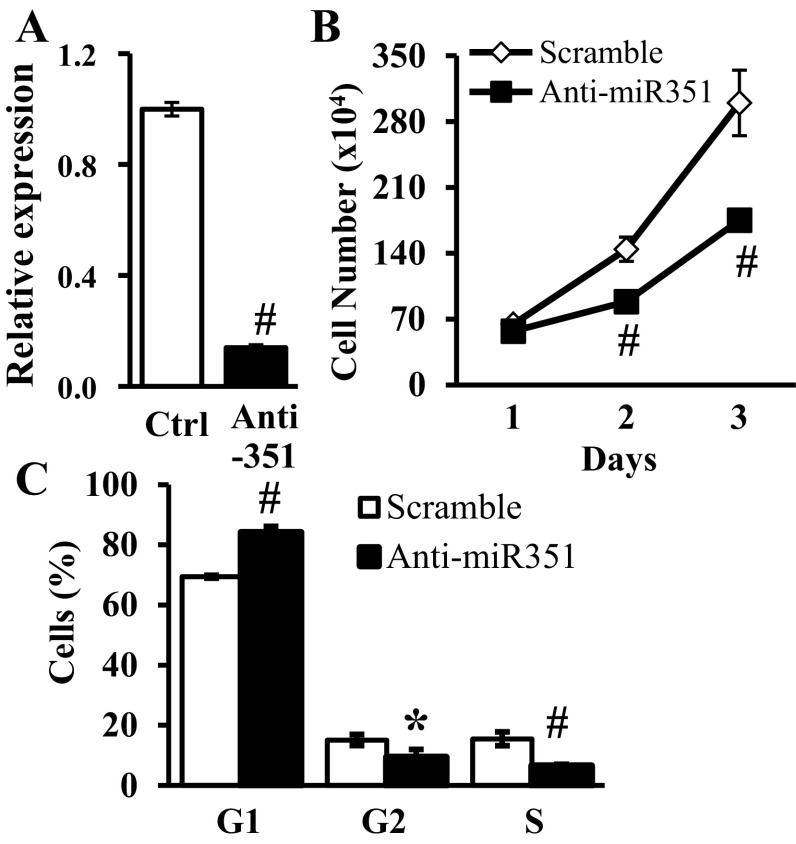
Transfection of proliferating MPC with miR-351 antisense oligonucleotides inhibited (P < 0.001) miR-351 expression compared with a scramble sequence used as a control (Fig. 4A). In parallel, MPC proliferation at days 2–3 posttransfection was reduced (P ≤ 0.004) (Fig. 4B). At day 3, the percentages of S and G2 phase cells were reduced (P ≤ 0.011) and G1 phase cells were correspondingly increased following miR-351 antisense transfection (Fig. 4C). However, no marked cell death was observed by caspase assays in either miR-351 antisense or scramble control transfected MPC (3.7 ± 1.2% vs. 2.9 ± 0.9% caspase-positive nuclei, miR-351 antisense vs. scrambled oligonucleotide control, respectively).
Fig. 4.
Effects of inhibition of endogenous miR-351 in proliferating MPC. MPC were transfected with either miR-351 antisense or scramble control (Ctrl) oligonucleotides on day 0 and cultured in growth medium. A: miR-351 expression was measured by quantitative real time-PCR 2 days after transfection. B: total cell number determined at the indicated time. C: percent of cells at stages of the cell cycle determined at 3 days after transfection. Data represent means ± SD, n = 3 different cultures/assay. *P ≤ 0.01 compared with scramble oligonucleotide.
MiR-351 protected MPC from apoptosis during differentiation.
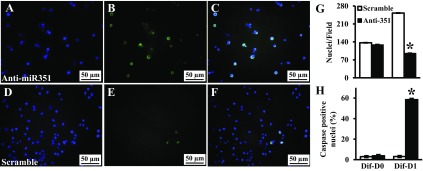
MPC differentiation was induced in proliferating MPC by exposure to low serum 1 day after miR-351 antisense transfection (Fig. 5, A–H). Compared with cells treated with scramble oligonucleotides, the cell number was decreased (P < 0.001) at differentiation day 1 in miR-351 antisense transfected cells (Fig. 5G), and few antisense-treated cells survived at differentiation day 3. Using a poly-caspase assay to detect apoptosis, caspase activation was similar between miR-351 antisense and scramble transfected cells when MPC were cultured for 24 h in growth medium (differentiation day 0, Fig. 5H). However, within 24 h of MPC differentiation, caspase activation was increased (P < 0.001) in miR-351 antisense transfected MPC, i.e., 58 ± 7% vs. 3 ± 2% caspase-positive nuclei, miR-351 antisense vs. scrambled oligonucleotide control, respectively (Fig. 5, A–F and H). Thus, miR-351 expression in cultured MPC has different consequences depending on whether the cells are proliferating or differentiating.
Fig. 5.
Effects of inhibition of miR-351 on apoptosis during MPC differentiation. MPC were subcultured and transfected with miRNA antisense (top) or scramble (bottom) oligonucleotide. Differentiation medium was added 1 day after transfection (differentiation day 0, Dif-D0). Caspase activity was measured at differentiation day 1 (Dif-D1) and labeled with a green fluorescence probe (FLICA; B and E); and nuclear integrity was monitored by Hoechst 33342 counterstaining (blue, A and D). C and F show merged images from green and blue fluorescence. Cell number was determined using images obtained with a fluorescent microscope to determine the nuclei/field (G) and the percent of caspase-positive cells (H). Data represent means ± SD of 3 different MPC cultures. *P < 0.001 compared with the scramble oligonucleotide.
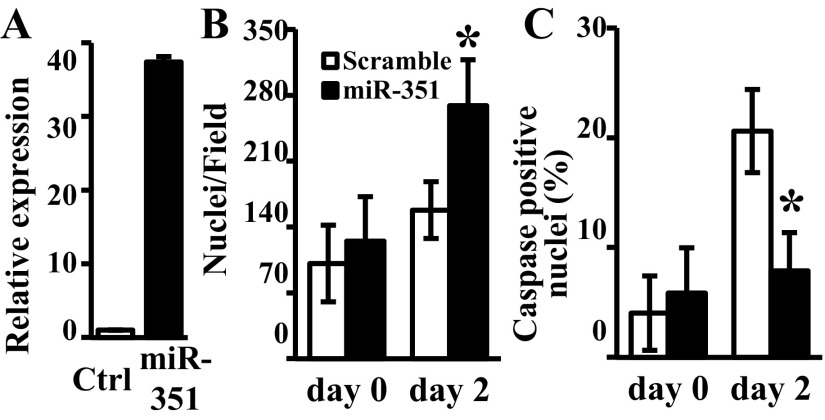
To further assess the antiapoptotic effect of miR-351 during MPC differentiation, MPC were transfected with miRNA mimics and cultured under serum-free conditions. Not surprisingly, miR-351 mimic resulted in a >30-fold overexpression of miR-351 (Fig. 6A) and a significant increase of cell number at differentiation day 2 compared with scramble control (Fig. 6B, P = 0.014). In parallel, 2 days in serum-free medium resulted in 21 ± 4% of caspase-positive nuclei in scramble-transfected MPC, whereas apoptosis was reduced (P = 0.001) to only 8 ± 3% caspase-positive nuclei with miR-351 overexpression (Fig. 6C). Thus, overexpression of miR-351 protected differentiating MPC from apoptosis in serum-free conditions.
Fig. 6.
Effects of miR-351 overexpression on MPC apoptosis in serum-free conditions. MPC were subcultured and transfected with miR-351 mimic or scramble control (Ctrl) oligonucleotide. Serum-free medium was added 1 day after transfection (day 0). A: miR-351 expression was measured by qRT-PCR in serum-free medium (day 2). Caspase activity in individual nuclei was assessed and the cells identified in images obtained from a fluorescent microscope to determine the nuclei/field (B) and the percent of caspase-positive cells (C). Data represent means ± SD of 3 different MPC cultures; *P ≤ 0.01 compared with scramble oligonucleotide.
E2f3 is a direct target of miR-351 in myoblasts.
To gain insight into the biological function of miR-351 in muscle regeneration, we evaluated the predicted target genes of miR-351, as identified by TargetScan Mouse 5.1 algorithm (http://www.targetscan.org/) and subjected this list to functional annotation by DAVID (http://david.abcc.ncifcrf.gov/). Annotation of 472 highly conserved target genes revealed enrichment of genes involved with protein catabolism, cellular synthesis, regulation of transcription, cell migration, and apoptosis (Table 1). Of interest, E2f3 mRNA has a target site in its 3′-UTR with high complementarity to miR-351 (Fig. 7A). We were particularly interested in E2f3 because of its essential roles in cell proliferation, apoptosis, and myogenic differentiation (3, 10, 37, 44).
Table 1.
Gene ontology enrichment of miR-351
| Term | P Value | Genes |
|---|---|---|
| Cellular protein catabolic process | 7.49E-04 | SYVN1, USP2, UBE2G1, PPP2R5C, MAN1B1, ASB13, ZNRF1, EDEM1, UBE2R2, FBXW4, USP38, USP12, USP37, YOD1, FBXO42, TRAF6, FBXO45, RNF144A, UBE2J1, UBE2L3, ATG4D, UBR7, 1110018G07RIK, SMURF1, TNFAIP3, USP45 |
| Regulation of cellular biosynthetic process | 0.010 | E2F3, ESRRA, CREB1, SOX11, ESRRG, ZFP148, SIRT7, RFXANK, STAT3, PLAGL1, EAF1, MTF1, ETS1, DCP1A, MAPK14, TEAD4, TEF, SERTAD3, IRF4, TRAF6, EIF5A2, PLAGL2, NFIB |
| Regulation of transcription | 0.008 | E2F3, ESRRA, CREB1, SOX11, ESRRG, ZFP148, SIRT7, RFXANK, STAT3, PLAGL1, EAF1, MTF1, ETS1, DCP1A, MAPK14, TEAD4, TEF, SERTAD3, IRF4, PLAGL2, NFIB |
| Cell migration | 0.024 | SGPL1, PHOX2B, JUB, RET, IL16, PODXL, BARHL1, PAFAH1B1, SCARB1, ENPEP, GDNF, APC |
| Regulation of apoptosis | 0.025 | SYVN1, MCL1, BARHL1, BCL2L13, GDNF, ACVR1C, KCNIP3, VDR, BAG4, TRIAP1, BAK1, SARM1, ERCC6, TRP53INP1, TRAF6, CASP2, BMF, PLAGL2, APC |
Top 5 lowest P value.
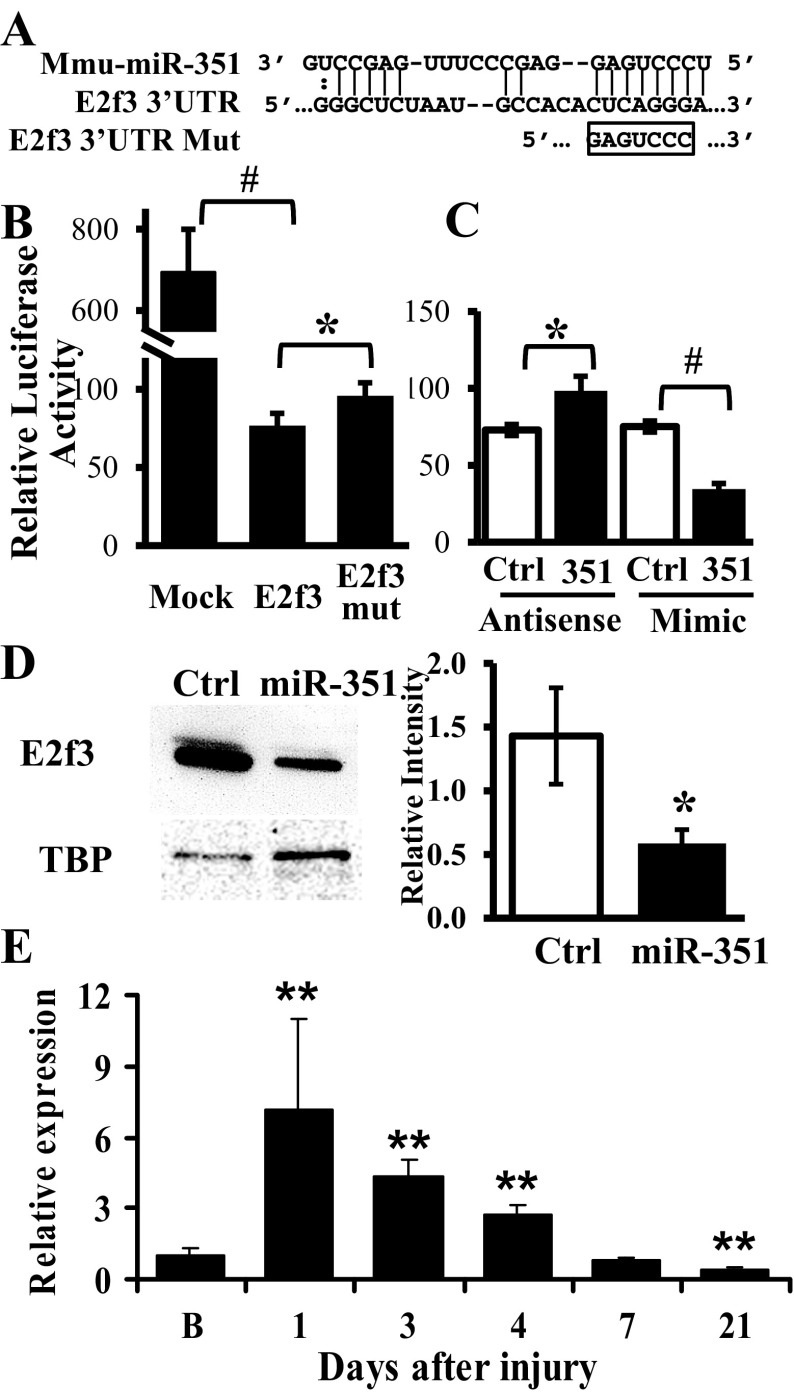
Fig. 7.
E2f3 is a putative target of miR-351 in proliferating C2C12 myoblasts. A: predicted target site of miR-351 in the 3′-untranslated region (UTR) of mouse E2f3. An E2f3 3′-UTR mutant (E2f3 mut) was produced by introducing mutation in the seed sequence as indicated. B and C: luciferase assays were performed at day 2 after transfection of a mock control or luciferase reporters bearing intact or mutant E2f3 3′-UTR into myoblasts. To measure the effect of miR-351 on luciferase activity, the luciferase reporter fused to an intact E2f3 3′-UTR was cotransfected with either miRNA antisense or mimic and compared with that of scramble control (Ctrl). The results were expressed as firefly luciferase activity relative to the renilla luciferase expressed from a cotransfected plasmid and serving as a transfection control. D: endogenous E2f3 protein levels were determined by Western blot 2 days after transfection of miR-351 mimic or a scramble control (Ctrl) in myoblasts. Quantification of Western blot results was performed using scanning and ImageJ software. Results are expressed as integrated optical density normalized to TATA box binding protein (TBP) content; *P ≤ 0.04, #P ≤ 0.004. E: E2f3 mRNA expression was determined during in vivo muscle regeneration at 1, 3, 4, 7, and 21 days postinjury by qRT-PCR. Data are reported as fold change compared with uninjured baseline (B) muscle, 95% confidence intervals, 4–6 per mice/time point, and **P < 0.001.
To determine if E2f3 is a direct target of miR-351, a luciferase reporter with E2f3 3′-UTR was transfected into C2C12 myoblasts. Compared with control, luciferase activity decreased (P = 0.001) in cells transfected by reporters with E2f3 3′-UTR (Fig. 7B). Mutation in the miR-351 seed target sequence increased (P = 0.011) luciferase activity but did not fully restore the repressed luciferase activity (Fig. 7B), suggesting either that the E2f3 3′-UTR has multiple miR-351 target sites with nonseed matching sequence(s) that have not been identified (33) or that the E2f3 3′-UTR is targeted by multiple miRNAs in addition to miR-351. To differentiate these possibilities, the luciferase reporter with the E2f3 3′-UTR was cotransfected with either miR-351 antisense or mimic. MiR-351 antisense rescued the luciferase activity to similar levels compared with C2C12 cells transfected with the mutated E2f3 target site, whereas, miR-351 mimics decreased luciferase activity >50% (Fig. 7C). These results are consistent with the working hypothesis that E2f3 expression is regulated by multiple miRNAs such that miR-351 knockdown or target sequence mutation could not fully recover luciferase activity. Interestingly, E2f3 is also a predicted target of both miR-433 and -298, miRNAs that exhibited similar expression patterns as miR-351 during the time course of muscle regeneration (Supplemental Table S1).
To determine if miR-351 regulated endogenous E2f3 expression, C2C12 myoblasts were transfected with miR-351 mimic and E2f3 protein was decreased (P = 0.021) (Fig. 7D) without changes in E2f3 mRNA levels (1.1 ± 0.1-fold compared with scramble control). Taken together, these results indicate that miR-351 represses E2f3 expression in myoblasts by translational inhibition. Interestingly, E2f3 mRNA exhibits a temporary expression pattern during in vivo muscle regeneration (Fig. 7E). The mRNA of E2F3 had peak expression (7.2 ± 3.8-fold compared with baseline, P = 2.9 × 10−15) 1 day following injury, gradually returns back to baseline by day 7, and decreases below baseline by day 21 (P = 1.9 × 10−8). These results suggest that E2f3 expression is precisely regulated by both transcriptional and posttranscriptional mechanisms during muscle regeneration.
DISCUSSION
Skeletal muscle repair is a highly synchronized process involving the activation of various cellular responses. The initial phase of muscle repair is characterized by necrosis of the damaged tissue and activation of an inflammatory response including neutrophils and macrophages. The initial phase is rapidly followed by activation of satellite cells to proliferate, differentiate, and fuse, leading to new myofiber formation and reconstitution of a functional contractile apparatus. Dynamic morphological changes were observed during the time course of CTX-induced mouse muscle injury and regeneration. In parallel, a temporal miRNA expression pattern was identified during the process of muscle regeneration (clusters A–C, Fig. 2B), indicating distinct roles of each group of miRNAs at different regenerative stages. These differentially expressed miRNAs will provide useful data sets in identifying biological pathways and individual molecules that may be important mediators of muscle regeneration. Toward this goal, functional analysis of miR-351, one of the miRNAs in cluster C, indicated regulatory roles in MPC proliferation and survival during differentiation, and identified E2f3 as a novel gene target. Thus, we present miR-351 as a novel regulatory component in muscle regeneration via control of E2f3 expression.
After muscle damage, inflammatory cells, initially neutrophils then macrophages, infiltrate the area. The onset and the extent of infiltrating inflammatory cells played active roles in muscle injury and repair (55, 57). Macrophages are essential for the removal of necrotic tissue, assisting in the conversion of the hostile, postinjury milieu into an environment that is more conducive to regeneration. Macrophages also produce a vast array of growth factors and enzymes that influence many aspects of the regenerative process including angiogenesis and the chemotaxis, proliferation, and differentiation of myoblasts (20). Corresponding to the initial phase of muscle injury, a group of miRNAs, upregulated immediately after injury (cluster A, Fig. 2B), including miR-147 and -223, were induced in multiple immune cells, such as neutrophils and macrophages (29, 35). MiR-200 family members, miR-141, -200a, and -429, also clustered in this group, were upregulated in response to oxidative stress and were involved in insulin-like growth factor-signaling pathway (26, 61). Additionally, inflammation-related gene functions were found to be significantly enriched by predicted gene targets of miRNAs in cluster A (Supplemental Table S2). Thus, cluster A miRNAs may contribute to specific changes in gene expression associated with inflammatory reaction in the early phase of muscle injury.
In contrast to the immediate increased miRNA expression in cluster A, the expression of muscle specific miR-1, -133, and -499 decreased after muscle injury and returned toward baseline in association with new myofiber formation (cluster B, Fig. 2B). The muscle-specific miRNAs regulate fundamental processes of satellite cell proliferation and differentiation under the control of myogenic transcription factors (6, 58, 62). Additional miRNAs reported to function in skeletal myogenesis, including miR-26a (64), -29 (60), and -486 (51), were also grouped in this cluster. The expression pattern of the miRNAs in cluster B was consistent with roles in regulating muscle differentiation and/or fiber type. These roles were further supported by the enrichment of myogenesis and muscle development-related gene functions enriched by predicted gene targets in several miRNAs in cluster B (Supplemental Table S3). These findings support the use of our in vivo animal model for the identification of essential miRNAs in the regulation of muscle regeneration.
In skeletal muscle, the processes of proliferation and differentiation are mutually exclusive. The decision of whether myoblasts should continue to proliferate or switch into a differentiation pathway is regulated by the balance of positive and negative cell-cycle regulators (11). MiRNAs have been functionally integrated into many crucial cell-cycle control pathways in both physiologic settings and in disease states (9). Interestingly, we identified a group of miRNAs transiently upregulated in the early days of muscle regeneration (cluster C, Fig. 2B) including cell cycle-related miR-34c, -107, and -292 (2, 18, 34). The miR-34 family of miRNAs have been repeatedly implicated as important regulators of the cell cycle and are directly induced by p53 (24). MiR-34 is able to induce cell cycle arrest by decreasing CDK4 and Cyclin E/CCNE2 protein levels (23). In addition, miR-34 modulates the expression of different E2f family members linking the p53 pathway with the progression through the G1/S transition in a pRB-independent manner. Similarly, miR-290–295 cluster promotes pluripotency maintenance by regulating cell cycle phase distribution in mouse embryonic stem cells (34). The temporal expression pattern of cluster C miRNAs of a transient increase at days 3–4 after injury suggests possible roles for these miRNAs in controlling the transition from MPC proliferation to differentiation.
Supporting this hypothesis, miR-351 exhibited a similar expression pattern during in vitro MPC differentiation, with a peak expression at early phase of MPC differentiation. Consistent with the temporal expression pattern, knockdown of miR-351 expression inhibited MPC proliferation with significant reduction in S and G2 phase cells with increased G1 phase cells. These results indicated that miR-351 was involved in MPC proliferation by cell cycle regulation. Interestingly, using computer prediction tools and experimental approaches, we demonstrated E2f3 as a novel target of miR-351. The E2f family of transcription factors function as key regulators of cell cycle progression and proliferation (27, 44). The classic view is that E2Fs determine the timely expression of many genes required for entry into and progression through the S phase of the cell cycle (4, 46, 53). In line with this regulation, overexpression of E2F1, E2F2, or E2F3a induces quiescent immortalized cells to enter S phase (14). However, it has become clear that transcriptional activation of S phase-related genes is only one facet of E2F activity; it is now known that E2Fs both transactivate and repress gene expression. E2F1 overexpression in primary fibroblasts does not lead to S-phase entry but instead promotes senescence (15, 36). This indicates that the proliferative activity of E2fs is cell context-dependent. In addition, E2f1-deficient mice develop a wide variety of cancers, which indicate an in vivo tumor-suppressor role for activating E2fs (66). Thus, E2f3 might be a critical target that mediated the antiproliferative effects of miR-351 antisense in MPC.
During muscle development, somite-derived myoblasts differentiate into multinucleated skeletal muscle fibers. Myoblasts that fail to form muscle fibers initiate apoptosis and are rapidly lost (45, 49). The state of myogenic differentiation influences the propensity of MPC to undergo apoptosis (59). The coordinated regulation of cell proliferation, differentiation, and apoptosis is necessary to regulate muscle mass during myogenesis. The molecules controlling the apoptosis cascade, such as caspase-3 and caspase-8, are also involved in differentiation in skeletal muscle fibers (13, 19). Recent work demonstrated that MyoD regulates apoptosis of MPC through miR-1 and -206-mediated downregulation of Pax3 (25). In this study, we demonstrated that decreased miR-351 expression during MPC differentiation-induced apoptosis through caspase activation, whereas miR-351 overexpression protected MPC from apoptosis in serum-free culture conditions, potentially mimicking the hostile environment present after muscle injury. Although miR-351 is present only in mice and rats, it belongs to the miR-125 family, the human ortholog of miR-351. MiR-125 family performs varied roles in human embryonic stem cell differentiation, cancer, and inflammation (54, 56, 65). Recent studies have demonstrated that miR-125b inhibited p53-dependent apoptosis pathway and mediated antiapoptotic effect in breast cancer cells by suppression of proapoptotic Bcl-2 antagonist killer 1 (Bak1) expression (32, 70). The complementary expression of miR-351 and miR-125b in mice and marmosets suggests a parallel role played by these miRNAs in mice and primates (39).
It has become clear that E2fs function is cell context dependent; E2f1–3 switch from transcriptional activators in progenitor cells to repressors in differentiating cells, revealing distinct roles in cell growth and differentiation (10). Furthermore, E2f3, and in some settings E2f1, induce apoptosis through p53-dependent or -independent pathways (37, 44). The complexity of such regulatory mechanisms indicates that miR-351 might regulate, depending on context, either MPC proliferation or apoptosis partially through posttranscriptional regulation of E2f3. Because miR-351 is predicted to target multiple genes involved in apoptosis (Table 1), the antiapoptotic effect of miR-351 may be mediated by coordination of multiple targets.
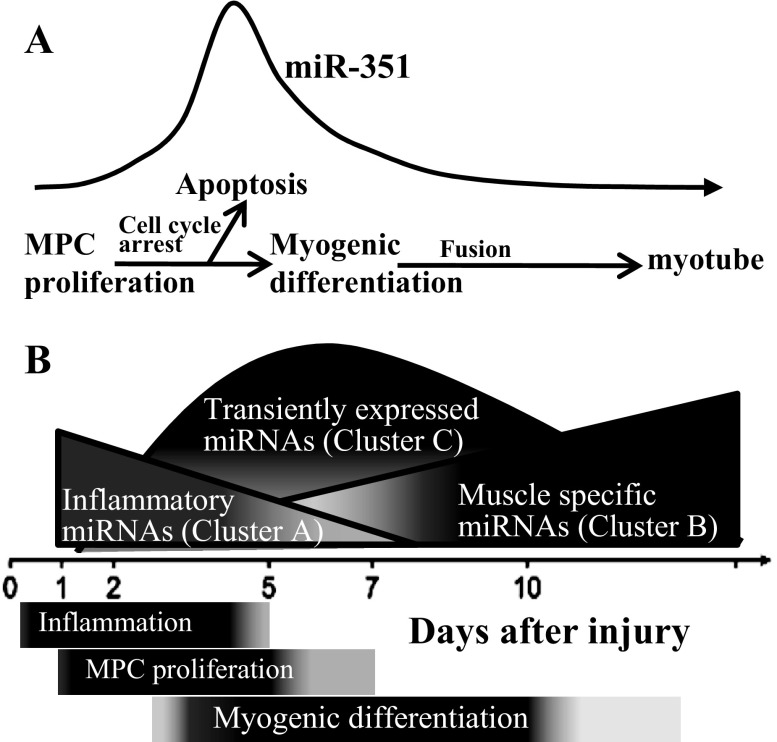
In conclusion, our results suggest that transient in vivo expression patterns of miRNAs play important roles during the time course of muscle regeneration; a model of these events is provided in Fig. 8. In vitro, miR-351 expression was required for MPC proliferation; increased expression protected MPC from apoptosis when mitogenic signals were removed and differentiation signals were present (Fig. 8A), protecting early differentiating MPC from a hostile, serum-free environment. E2f3 as a direct target may partially mediate miR-351 function in MPC. During in vivo muscle regeneration, the initial change of miRNAs (cluster A) regulated inflammatory responses; macrophages remove necrotic tissue, converting the postinjury milieu into an environment more conducive to muscle regeneration. Muscle-specific and other miRNAs (cluster B) were upregulated in the late phase of muscle regeneration and are implicated in myofiber formation and identity (Fig. 8B). A group of miRNAs transiently expressed during regeneration (cluster C) may be involved in MPC proliferation and differentiation by cell cycle regulation. In cluster C, miR-351 mediated the cell fate decision of MPC proliferation to differentiation while protecting early differentiating MPC from apoptosis in the hostile, postinjury environment, allowing the survival of early differentiating muscle fibers. Future studies will further determine the regulatory mechanisms of how miR-351 and its mRNA targets may play important roles during muscle regeneration.
Fig. 8.
Model of miRNA temporal expression during muscle injury and regeneration. A: the transient upregulation of miR-351 protects MPC from apoptosis during muscle regeneration. B: relative changes in the amount of different clusters of miRNAs during muscle regeneration. The inflammatory miRNAs (cluster A) were upregulated immediately after muscle injury; muscle-specific and other miRNAs (cluster B) were upregulated in the late phase of muscle regeneration. A group of miRNAs transiently expressed during regeneration (cluster C) regulates cell cycle and the switch from MPC proliferation to differentiation and protects early differentiating cells from apoptosis.
GRANTS
These studies were supported, in part, by National Institutes of Health (NIH) Grants K01-AR-059096, KL2-R025766, R01-HL-074236, T32-HL-007446, and F30-HL-110743 and by Veterans Administration Merit Review. Data were generated in the Core Flow Cytometry Facility, which is supported by the University of Texas Health Science Center at San Antonio and NIH-NCI P30 CA-054174 (Cancer Therapy & Research Center).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.C., L.M.M., and P.K.S. conception and design of research; Y.C. and D.W.M. performed experiments; Y.C. and J.A.L.G. analyzed data; Y.C., D.W.M., J.A.L.G., L.M.M., and P.K.S. interpreted results of experiments; Y.C. prepared figures; Y.C. drafted manuscript; Y.C., D.W.M., J.A.L.G., L.M.M., and P.K.S. edited and revised manuscript; Y.C., D.W.M., J.A.L.G., L.M.M., and P.K.S. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the technical assistance Z. Sarwar and K. Oberheu.
Current address for Y. Chen: Dept. of Pathology, St. Louis Univ. School of Medicine, St. Louis, MO.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Anderson JE, Murray L. Barr Award Lecture. Studies of the dynamics of skeletal muscle regeneration: the mouse came back! Biochem Cell Biol 76: 13–26, 1998. [PubMed] [Google Scholar]
- 2.Antonini D, Russo MT, De Rosa L, Gorrese M, Del Vecchio L, Missero C. Transcriptional repression of miR-34 family contributes to p63-mediated cell cycle progression in epidermal cells. J Invest Dermatol 130: 1249–1257, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Asp P, Acosta-Alvear D, Tsikitis M, van Oevelen C, Dynlacht BD. E2f3b plays an essential role in myogenic differentiation through isoform-specific gene regulation. Genes Dev 23: 37–53, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cam H, Dynlacht BD. Emerging roles for E2F: beyond the G1/S transition and DNA replication. Cancer Cell 3: 311–316, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Chen JF, Callis TE, Wang DZ. microRNAs and muscle disorders. J Cell Sci 122: 13–20, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet 38: 228–233, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Gelfond J, McManus LM, Shireman PK. Temporal microRNA expression during in vitro myogenic progenitor cell proliferation and differentiation: regulation of proliferation by miR-682. Physiol Genomics 43: 621–630, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Gelfond JA, McManus LM, Shireman PK. Reproducibility of quantitative RT-PCR array in miRNA expression profiling and comparison with microarray analysis. BMC Genomics 10: 407, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chivukula RR, Mendell JT. Circular reasoning: microRNAs and cell-cycle control. Trends Biochem Sci 33: 474–481, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chong JL, Wenzel PL, Saenz-Robles MT, Nair V, Ferrey A, Hagan JP, Gomez YM, Sharma N, Chen HZ, Ouseph M, Wang SH, Trikha P, Culp B, Mezache L, Winton DJ, Sansom OJ, Chen D, Bremner R, Cantalupo PG, Robinson ML, Pipas JM, Leone G. E2f1–3 switch from activators in progenitor cells to repressors in differentiating cells. Nature 462: 930–934, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Falco G, Comes F, Simone C. pRb: master of differentiation. Coupling irreversible cell cycle withdrawal with induction of muscle-specific transcription. Oncogene 25: 5244–5249, 2006. [DOI] [PubMed] [Google Scholar]
- 12.de Hoon MJ, Imoto S, Nolan J, Miyano S. Open source clustering software. Bioinformatics 20: 1453–1454, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Dee K, Freer M, Mei Y, Weyman CM. Apoptosis coincident with the differentiation of skeletal myoblasts is delayed by caspase 3 inhibition and abrogated by MEK-independent constitutive Ras signaling. Cell Death Differ 9: 209–218, 2002. [DOI] [PubMed] [Google Scholar]
- 14.DeGregori J, Johnson DG. Distinct and overlapping roles for E2F family members in transcription, proliferation and apoptosis. Curr Mol Med 6: 739–748, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Dimri GP, Itahana K, Acosta M, Campisi J. Regulation of a senescence checkpoint response by the E2F1 transcription factor and p14(ARF) tumor suppressor. Mol Cell Biol 20: 273–285, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes Dev 18: 504–511, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenberg I, Alexander MS, Kunkel LM. miRNAS in normal and diseased skeletal muscle. J Cell Mol Med 13: 2–11, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng L, Xie Y, Zhang H, Wu Y. miR-107 targets cyclin-dependent kinase 6 expression, induces cell cycle G1 arrest and inhibits invasion in gastric cancer cells. Med Oncol 29: 856–863, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Fernando P, Kelly JF, Balazsi K, Slack RS, Megeney LA. Caspase 3 activity is required for skeletal muscle differentiation. Proc Natl Acad Sci USA 99: 11025–11030, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grounds MD. Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. Ann NY Acad Sci 854: 78–91, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Grounds MD, Yablonka-Reuveni Z. Molecular and cell biology of skeletal muscle regeneration. Mol Cell Biol Hum Dis Ser 3: 210–256, 1993. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y, Chen Y, Ito H, Watanabe A, Ge X, Kodama T, Aburatani H. Identification and characterization of lin-28 homolog B (LIN28B) in human hepatocellular carcinoma. Gene 384: 51–61, 2006. [DOI] [PubMed] [Google Scholar]
- 23.He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, Jackson AL, Linsley PS, Chen C, Lowe SW, Cleary MA, Hannon GJ. A microRNA component of the p53 tumour suppressor network. Nature 447: 1130–1134, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He L, He X, Lowe SW, Hannon GJ. microRNAs join the p53 network–another piece in the tumour-suppression puzzle. Nat Rev Cancer 7: 819–822, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirai H, Verma M, Watanabe S, Tastad C, Asakura Y, Asakura A. MyoD regulates apoptosis of myoblasts through microRNA-mediated down-regulation of Pax3. J Cell Biol 191: 347–365, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyun S, Lee JH, Jin H, Nam J, Namkoong B, Lee G, Chung J, Kim VN. Conserved MicroRNA miR-8/miR-200 and its target USH/FOG2 control growth by regulating PI3K. Cell 139: 1096–1108, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Iaquinta PJ, Lees JA. Life and death decisions by the E2F transcription factors. Curr Opin Cell Biol 19: 649–657, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivanov SV, Goparaju CM, Lopez P, Zavadil J, Toren-Haritan G, Rosenwald S, Hoshen M, Chajut A, Cohen D, Pass HI. Pro-tumorigenic effects of miR-31 loss in mesothelioma. J Biol Chem 285: 22809–22817, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD, Camargo FD. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature 451: 1125–1129, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Kim HK, Lee YS, Sivaprasad U, Malhotra A, Dutta A. Muscle-specific microRNA miR-206 promotes muscle differentiation. J Cell Biol 174: 677–687, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuang S, Rudnicki MA. The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med 14: 82–91, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Kumar M, Lu Z, Takwi AA, Chen W, Callander NS, Ramos KS, Young KH, Li Y. Negative regulation of the tumor suppressor p53 gene by microRNAs. Oncogene 30: 843–853, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lal A, Navarro F, Maher CA, Maliszewski LE, Yan N, O'Day E, Chowdhury D, Dykxhoorn DM, Tsai P, Hofmann O, Becker KG, Gorospe M, Hide W, Lieberman J. miR-24 Inhibits cell proliferation by targeting E2F2, MYC, and other cell-cycle genes via binding to “seedless” 3′UTR microRNA recognition elements. Mol Cell 35: 610–625, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lichner Z, Pall E, Kerekes A, Pallinger E, Maraghechi P, Bosze Z, Gocza E. The miR-290–295 cluster promotes pluripotency maintenance by regulating cell cycle phase distribution in mouse embryonic stem cells. Differentiation 81: 11–24, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Liu G, Friggeri A, Yang Y, Park YJ, Tsuruta Y, Abraham E. miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proc Natl Acad Sci USA 106: 15819–15824, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lomazzi M, Moroni MC, Jensen MR, Frittoli E, Helin K. Suppression of the p53- or pRB-mediated G1 checkpoint is required for E2F-induced S-phase entry. Nat Genet 31: 190–194, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Martinez LA, Goluszko E, Chen HZ, Leone G, Post S, Lozano G, Chen Z, Chauchereau A. E2F3 is a mediator of DNA damage-induced apoptosis. Mol Cell Biol 30: 524–536, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarthy JJ, Esser KA, Peterson CA, Dupont-Versteegden EE. Evidence of MyomiR network regulation of beta-myosin heavy chain gene expression during skeletal muscle atrophy Physiol Genomics 39: 219–226, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mor E, Cabilly Y, Goldshmit Y, Zalts H, Modai S, Edry L, Elroy-Stein O, Shomron N. Species-specific microRNA roles elucidated following astrocyte activation. Nucleic Acids Res 39: 3710–3723, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naguibneva I, Ameyar-Zazoua M, Polesskaya A, Ait-Si-Ali S, Groisman R, Souidi M, Cuvellier S, Harel-Bellan A. The microRNA miR-181 targets the homeobox protein Hox-A11 during mammalian myoblast differentiation. Nat Cell Biol 8: 278–284, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Nakasa T, Ishikawa M, Shi M, Shibuya H, Adachi N, Ochi M. Acceleration of muscle regeneration by local injection of muscle-specific microRNAs in rat skeletal muscle injury model. J Cell Mol Med 14: 2495–2505, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pearce GW. Histopathology of voluntary muscle. Postgrad Med J 41: 294–312, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearson CM. Histopathological features of muscle in the preclinical stages of muscular dystrophy. Brain 85: 109–120, 1962. [DOI] [PubMed] [Google Scholar]
- 44.Polager S, Ginsberg D. E2F - at the crossroads of life and death. Trends Cell Biol 18: 528–535, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature 435: 948–953, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Ren B, Cam H, Takahashi Y, Volkert T, Terragni J, Young RA, Dynlacht BD. E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev 16: 245–256, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ridgeway AG, Skerjanc IS. Pax3 is essential for skeletal myogenesis and the expression of Six1 and Eya2. J Biol Chem 276: 19033–19039, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Saldanha AJ. Java Treeview–extensible visualization of microarray data. Bioinformatics 20: 3246–3248, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz LM, Gao Z, Brown C, Parelkar SS, Glenn H. Cell death in myoblasts and muscles. Methods Mol Biol 559: 313–332, 2009. [DOI] [PubMed] [Google Scholar]
- 50.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell 102: 777–786, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Small EM, O'Rourke JR, Moresi V, Sutherland LB, McAnally J, Gerard RD, Richardson JA, Olson EN. Regulation of PI3-kinase/Akt signaling by muscle-enriched microRNA-486. Proc Natl Acad Sci USA 107: 4218–4223, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smyth GK. Limma: linear models for microarray data. In: Bioinformatics and Computational Biology Solutions using R and Bioconductor, edited by Gentleman VC, Dudoit S, Irizarry R, Huber W. New York: Springer, 2005, p. 397–420. [Google Scholar]
- 53.Stevens C, La Thangue NB. E2F and cell cycle control: a double-edged sword. Arch Biochem Biophys 412: 157–169, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Sun D, Lee YS, Malhotra A, Kim HK, Matecic M, Evans C, Jensen RV, Moskaluk CA, Dutta A. miR-99 family of microRNAs suppresses the expression of prostate-specific antigen and prostate cancer cell proliferation. Cancer Res 71: 1313–1324, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun D, Martinez CO, Ochoa O, Ruiz-Willhite L, Bonilla JR, Centonze VE, Waite LL, Michalek JE, McManus LM, Shireman PK. Bone marrow-derived cell regulation of skeletal muscle regeneration. FASEB J 23: 382–395, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Surdziel E, Cabanski M, Dallmann I, Lyszkiewicz M, Krueger A, Ganser A, Scherr M, Eder M. Enforced expression of miR-125b affects myelopoiesis by targeting multiple signaling pathways. Blood 117: 4338–4348, 2011. [DOI] [PubMed] [Google Scholar]
- 57.Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol 288: R345–R353, 2005. [DOI] [PubMed] [Google Scholar]
- 58.van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA, Kelm RJ, Jr, Olson EN. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell 17: 662–673, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walsh K. Coordinate regulation of cell cycle and apoptosis during myogenesis. Prog Cell Cycle Res 3: 53–58, 1997. [DOI] [PubMed] [Google Scholar]
- 60.Wang H, Garzon R, Sun H, Ladner KJ, Singh R, Dahlman J, Cheng A, Hall BM, Qualman SJ, Chandler DS, Croce CM, Guttridge DC. NF-kappaB-YY1-miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer Cell 14: 369–381, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Z, Liu Y, Han N, Chen X, Yu W, Zhang W, Zou F. Profiles of oxidative stress-related microRNA and mRNA expression in auditory cells. Brain Res 1346: 14–25, 2010. [DOI] [PubMed] [Google Scholar]
- 62.Williams AH, Liu N, van Rooij E, Olson EN. MicroRNA control of muscle development and disease. Curr Opin Cell Biol 21: 461–469, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams BA, Ordahl CP. Pax-3 expression in segmental mesoderm marks early stages in myogenic cell specification. Development 120: 785–796, 1994. [DOI] [PubMed] [Google Scholar]
- 64.Wong CF, Tellam RL. MicroRNA-26a targets the histone methyltransferase Enhancer of Zeste homolog 2 during myogenesis. J Biol Chem 283: 9836–9843, 2008. [DOI] [PubMed] [Google Scholar]
- 65.Wong SS, Ritner C, Ramachandran S, Aurigui J, Pitt C, Chandra P, Ling VB, Yabut O, Bernstein HS. miR-125b promotes early germ layer specification through Lin28/let-7d and preferential differentiation of mesoderm in human embryonic stem cells. PLoS One 7: e36121, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamasaki L, Jacks T, Bronson R, Goillot E, Harlow E, Dyson NJ. Tumor induction and tissue atrophy in mice lacking E2F-1. Cell 85: 537–548, 1996. [DOI] [PubMed] [Google Scholar]
- 67.Yan Z, Choi S, Liu X, Zhang M, Schageman JJ, Lee SY, Hart R, Lin L, Thurmond FA, Williams RS. Highly coordinated gene regulation in mouse skeletal muscle regeneration. J Biol Chem 278: 8826–8836, 2003. [DOI] [PubMed] [Google Scholar]
- 68.Yuasa K, Hagiwara Y, Ando M, Nakamura A, Takeda S, Hijikata T. MicroRNA-206 is highly expressed in newly formed muscle fibers: implications regarding potential for muscle regeneration and maturation in muscular dystrophy. Cell Struct Funct 33: 163–169, 2008. [DOI] [PubMed] [Google Scholar]
- 69.Zacharias JM, Anderson JE. Muscle regeneration after imposed injury is better in younger than older mdx dystrophic mice. J Neurol Sci 104: 190–196, 1991. [DOI] [PubMed] [Google Scholar]
- 70.Zhou M, Liu Z, Zhao Y, Ding Y, Liu H, Xi Y, Xiong W, Li G, Lu J, Fodstad O, Riker AI, Tan M. MicroRNA-125b confers the resistance of breast cancer cells to paclitaxel through suppression of pro-apoptotic Bcl-2 antagonist killer 1 (Bak1) expression. J Biol Chem 285: 21496–21507, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.