Abstract
A 7-month-old Australian kelpie dog and a 14-month-old Labrador retriever dog were diagnosed with an uncommon form of cerebellar abiotrophy called cerebellar granuloprival degeneration. This was characterized by a loss of the granular neurons with relative sparing of the Purkinje neurons.
Résumé
Dégénérescence cérébelleuse granuloprive chez un chien Kelpie australien et un chien Labrador retriever. Un chien Kelpie australien âgé de 7 mois et un chien Labrador retriever âgé de 14 mois ont été diagnostiqués avec une forme rare d’abiotrophie cérébelleuse appelée dégénérescence cérébelleuse granuloprive. Elle a été caractérisée par la perte de neurones granulaires en épargnant en grande partie les neurones de Purkinje.
(Traduit par Isabelle Vallières)
Cerebellar abiotrophy (CA) is defined as the premature degeneration of formed cerebellar tissue, representing an intrinsic metabolic anomaly that results in progressive signs of cerebellar dysfunction (1). Cerebellar cortical degeneration (CCD) is a synonymous term more recently introduced to reflect the inability to completely rule out effects of extrinsic factors leading to degenerative changes. In dogs, CA/CCD has been widely described in the following breeds: kerry blue terrier (2,3), Gordon setter (4,5), old English sheepdog (6), Australian kelpie (7–9), American Staffordshire terrier (10–12), English bulldog (13), Scottish terrier (14,15), Labrador retriever (16,17), boxer (18), Rhodesian ridgeback (19), and the miniature schnauzer (20).
While a neonatal form has been described, for instance in the beagle (21), most cases of CA/CCD are postnatal with affected animals being normal at birth and clinical signs occurring early in life (< 6 mo of age) or late in adulthood (late-onset) with varying rates of progression. An autosomal recessive pattern of inheritance is usually suspected (1,2,4,6,7,11,22).
The most frequently reported form of CA/CCD is characterized by a loss of Purkinje cell neurons, often with retrograde degeneration of the granular cells (23). However, loss of the granular cell layer with relative sparing of Purkinje cells, called cerebellar granuloprival degeneration, has been sporadically reported in dogs (9,22,24–28).
This article describes 2 cases of histopathologically confirmed cerebellar granuloprival degeneration in an Australian kelpie dog and a Labrador retriever dog.
Case descriptions
Case 1
A 7-month-old, female intact, Australian kelpie dog (Case 1) was presented to the Neurology Service of the Ontario Veterinary College Health Science Center (OVC-HSC) due to progressive ataxia and abnormal behavior. Clinical signs began at 6 wk of age with wide, lateral excursions of the head that progressed to dysmetria, intermittent circling to the right, intention head tremors, and compulsive behavior (licking and fly biting). No traumatic episode was noted nor was there any known exposure to toxins. At 11 and 14 wk of age neurological examination revealed an absent menace response with the left eye, and intention head tremors. Galactocerebroside activity analysis was performed on whole blood for suspected globoid cell leukodystrophy and results were within normal reference range (0.9 nmol/h/mg protein). Toxoplasma gondii (IgG immunofluorescent test) and Neospora caninum enzyme-linked immunosorbent assay (ELISA) serum titers were negative.
On presentation at the OVC-HSC, the results of a general examination were unremarkable. Neurological examination revealed wide lateral head excursions, circling to the left and right sides, intention head tremors, hypermetria in all 4 limbs, and decreased menace response with the left eye. The results of the rest of the neurological examination were unremarkable. Neuroanatomical localization was mostly consistent with a cerebellar lesion. The episodic compulsive behavior reported by the owners suggested involvement of the thalamocortex (limbic system), although it was uncertain if this was an unrelated behavioral non-neurological dysfunction. Further diagnostics were declined by the client. The patient was presented again 1 mo later due to progression of the described clinical signs including a bilateral menace response deficit. At that time, the owner opted for euthanasia. A postmortem examination was performed.
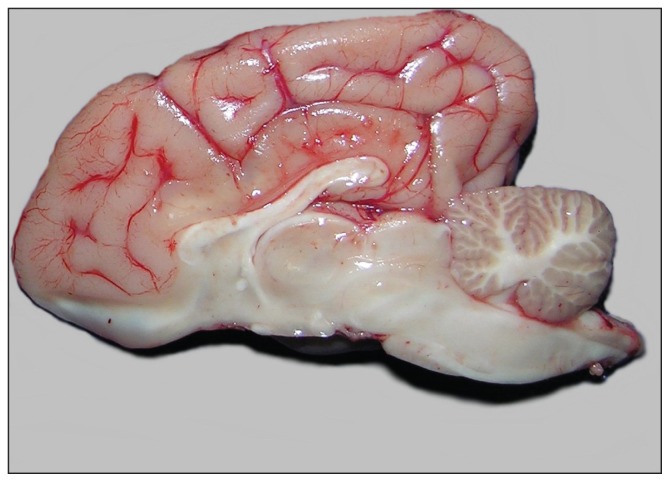
At postmortem examination, there was a subtle dorsoventral flattening of the cerebellum and cerebrum, both of which conformed to the shape of the cranial vault (Figure 1). This suggested that the calvarium and central nervous system developed simultaneously and sequentially. The brain was otherwise unremarkable. The brain was fixed in 10% neutral-buffered formalin, sectioned and embedded in paraffin wax. The cerebellum was sectioned midsagittally through the vermis, followed by bilateral transverse sections in order to evaluate the respective cerebellar lobes. Tissue sections were stained with hematoxylin and eosin (H&E), periodic acid Schiff (PAS), and Luxol fast blue (LFB-Holmes) for histological evaluation.
Figure 1.
Mid-sagittal section of the brain of the dog in case 1. Note the dorsoventral flattening of the cerebellum.
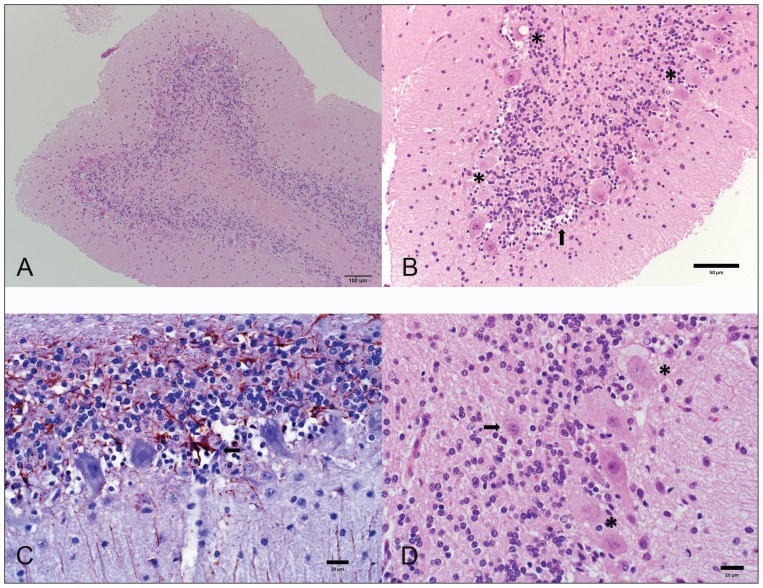
Microscopically, all cerebellar folia were small and had wide cerebellar sulci. There was an extensive and bilaterally asymmetrical thinning of all the cerebellar cortex, including the granular, Purkinje, and molecular layers, such that the cortex ranged from 340 to 500 μm thick; normal thickness should be approximately 650 μm at the end of cerebellar development (29). The thickness of the granular layer varied from 47 μm at its thinnest point to 180 μm at its widest; normal thickness is approximately 200 μm (29). There was marked hypocellularity of especially the granular layer, and also a marked reduction in the number of granule neurons, Golgi cells, Purkinje neuron axons, and myelinated fibers of the white matter (Figure 2). The remaining granule cells were loosely arranged and cell nuclei of the granular layer in these areas were small and condensed with euchromatic chromatin. Purkinje cells were tightly packed with approximately 9 Purkinje neuronal cell bodies present in a 350 μm length of the Purkinje cell layer as opposed to 4 Purkinje neuronal cell bodies in an age matched normal cerebellum, suggesting collapse of the neuropil. There were randomly scattered groups of individual empty Purkinje cell baskets (empty baskets) suggesting that about 30% of Purkinje cells were missing. Varied stages of cellular degeneration of the remaining Purkinje cells were identified, including central chromatolysis, karyolysis, and pyknosis of nuclei, affecting approximately 40% of the remaining Purkinje cells (Figure 2B). Their cytoplasm commonly contained irregular, pale, homogenous and glassy, basophilic material and often had overlying clear microvesicular vacuolization. Astrocytic processes surrounding empty baskets were accentuated with a glial fibrillary acidic protein (GFAP) immunohistochemical (IHC) stain (Figure 2C). Baskets were occasionally filled with small numbers of hypertrophic plump astrocytes with large vesicular nuclei (Bergmann’s glia proliferation). Rare randomly scattered, small, 15 × 20 μm, angular polygonal neuronal cell bodies, with small amounts of amphophilic, finely granular cytoplasm, and large, round nuclei with euchromatic chromatin and a single prominent nucleolus were present within the granular and rarely molecular layer of the cerebellar cortex (Figure 2D). Although those were interpreted as Golgi cells (appeared to be more prominent due to the granular cell depletion), they could not definitively be differentiated from Purkinje cells without the use of immunohistochemistry. No changes were observed in the cerebellar nuclei, cerebellar peduncles, pontine nuclei, vestibular nuclei, olivary nuclei, spinocerebellar tracts, or the limbic system. Staining with PAS and LFB-Holmes did not identify any further abnormalities. Microscopic findings were consistent with cerebellar granuloprival degeneration.
Figure 2.
Photomicrographs of the cerebellum of the dog in case 1. (A,B) Severe depletion of granular neurons with secondary degeneration of the Purkinje cells (asterisk) and empty Purkinje cell baskets with Bergmann’s glia proliferation. Hematoxylin and eosin (H&E). (C) Astrocytic processes surround empty baskets (arrow). Glial fibrillary acid protein (GFAP) immunohistochemical (IHC) stain. (D) Neuronal cell body within the granular cell layer interpreted as a Golgi cell (arrow), and presence of a degenerate Purkinje cell (asterisk).
Case 2
A 14-month-old, spayed female, Labrador retriever dog was presented to the Neurology Service of the Southern Counties Veterinary Specialists due to a 4-week history of progressive lack of coordination involving all 4 limbs. The owner reported that the dog initially exhibited pelvic limb ataxia which progressed to the thoracic limbs after approximately 1 wk. No traumatic episode was noted nor was there any known exposure to toxins. On initial presentation, the results of a general physical examination were unremarkable. Neurological examination revealed a wide-based stance in the thoracic limbs, intermittent swaying of the body from side to side, moderate spasticity and hypermetric ataxia of all 4 limbs (worse in the pelvic limbs), fine intention tremors of the head, and decreased menace responses bilaterally. The results of the remainder of the neurological examination were normal. The neuroanatomical localization was consistent with a cerebellar lesion.
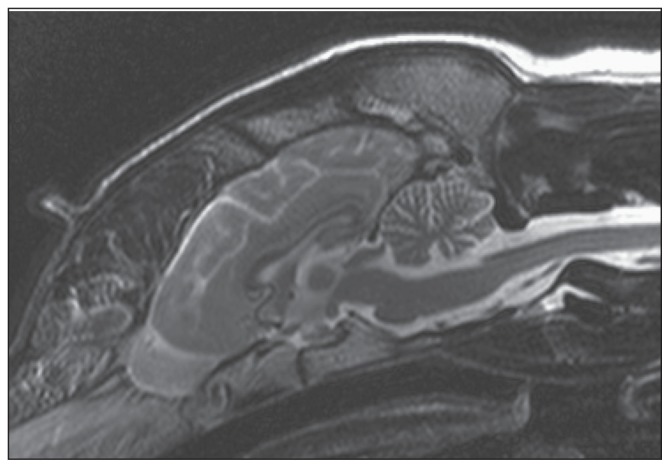
Complete blood (cell) count, serum biochemistry profile, urinalysis, and thoracic radiographs were unremarkable. A magnetic resonance imaging (MRI) study of the brain was performed (Philips Intera 1.0 T; Philips Healthcare, Guildford, Surrey, UK). Routine T1-weighted and T2-weighted sequences in sagittal, transverse, and dorsal planes were obtained (3-mm slice thickness) and revealed an increase in the amount of cerebrospinal fluid (CSF) between the folia around the cerebellum due to thinner cerebellar folia and wider sulci, suggesting cerebellar cortical atrophy. Those changes were particularly evident on sagittal T2-weighted images (Figure 3). Cerebrospinal fluid (CSF) analysis did not reveal abnormalities (0 nucleated cells/μL, reference range: 0 to 3 nucleated cells/μL; total protein concentration: 0.17 g/L; reference value: < 0.3 g/L). Polymerase chain reaction (PCR) for Neospora caninum, canine distemper virus, and Toxoplasma gondii were negative on both blood and CSF samples. A presumptive diagnosis of CA/CCD was made.
Figure 3.
T2-weighted sagittal magnetic resonance image (MRI) showing an increased amount of cerebrospinal fluid within the cerebellar folia suggestive of cerebellar cortical atrophy in the dog in case 2.
The dog was presented 4 mo later due to worsening of the clinical signs. At that time, the animal had severe generalized ataxia; the dog was still able to walk but fell down or collapsed frequently. Due to the guarded prognosis the owners opted for euthanasia and postmortem examination.
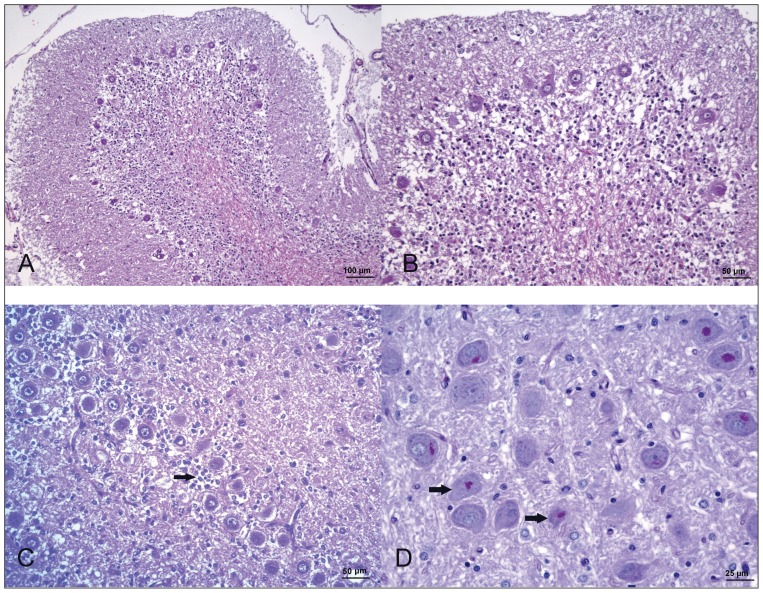
At the postmortem examination, the cerebellum appeared small with wide cerebellar cortical sulci. The brain was otherwise unremarkable. The brain was fixed in 10% neutral-buffered formalin, then sectioned and embedded in paraffin wax. Tissue sections were stained with H&E, and PAS for histological evaluation. Microscopic examination revealed a generalized and marked loss of granule neurons, affecting mainly the vermis, and with a minor intensity, the cerebellar hemispheres (Figures 4A–C). Few Golgi neurons were observed in the sparse granular layer without showing any morphological change. The Purkinje cell layer had a mild decrease in cell number, showing focal clusters of these neurons and several empty baskets. Most of the Purkinje cells showed central chromatolysis. Spheroids corresponding to axons of Purkinje cells were detected in the sparse granular layer. Bergman’s glia proliferation and hypertrophy were also present (Figure 4C). A mild spongiosis of the white matter was observed affecting mainly cerebellum and cerebellar peduncles. No changes were observed in the cerebellar nuclei, pontine nuclei, and spinocerebellar tracts. Eosinophilic, PAS-positive, granular material was detected in some of the remaining cerebellar granular neurons and also in the perikarya of neurons of vestibular and olivary nuclei in a bilateral and symmetrical pattern (Figure 4D). Based on those findings, cerebellar granuloprival degeneration was diagnosed.
Figure 4.
Photomicrographs of the cerebellum of the dog in case 2. (A–C) Severe depletion of granular neurons with mild decrease in Purkinje cell number, empty baskets, and Bermann’s glia proliferation (arrow) H&E. (D) Presence of PAS-positive material in the perikarya of vestibular nucleus neurons (arrow). PAS.
Discussion
The clinical signs in both patients, including the loss of menace response in case 1, were attributed to cerebellar disease classified as CA/CCD. Although loss of the Purkinje cell neurons is the most commonly described form of CA/CCD, another form called cerebellar granuloprival degeneration characterized by a specific degeneration of the granular cell neurons while sparing the Purkinje cells has been sporadically reported in several canine breeds: border collie (22), Brittany spaniel (24), Italian hound (25), coton de Tuléar (26), lagotto Romagnolo (27), Bavarian mountain dog (28), and, recently, the Australian kelpie (9). Other degenerative changes affecting cerebellar and related brainstem nuclei, such as neuronal eosinophilic granules, have also been described in some of those cases (15,24,26).
A report of 3 Labrador retriever puppies from the same litter as the dog herein (16) described histological findings of a predominantly Purkinje neuron degeneration throughout the entire cerebellum, with the rostral vermis most severely affected. The onset of clinical signs ranged from 9 to 17 wk of age with rapid deterioration, in contrast to the slow progression of clinical signs observed in an 8-month-old Labrador retriever with comparable selective degeneration of Purkinje cells (17). Those findings are in contrast to our cases in which sparing of the Purkinje cell layer and specific degeneration of the granular cells was diagnosed, thus representing the first report of this cerebellar granuloprival degeneration form of CA/CCD in the Labrador retriever.
Thomas and Robertson (7) described the findings in 5 Australian kelpie littermates with clinical signs of progressive ataxia, hypermetria, and head tremors beginning between 5 to 12 wk of age. Histopathologically, the more common CA/CCD form with loss of Purkinje cells and varying degrees of reduction in the granular cell layer was diagnosed in those affected puppies between 8 wk and 5.5 mo of age. However, recent findings suggest that the granuloprival form occurs in Australian kelpies as well, particularly in young individuals (9). Those authors suggested that Purkinje cells are lost in more severe, long-surviving dogs with more pronounced cerebellar changes secondary to the granule cell depletion since granule neurons are facilitatory to Purkinje cells. Preservation of Purkinje cells, therefore, could be found in younger puppies and depletion of both cell groups would be evident in older dogs. Nevertheless, the first description of the CA/CCD in the Australian kelpie (7) included young puppies (8 wk of age) in which regional loss of Purkinje cells was detected. This suggests that both forms coexist in the Australian kelpie, though the incidence of each one remains to be investigated as well as the potential for different underlying etiologies.
The reason for a selective depletion of granular cells still needs to be elucidated. It is hypothesized that cerebellar granuloprival degeneration is usually the result of an inherited disturbance of granular cell development, but Purkinje cells survive retrograde degeneration since their major excitatory input is received from the climbing fibers coming from the olivary nucleus (1,24,28). However, the granular neurons are considered facilitatory to Purkinje cells as well, and some authors have suggested that Purkinje cells can be lost as result of granular cell depletion in longer-affected individuals (9).
Inflammatory-infectious etiologies should be considered, although unlikely in our cases due to the lack of evidence of inflammation. Inflammatory foci were found in coton de Tuléar dogs and a genetically based immune-mediated granule cell destruction pathophysiology was proposed (26). Canine herpes virus infection has been reported to cause similar reduction of the granular cell layer but with associated loss of Purkinje cells and foci of infiltrating mononuclear cells and malacia (30). Intrauterine or perinatal infection with feline parvovirus (panleukopenia virus) in cats can cause hypoplasia of the granular cell layer resulting in clinical signs of cerebellar dysfunction (31,32). Canine parvovirus has been identified in dogs with cerebellar hypoplasia but not with CA/CCD (33). None of these features were present in our 2 cases, showing changes more consistent with previous reports of a suspected non-inflammatory probably inherited process (24,25,28).
A definitive diagnosis of CA/CCD requires histopathological confirmation. Nevertheless, CA/CCD can be suspected when other differential diagnoses have been ruled out (as intracranial neoplasia, encephalitis, or toxicities) and supported by the MRI findings. Although MRI images can be absolutely normal, abnormalities could be particularly detected in mid-sagittal T2-weighted sequences, characterized by a decreased cerebellar size and a reduced thickness of the cerebellar folia associated with an increased amount of CSF in the sulci (11,12,14,28,34).
Interestingly, seizures were reported in 1 of the Australian kelpie puppies included by Thomas and Robertson (7), although a more extended description of the episodes was missing. The episodic compulsive behavior reported by the owners in Case 1 herein could certainly be a seizure. However, on the postmortem examination no abnormal histopathological findings were detected in either case in the cerebrum and it is uncertain if those episodes were truly an epileptic seizure. Interestingly, behavioral changes, including lack of confidence and aggression towards other dogs in the household, were reported by owners in 2 of the 63 dogs included in a retrospective study on CCD in the American Staffordshire terrier (11). Although neuronal death was restricted to the cerebellar cortex in those dogs, in 1 dog MRI was suggestive of cerebral atrophy. Those authors noted that cognitive deficits and a more diffusely neuronal loss can occur in humans with adult-onset spinocerebellar ataxia (the suggested human counterpart for CCD in the American Staffordshire terrier).
Given the paucity of literature with histological descriptions of CA/CCD in the Australian kelpie and Labrador retriever, these 2 cases could represent a subclass of the disease or be a completely different form for these breeds. While the previously reported CA/CCD cases in the Australian kelpie are consistent with an autosomal recessive pattern of inheritance (7), no pedigree analysis was available for this case, though the bitch and sire were believed to be clinically normal.
A recent study identified the mutation causing CA/CCD in American Staffordshire terriers, confirming that CA/CCD in this breed represents a model of a late-onset form of neuronal ceroid lipofuscinosis, a neurodegenerative lysosomal disorder (35). In the Australian kelpie, 3 candidate genes have been eliminated (8); a more recent study identified a candidate region containing 29 genes, although none of them are known to cause ataxia (9).
To the authors’ knowledge, this is the first report of a granuloprival form of cerebellar cortical degeneration in the Labrador retriever, and the first case reported in an Australian kelpie in North America. With the search for causative mutations, either for screening purposes or targets for future gene therapy, the exact histological classification will be necessary to ensure accuracy as the mutation responsible for cerebellar granuloprival degeneration might differ from that which causes CA/CCD, primarily affecting the Purkinje cell layer. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.De Lahunta A, Glass E, editors. Veterinary Neuroanatomy and Clinical Neurology. 3rd ed. St Louis, Missouri: Saunders Elsevier; 2009. pp. 363–388. [Google Scholar]
- 2.Deforest ME, Eger CE, Basrur PK. Hereditary cerebellar neuronal abiotrophy in a Kerry Blue Terrier dog. Can Vet J. 1978;19:198–202. [PMC free article] [PubMed] [Google Scholar]
- 3.Montgomery DL, Storts RW. Hereditary striatonigral and cerebello-olivary degeneration of the Kerry Blue Terrier. I. Gross and light microscopic central nervous system lesions. Vet Pathol. 1983;20:143–159. doi: 10.1177/030098588302000202. [DOI] [PubMed] [Google Scholar]
- 4.De Lahunta A, Fenner WR, Indrieri RJ, Mellik PW, Gardner S, Bell JS. Hereditary cerebellar cortical abiotrophy in the Gordon Setter. J Am Vet Med Assoc. 1980;177:538–541. [PubMed] [Google Scholar]
- 5.Steinberg HS, Troncoso JC, Cork LC, Price DL. Clinical features of inherited cerebellar degeneration in Gordon setters. J Am Vet Med Assoc. 1981;179:886–890. [PubMed] [Google Scholar]
- 6.Steinberg HS, Van Winkle T, Bell JS, de Lahunta A. Cerebellar degeneration in Old English Sheepdogs. J Am Vet Med Assoc. 2000;217:1162–1165. doi: 10.2460/javma.2000.217.1162. [DOI] [PubMed] [Google Scholar]
- 7.Thomas JB, Robertson D. Hereditary cerebellar abiotrophy in Australian kelpie dogs. Aust Vet J. 1989;66:301–302. doi: 10.1111/j.1751-0813.1989.tb13959.x. [DOI] [PubMed] [Google Scholar]
- 8.Shearman JR, Lau VM, Wilton AN. Elimination of SETX, SYNE1 and ATCAY as the cause of cerebellar abiotrophy in Australian Kelpies. Anim Genet. 2008;39:573. doi: 10.1111/j.1365-2052.2008.01753.x. [DOI] [PubMed] [Google Scholar]
- 9.Shearman JR, Cook RW, McCowan C, Fletcher JL, Taylor RM, Wilton AN. Mapping cerebellar abiotrophy in Australian Kelpies. Anim Genet. 2011;42:675–678. doi: 10.1111/j.1365-2052.2011.02199.x. [DOI] [PubMed] [Google Scholar]
- 10.Hanzlicek D, Kathmann I, Bley T, et al. Cerebellar cortical abiotrophy in American Staffordshire terriers: Clinical and pathological features of 3 cases. Schweiz Arch Tierheilkd. 2003;145:369–375. doi: 10.1024/0036-7281.145.8.369. [DOI] [PubMed] [Google Scholar]
- 11.Olby N, Blot S, Thibaud JL, et al. Cerebellar cortical degeneration in adult American Staffordshire Terriers. J Vet Intern Med. 2004;18:201–208. doi: 10.1892/0891-6640(2004)18<201:ccdiaa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Henke D, Böttcher P, Doherr MG, Oechtering G, Flegel T. Computer-assisted magnetic resonance imaging brain morphometry in American Staffordshire Terriers with cerebellar cortical degeneration. J Vet Intern Med. 2008;22:969–975. doi: 10.1111/j.1939-1676.2008.0138.x. [DOI] [PubMed] [Google Scholar]
- 13.Gandini G, Botteron C, Brini E, Fatzer R, Diana A, Jaggy A. Cerebellar cortical degeneration in three English bulldogs: Clinical and neuro-pathological findings. J Small Anim Pract. 2005;46:291–294. doi: 10.1111/j.1748-5827.2005.tb00323.x. [DOI] [PubMed] [Google Scholar]
- 14.Van der Merwe LL, Lane E. Diagnosis of cerebellar cortical degeneration in a Scottish terrier using magnetic resonance imaging. J Small Anim Pract. 2001;42:409–412. doi: 10.1111/j.1748-5827.2001.tb02491.x. [DOI] [PubMed] [Google Scholar]
- 15.Urkasemsin G, Linder KE, Bell JS, de Lahunta A, Olby NJ. Hereditary cerebellar degeneration in Scottish Terriers. J Vet Intern Med. 2010;24:565–570. doi: 10.1111/j.1939-1676.2010.0499.x. [DOI] [PubMed] [Google Scholar]
- 16.Perille AL, Baer K, Joseph RJ, Carillo JM, Averill DR. Postnatal cerebellar cortical degeneration in Labrador Retriever puppies. Can Vet J. 1991;32:619–621. [PMC free article] [PubMed] [Google Scholar]
- 17.Bildfell RJ, Mitchell SK, de Lahunta A. Cerebellar cortical degeneration in a Labrador retriever. Can Vet J. 1995;36:570–572. [PMC free article] [PubMed] [Google Scholar]
- 18.Gumber S, Cho DY, Morgan TW. Late onset cerebellar abiotrophy in a Boxer dog. Vet Med Int. 2010;2010:Article ID 406275. doi: 10.4061/2010/406275. 4 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chieffo C, Stalis IH, Van Winkle TJ, Haskins ME, Patterson DF. Cerebellar Purkinje’s cell degeneration and coat colour dilution in a family of Rhodesian Ridgeback dogs. J Vet Intern Med. 1994;8:112–116. doi: 10.1111/j.1939-1676.1994.tb03207.x. [DOI] [PubMed] [Google Scholar]
- 20.Berry ML, Blas-Machado U. Cerebellar abiotrophy in a miniature schnauzer. Can Vet J. 2003;44:657–659. [PMC free article] [PubMed] [Google Scholar]
- 21.Kent M, Glass E, de Lahunta A. Cerebellar cortical abiotrophy in a beagle. J Small Anim Pract. 2000;41:321–323. doi: 10.1111/j.1748-5827.2000.tb03210.x. [DOI] [PubMed] [Google Scholar]
- 22.Sandy JR, Slocombe RE, Mitten RW, Jedwab D. Cerebellar abiotrophy in a family of Border Collie dogs. Vet Pathol. 2002;39:736–738. doi: 10.1354/vp.39-6-736. [DOI] [PubMed] [Google Scholar]
- 23.De Lahunta A. Abiotrophy in domestic animals: A review. Can J Vet Res. 1990;54:65–76. [PMC free article] [PubMed] [Google Scholar]
- 24.Tatalick LM, Marks SL, Baszler TV. Cerebellar abiotrophy characterized by granular cell loss in a Brittany. Vet Pathol. 1993;30:385–388. doi: 10.1177/030098589303000411. [DOI] [PubMed] [Google Scholar]
- 25.Cantile C, Salvadori C, Modenato M, Arispici M, Fatzer R. Cerebellar granuloprival degeneration in an Italian hound. J Vet Med A Physiol Pathol Clin Med. 2002;49:523–525. doi: 10.1046/j.1439-0442.2002.00495.x. [DOI] [PubMed] [Google Scholar]
- 26.Tipold A, Fatzer R, Jaggy A, Moore P, Vandevelde M. Presumed immune-mediated cerebellar granuloprival degeneration in the Coton de Tuléar breed. J Neuroimmunol. 2000;110:130–133. doi: 10.1016/s0165-5728(00)00330-1. [DOI] [PubMed] [Google Scholar]
- 27.Jokinen TS, Rusbridge C, Steffen F, et al. Cerebellar cortical abiotrophy in Lagotto Romagnolo dogs. J Small Anim Pract. 2007;48:470–473. doi: 10.1111/j.1748-5827.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- 28.Flegel T, Matiasek K, Henke D, Grevel V. Cerebellar cortical degeneration with selective granule cell loss in Bavarian mountain dogs. J Small Anim Pract. 2007;48:462–465. doi: 10.1111/j.1748-5827.2006.00257.x. [DOI] [PubMed] [Google Scholar]
- 29.Phemister RD, Young S. The postnatal development of the canine cerebellar cortex. J Comp Neurol. 1968;134:243–254. doi: 10.1002/cne.901340209. [DOI] [PubMed] [Google Scholar]
- 30.Percy DH, Carmichael LE, Albert DM, King JM, Jonas AM. Lesions in puppies surviving infection with canine herpesvirus. Vet Pathol. 1971;8:37–53. doi: 10.1177/030098587100800106. [DOI] [PubMed] [Google Scholar]
- 31.Johnson RH, Margolis G, Kilham L. Identity of feline ataxia virus with feline panleucopenia virus. Nature. 1967;214:175–177. doi: 10.1038/214175a0. [DOI] [PubMed] [Google Scholar]
- 32.Summers BA, Cummings JF, de Lahunta A, editors. Veterinary neuropathology. St. Louis, Missouri: Mosby-Year Book; 1995. pp. 300–307. [Google Scholar]
- 33.Schatzberg SJ, Haley NJ, Barr SC, et al. Polymerase chain reaction (PCR) amplification of parvoviral DNA from the brains of dogs and cats with cerebellar hypoplasia. J Vet Intern Med. 2003;17:538–544. doi: 10.1111/j.1939-1676.2003.tb02475.x. [DOI] [PubMed] [Google Scholar]
- 34.Thames RA, Robertson ID, Flegel T, et al. Development of a morphometric magnetic resonance image parameter suitable for distinguishing between normal dogs and dogs with cerebellar atrophy. Vet Radiol Ultrasound. 2010;51:246–253. doi: 10.1111/j.1740-8261.2009.01655.x. [DOI] [PubMed] [Google Scholar]
- 35.Abitbol M, Thibaud JL, Olby NJ, et al. A canine Arylsulfatase G (ARSG) mutation leading to a sulfatase deficiency is associated with neuronal ceroid lipofuscinosis. Proc Natl Acad Sci USA. 2010;107:14775–14780. doi: 10.1073/pnas.0914206107. [DOI] [PMC free article] [PubMed] [Google Scholar]