Abstract
A cluster of canine parainfluenza virus infections was identified in a veterinary referral hospital. While hospital-associated outbreaks of canine parainfluenza virus infection have not been previously reported, veterinary hospitals possess some of the same risk factors that may be present in traditional high-risk sites such as kennels. Hospital-associated transmission of canine respiratory pathogens, therefore, must be considered.
Résumé
Éclosion de maladies respiratoires dans une clinique vétérinaire associée à l’infection par le virus parainfluenza canin. Un groupe d’infections par le virus parainfluenza canin a été identifié dans une clinique vétérinaire spécialisée. Même si des éclosions d’infections par le virus parinfluenza canin n’ont pas été signalées antérieurement, les cliniques vétérinaires présentent certains des mêmes facteurs de risque qui peuvent être présents dans des lieux à risque traditionnellement élevé, comme les chenils. Par conséquent, il est nécessaire de considérer comme possible la transmission d’agents pathogènes respiratoires canins associée aux cliniques.
(Traduit par Isabelle Vallières)
Hospital-associated (HA) infections are an ever-present risk in veterinary healthcare facilities, and there has been increasing concern about these infections in recent years, largely due to the emergence and dissemination of multidrug resistant pathogens such as methicillin-resistant staphylococci and multi-drug resistant Gram-negative bacteria (1–4). There are limited reports of HA transmission and outbreaks of other pathogens such as Clostridium difficile(5) and Salmonella(6,7) but a wide range of potential HA pathogens can be encountered.
Canine infectious respiratory disease complex (CIRDC) (previously referred to as infectious tracheobronchitis or kennel cough) is a syndrome that can be caused by a variety of pathogens, most notably canine adenovirus type 2, canine parainfluenza virus (CPiV), canine influenza, canine herpesvirus, canine respiratory coronavirus, Bordetella bronchiseptica, Streptococcus zooepidemicus, and Mycoplasma spp., sometimes in combination (8,9). This disease complex (CIRDC) typically causes mild to moderate, self-limiting upper respiratory tract infection, mainly characterized by cough with a relatively small percentage of cases developing complications such as pneumonia. Fatal infections are uncommon (8).
Canine infectious respiratory disease complex is most common in situations in which large numbers of dogs are kept together, and outbreaks are classically associated with kennels and shelters (10,11). Because veterinary clinics may present the same opportunities for mixing of large numbers of dogs, HA-CIRDC is also a potential problem, and HA-CIRDC caused by canine herpesvirus has recently been reported (8). This report describes an outbreak of respiratory disease in a veterinary hospital associated with CPiV infection.
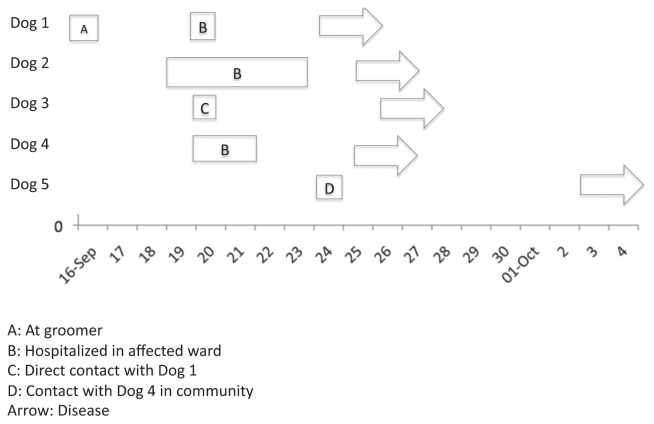
On September 20, 2011, a 1-year-old male castrated standard poodle dog was presented to the Ontario Veterinary College Health Sciences Centre (OVC-HSC) for an ophthalmological examination (Figure 1). He was an otherwise healthy dog that had been vaccinated against canine distemper, canine adenovirus type 2, CPiV, and canine parvovirus (DA2PP) with a 3-dose parenteral series on February 28, March 30, and May 12, 2011. He had not recently been at a kennel or shelter, but had direct contact with other dogs at a dog park 5 days earlier and had been at a grooming facility 4 days earlier. No evidence of respiratory disease was present at initial examination. He was housed in a general hospital ward in a floor-level cage and discharged later that day. On September 24, 2011, he was re-admitted for examination of an acute onset of fever and cough. He was housed in an isolation unit during this visit and handled with contact barrier precautions. Radiographic changes suggestive of bacterial pneumonia were noted, as was esophageal dilation. Empirical treatment with amoxicillin/clavulanic acid (Clavamox, Pfizer, Mississauga, Ontario), 14 mg/kg body weight (BW), PO, q12h for 10 d was initiated. Pasteurella sp. susceptible to amoxicillin/clavulanic acid was isolated from a pharyngeal swab, although given the sampling site, the relevance was unclear. At recheck, 10 days later, the dog was improving both clinically and radiographically. Esophageal dilation was no longer present. Full recovery, occurring approximately 6 wk after disease onset, was subsequently uneventful.
Figure 1.
Outbreak timeline.
Dog 2, a 1-year-old spayed female bulldog, was hospitalized September 19 to 23 because of diffuse urticaria of unknown origin. The dog had diarrhea, pyrexia, facial swelling, and hives. It had received an appropriate initial series of DA2PP in 2010 and a booster on June 4, 2011. No signs of respiratory disease were identified. It was housed in the same ward in which Dog 1 had spent the day during its initial visit (September 20), in a floor-level cage approximately 3 m from Dog 1. Brief nose-to-nose contact with Dog 1 occurred through the cage bars when Dog 1 was walked through the ward. On September 25, 5 days after exposure to Dog 1, there was an acute onset of cough without fever. As with dog 1, this dog was isolated immediately upon re-admission on September 25 and handled with contact barrier precautions. Thoracic radiographs were unremarkable. Treatment with hydrocodone (Pharmascience, Montreal, Quebec), 0.2 mg/kg BW, PO, q6h for 14 d and doxycycline (Apo-Doxy; Apotex, Toronto, Ontario), 20 mg/kg BW, PO, q12h for 10 d was administered. Recovery was uneventful, with cough persisting for approximately 14 d.
Dog 3 was a 1-year-old male standard poodle which was a littermate to Dog 1 but owned by a separate owner in a different household. The dog was at the OVC-HSC on September 20 for castration and was housed in a different ward; however, contrary to facility policy, it was allowed to play with Dog 1 in its run for approximately 5 min on September 20. It had received an appropriate initial DA2PP series, with the 3rd dose administered on May 25, 2011. Cough was noted on September 26 and Dog 3 was presented for examination on September 28. The dog was housed in isolation and handled with contact barrier precautions. Harsh coughing was the only clinical abnormality. Thoracic radiographs were normal. Doxycycline and hydrocodone were prescribed as for Dog 2. Clinical signs abated after 3 d.
Dog 4 was a 12-year-old castrated male cocker spaniel dog that was hospitalized September 20 to 21 for surgical excision of a limb mass and was housed in the same ward as Dog 1 in a floor-level cage approximately 2 m from Dog 1. No direct contact with Dogs 1, 2, or 3 was recalled by hospital personnel; however, it cannot be completely excluded. Dog 4 had received its triennial vaccination booster, which included CPiV, 3 wk prior to presentation. Coughing was reported to have started on September 25. The dog was returned for collection of diagnostic samples, but because disease was mild, he was not admitted and no further diagnostic testing was performed. No treatment was prescribed and no complications developed.
Dog 5 was not at the OVC-HSC but was a suspected secondary case. This dog was owned by the neighbor of Dog 2 and there was periodic direct contact between the dogs in their yards. This dog was suspected to have had contact with Dog 5 on September 24 and had an onset of cough on October 3. No further clinical information was available.
An infection control response was initiated on September 26 immediately after reports of potential infections of dogs 2 and 3. The ward that had housed dogs 1, 2, and 4 was identified and quarantined. Patient follow-up was intensified and monitoring of hospitalized patients for signs of respiratory disease or fever of unknown origin was increased. Acute and convalescent serum samples were collected from dogs 1–4 and tested for canine influenza and CPiV antibodies by hemagglutination inhibition. Nasal swabs were also collected for canine influenza polymerase chain reaction (PCR) and pharyngeal swabs were collected for aerobic bacterial culture. Canine parainfluenza infection was diagnosed through identification of a 4-fold or greater increase in CPiV antibody titer in all tested dogs (Table 1). Antibodies against canine influenza were not detected on acute or convalescent samples and canine influenza virus PCR was negative. No bacterial pathogens were identified. No samples were collected from dog 5.
Table 1.
Canine parainfluenza virus specific antibody titers as determined by hemagglutination inhibition
| Dog | Acute | Convalescenta | Titer increase |
|---|---|---|---|
| 1 | 1:384 | 1:1536 | 4-fold |
| 2 | 1:32 | 1:1536 | 48-fold |
| 3 | 1:96 | 1:1536 | 16-fold |
| 4 | 1:96 | 1:512 | 5.3-fold |
Collected 10 to 14 d after acute phase samples.
Canine parainfluenza virus is an enveloped RNA virus that belongs to the Paramyxoviridae family (12). It is highly contagious, being excreted from the respiratory tract of acutely infected animals, and is an important component of CIRDC (10,12,13). Signs of disease typically occur 2 to 8 d after exposure (12), and the typical clinical presentation is the presence of a dry, hacking cough that persists for 2 to 6 d (14). Nasal discharge, pharyngitis, and tonsillitis may also be present, but pyrexia is usually mild or absent (12,14). Viral shedding typically starts 2 to 10 d after exposure and typically begins prior to the onset of clinical signs (12,14). Therefore, while the clinical syndrome of CIRDC is readily identifiable, not all dogs shedding the causative agents display signs of infection. CPiV is highly transmissible and infection rates among exposed susceptible individuals are high (15,16). Transmission is predominantly through infectious aerosols, although the role of fomites may be underestimated (14). There is no long-term carrier state.
Given the common involvement of CPiV in CIRDC and the potentially large number of dogs in close proximity in veterinary hospitals, either as outpatients in common areas or as inpatients, it is perhaps surprising that there are few reports of HA-CIRDC, particularly in light of the widespread reports of human PiV outbreaks in human hospitals (17–19). It is unclear whether this is because they rarely occur (perhaps because a vaccine is available for dogs, as opposed to humans), are rarely diagnosed or are rarely reported. Under-identification could certainly be possible considering the infrequent testing of dogs with respiratory disease, the likelihood that onset of disease would be in the community (and therefore be less likely to be recognized by veterinary hospitals), and the possible reluctance of facilities to report HA infections. Regardless, this report indicates that CPiV transmission can occur within a veterinary facility, including between dogs with no known direct contact.
The origin of CPiV in this case series cannot be definitively established. The 4 to 6 day onset of clinical signs after exposure of Dogs 2–4 to Dog 1 is consistent with CPiV infection acquired during hospitalization. No other cases of potentially infectious respiratory disease were identified in dogs at the OVC-HSC in the preceding month. Considering the typical incubation period (with the potential for shedding of CPiV for 4 or more days prior to the onset of disease) (14), the timing of this dog’s exposure to other dogs of unknown health status at the grooming facility or dog park is consistent with exposure at that time. However, it cannot be definitively established that this was the index case or that the dog park or grooming facility was the origin of infection.
Two of the 3 dogs (dogs 2 and 3) with HA-infections had direct, albeit relatively short-term, contact with the index case (Dog 1). This was against standard hospital infection control protocols and highlights the need for preventing contact between animals within facilities. Certainly, one cannot guarantee that transmission would not have occurred if direct contact was prevented, particularly since one other affected dog had no known direct contact; however, the closeness and duration of contact are presumably key factors in determining the likelihood of pathogen transmission. Indirect transmission via personnel, from hands or clothing, must also be considered. There was no overlap in clinical personnel between the 4 dogs; however, it is possible that kennel staff handled both dogs. General environmental fomites must also be considered, but items that might have been used on multiple dogs were not identified. Environmental exposure from sites contaminated by Dog 1 such as hallways or the dog walking area is also possible but unlikely. The authors are unaware of data describing environmental persistence of CPiV; however, being an enveloped virus, CPiV would not be expected to persist for a prolonged length of time and would be readily inactivated by accelerated hydrogen peroxide, the disinfectant used at this facility. Environmental infection was therefore considered unlikely given the greater potential of aerosol or personnel-borne transmission and the lack of infections identified in dogs outside of that ward that did not have direct contact with the index case.
While no testing was performed to confirm CPiV infection in Dog 5, it was considered a suspected case based on exposure to Dog 4 in the community and a timeframe and clinical presentation consistent with CPiV infection. The dog was exposed 9 d prior to the onset of clinical signs, which is near the end of the incubation period window, but transmission from Dog 4 is certainly possible.
In this outbreak, all of the suspected HA-associated transmission occurred before the onset of disease in the first case. First generation transmission (transmission of the infectious agent from the index case to the first group of subsequent cases) can be difficult to prevent with a virus that can be shed prior to the onset of clinical signs. However an outbreak assessment is critical to determine if any measures could have prevented transmission in some or all cases. The breaches of standard infection control practices, with direct contact allowed between dogs 1, 2, and 3, may have played a role in this outbreak. Once the outbreak was recognized, no further cases were identified. This may have been, in part, because of the short duration of hospitalization of the index case; however, immediate isolation of dogs upon re-admission and enhancement of infection control practices may have played a role. Despite a lack of proof of efficacy of outbreak interventions, these data suggest that a rapid yet practical response may help contain this infectious agent within a facility. Second generation transmission was suspected outside of the hospital, with potential infection of a neighbor’s dog (Dog 5). While not involving the hospital, this is of concern because of the potential for facilitating further community spread of CPiV. Although in this situation Dog 4 was not showing signs of illness when it was permitted to interact with Dog 5, the suggested occurrence of disease spread into the community highlights the importance of counseling owners of infectious animals that are being discharged from hospitals.
Modified live CPiV is included in common parenteral vaccines. While CPiV is not itself considered a “core” vaccine component (20), the other agents in the combination vaccine are; therefore, CPiV vaccination is common. Dogs 1–4 had been vaccinated appropriately, with boosters within the preceding 5 mo. Parenteral vaccination is used to prevent clinical disease but has not been shown to have an impact on CPiV shedding and does not confer absolute protection from disease, as was evident here. Intranasal CPiV vaccines are also available in combination with other CIRDC agents, and this approach is preferable because it can have a greater impact on both disease and viral shedding (21). These are not part of the recommended “core” canine vaccines (20), typically being reserved for dogs at perceived higher risk for exposure (e.g., dogs going to kennels) and had not been administered to any of these dogs.
This outbreak of CPiV infection should serve as a reminder of the ever-present risk of HA-infections, including outbreaks that originate from clinically normal individuals. Prompt recognition of potential outbreaks, the presence of a good standard infection control program, and the implementation of enhanced infection control practices may be important to reduce the impact on patients and the hospital.
Acknowledgment
Canine influenza testing was funded by Merck Animal Health. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Walther B, Wieler LH, Friedrich AW, Kohn B, Brunnberg L, Lübke-Becker A. Staphylococcus aureus and MRSA colonization rates among personnel and dogs in a small animal hospital: Association with nosocomial infections. Berl Munch Tierarztl Wochenschr. 2009;122:178–185. [PubMed] [Google Scholar]
- 2.Weese JS, Faires M, Rousseau J, Bersenas AM, Mathews KA. Cluster of methicillin-resistant Staphylococcus aureus colonization in a small animal intensive care unit. J Am Vet Med Assoc. 2007;231:1361–1364. doi: 10.2460/javma.231.9.1361. [DOI] [PubMed] [Google Scholar]
- 3.Francey T, Gaschen F, Nicolet J, Burnens AP. The role of Acinetobacter baumannii as a nosocomial pathogen for dogs and cats in an intensive care unit. J Vet Intern Med. 2000;14:177–183. doi: 10.1892/0891-6640(2000)014<0177:trobaa>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez S, McCrackin Stevenson MA, Hudson CR, et al. Characterization of multidrug-resistant Escherichia coli isolates associated with nosocomial infections in dogs. J Clin Microbiol. 2002;40:3586–3595. doi: 10.1128/JCM.40.10.3586-3595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weese J, Armstrong J. Outbreak of Clostridium difficile-associated disease in a small animal veterinary teaching hospital. J Vet Intern Med. 2003;17:813–816. doi: 10.1111/j.1939-1676.2003.tb02519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherry B, Burns A, Johnson GS, et al. Salmonella Typhimurium outbreak associated with veterinary clinic. Emerg Infect Dis. 2004;10:2249–2251. doi: 10.3201/eid1012.040714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright JG, Tengelsen LA, Smith KE, et al. Multidrug-resistant Salmonella Typhimurium in four animal facilities. Emerg Infect Dis. 2005;11:1235–1241. doi: 10.3201/eid1108.050111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawakami K, Ogawa H, Maeda K, et al. Nosocomial outbreak of serious canine infectious tracheobronchitis (kennel cough) caused by canine herpesvirus infection. J Clin Microbiol. 2010;48:1176–1181. doi: 10.1128/JCM.02128-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mochizuki M, Yachi A, Ohshima T, Ohuchi A, Ishida T. Etiologic study of upper respiratory infections of household dogs. J Vet Med Sci. 2008;70:563–569. doi: 10.1292/jvms.70.563. [DOI] [PubMed] [Google Scholar]
- 10.Erles K, Dubovi EJ, Brooks HW, Brownlie J. Longitudinal study of viruses associated with canine infectious respiratory disease. J Clin Microbiol. 2004;42:4524–4529. doi: 10.1128/JCM.42.10.4524-4529.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Englund L, Jacobs AA, Klingeborn B, Chriél M. Seroepidemiological survey of Bordetella bronchiseptica and canine parainfluenza-2 virus in dogs in Sweden. Vet Rec. 2003;152:251–254. doi: 10.1136/vr.152.9.251. [DOI] [PubMed] [Google Scholar]
- 12.Buonavoglia C, Martella V. Canine respiratory viruses. Vet Res. 2007;38:355–373. doi: 10.1051/vetres:2006058. [DOI] [PubMed] [Google Scholar]
- 13.Ueland K. Serological, bacteriological and clinical observations on an outbreak of canine infectious tracheobronchitis in Norway. Vet Rec. 1990;126:481–483. [PubMed] [Google Scholar]
- 14.Ellis JA, Krakowka GS. A review of canine parainfluenza virus infection in dogs. J Am Vet Med Assoc. 2012;240:273–284. doi: 10.2460/javma.240.3.273. [DOI] [PubMed] [Google Scholar]
- 15.Binn LN, Alford JP, Marchwicki RH, Keefe TJ, Beattie RJ, Wall HG. Studies of respiratory disease in random-source laboratory dogs: Viral infections in unconditioned dogs. Lab Anim Sci. 1979;29:48–52. [PubMed] [Google Scholar]
- 16.Binn LN, Lazar EC. Comments on epizootiology of parainfluenza SV-5 in dogs. J Am Vet Med Assoc. 1970;156:1774–1777. [PubMed] [Google Scholar]
- 17.Teo WY, Rajadurai VS, Sriram B. Morbidity of parainfluenza 3 outbreak in preterm infants in a neonatal unit. Ann Acad Med Singap. 2010;39:837–836. [PubMed] [Google Scholar]
- 18.Lee AV, Bibby DF, Oakervee H, et al. Nosocomial transmission of para-influenza 3 virus in hematological patients characterized by molecular epidemiology. Transpl Infect Dis. 2011;13:433–437. doi: 10.1111/j.1399-3062.2011.00603.x. [DOI] [PubMed] [Google Scholar]
- 19.Piralla A, Percivalle E, Di Cesare-Merlone A, Locatelli F, Gerna G. Multicluster nosocomial outbreak of parainfluenza virus type 3 infection in a pediatric oncohematology unit: A phylogenetic study. Haematologica. 2009;94:833–839. doi: 10.3324/haematol.2008.003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paul MA, Carmichael LE, Childers H, et al. 2006 AAHA canine vaccine guidelines. J Am Anim Hosp Assoc. 2006;42:80–89. doi: 10.5326/0420080. [DOI] [PubMed] [Google Scholar]
- 21.Kontor EJ, Wegrzyn RJ, Goodnow RA. Canine infectious tracheo-bronchitis: Effects of an intranasal live canine parainfluenza-Bordetella bronchiseptica vaccine on viral shedding and clinical tracheobronchitis (kennel cough) Am J Vet Res. 1981;42:1694–1698. [PubMed] [Google Scholar]