Abstract
♦ Background, Objectives, and Methods: The number of patients on chronic peritoneal dialysis (CPD) is increasing rapidly on a global scale. We analyzed the International Pediatric Peritoneal Dialysis Network (IPPN) registry, a global database active in 33 countries spanning a wide range in gross national income (GNI), to identify the impact of economic conditions on CPD practices and outcomes in children and adolescents.
♦ Results: We observed close associations of GNI with the fraction of very young patients on dialysis, the presence and number of comorbidities, the prevalence of patients with unexplained causes of end-stage kidney disease, and the rate of culture-negative peritonitis. The prevalence of automated PD increased with GNI, but was 46% even in the lowest GNI stratum. The GNI stratum also affected the use of biocompatible peritoneal dialysis fluids, enteral tube feeding, calcium-free phosphate binders, active vitamin D analogs, and erythropoiesis-stimulating agents (ESAs). Patient mortality was strongly affected by GNI (hazard ratio per $10 000: 3.3; 95% confidence interval: 2.0 to 5.5) independently of young patient age and the number of comorbidities present. Patients from low-income countries tended to die more often from infections unrelated to CPD (5 of 9 vs 15 of 61, p = 0.1). The GNI was also a strong independent predictor of standardized height (p < 0.0001), adding to the impact of congenital renal disease, anuria, age at PD start, and dialysis vintage. Patients from the lower economic strata (GNI < $18 000) had higher serum parathyroid hormone (PTH) and lower serum calcium, and achieved lower hemoglobin concentrations. No impact of GNI was observed with regard to CPD technique survival or peritonitis incidence.
♦ Conclusions: We conclude that CPD is practiced successfully, albeit with major regional variation related to economic differences, in children around the globe. The variations encompass the acceptance of very young patients and those with associated comorbidities to chronic dialysis programs, the use of automated PD and expensive drugs, and the diagnostic management of peritonitis. These variations in practice related to economic difference do not appear to affect PD technique survival; however, economic conditions seem to affect mortality on dialysis and standardized height, a marker of global child morbidity.
Keywords: Children, economics, developing countries, patient survival, technique survival, growth, registry
Economic welfare is a key determinant of health and access to health services. The reported prevalence of end-stage kidney disease and the provision of renal replacement therapy are directly related to country-specific gross national income (GNI) (1-3). Dialysis programs covering a gradually increasing fraction of the population are typically initiated in developing countries when the annual GNI per capita reaches $3 000 - $5 000 (3). The provision of dialysis services is usually accomplished by the combined efforts of public health care programs and private charity organizations.
The number of dialysis patients in the developing world is growing rapidly. According to a recent survey, approximately 1.6 million patients are receiving hemodialysis (HD) and 200 000, peritoneal dialysis (PD) worldwide (4). The proportion of PD is higher in developing countries and growing at the same rate as HD. The prevalence of PD in the developing world has increased by 250% over the past 12 years. Currently, almost 60% of PD patients worldwide live in developing countries. Peritoneal dialysis obviously has certain advantages over HD in lower-income countries because of the simplicity of its application and its suitability for home use in remote and rural regions (5). The cost advantage of PD in developed countries is neutralized by lower HD staff costs in the developing world, but local production and facilitated importation of PD consumables may soon boost the further adoption of PD in emerging economies.
In the pediatric population, PD is a particularly attractive treatment option because of its compatibility with schooling and social life. Although solid global demographic data are lacking, the proportion of pediatric PD use typically ranges around 50% - 70% in developed countries (6,7) and is considered to be similar or even higher in the developing world. However, little information is available about the impact of economic conditions on global practice patterns in pediatric PD and treatment outcomes such as technique durability and patient morbidity and survival. In 2007, the International Pediatric Peritoneal Dialysis Network (IPPN) established a global registry active in 33 countries, which to date has collected comprehensive prospective information for 1900 children around the globe (8). We interrogated this unique database to assess the impact of the economic environment on various aspects of PD performance and outcomes.
METHODS
DATA COLLECTION
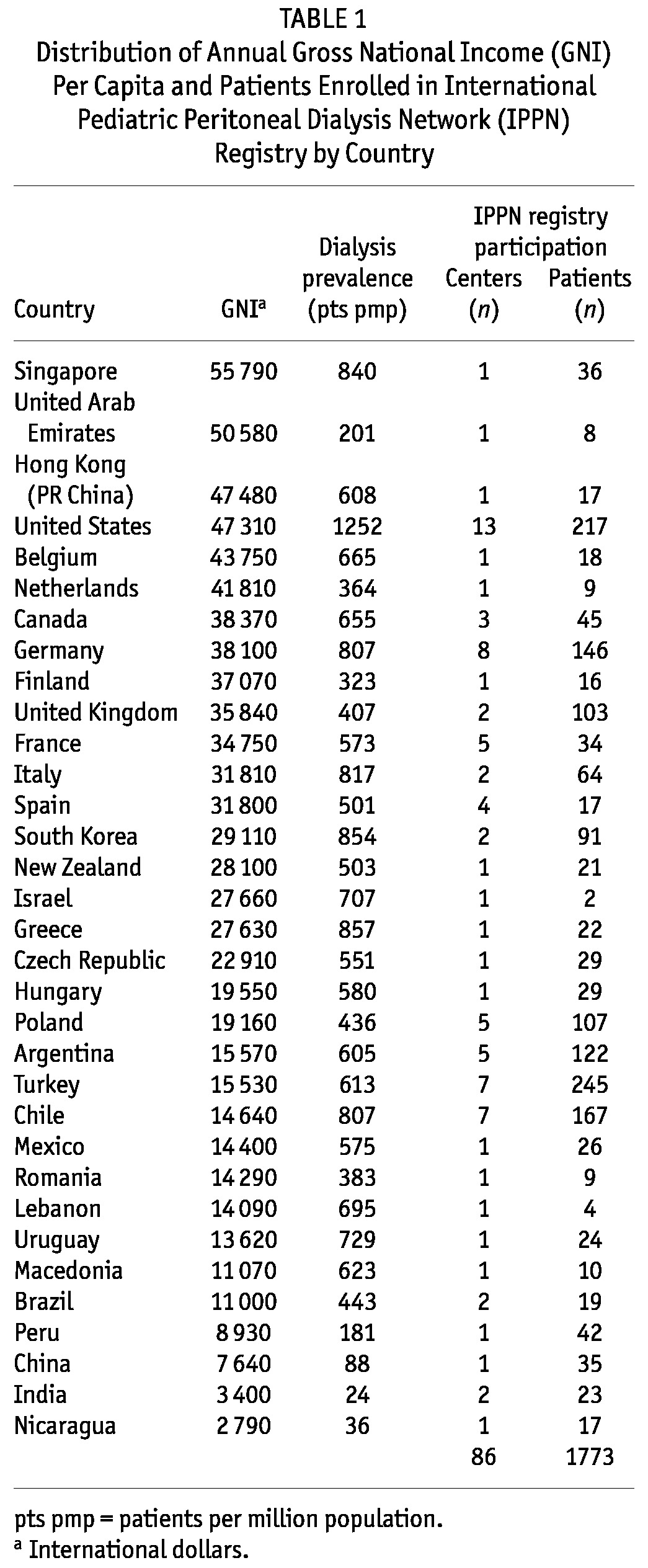
The IPPN is an international consortium of pediatric dialysis centers. In April 2007, the Network started an Internet-based registry that prospectively collects comprehensive clinical, laboratory, and treatment-related information for children undergoing chronic PD (CPD). Currently, 86 centers in 33 countries around the world regularly contribute patient data using the web platform (http://www.pedpd.org, Table 1). Because of the voluntary character of the registry, the nationwide coverage of pediatric PD patients varies between countries: for example, in the United States, it is 10%; in Turkey, 19%; in 12 European countries, 31%; in Canada, 59%; in Chile, Korea, and Argentina, 80% - 90%; in China, Finland, Macedonia, Nicaragua, Singapore, and Uruguay, 100%.
TABLE 1.
Distribution of Annual Gross National Income (GNI) Per Capita and Patients Enrolled in International Pediatric Peritoneal Dialysis Network (IPPN) Registry by Country
The basic patient characteristics collected at entry to the registry are these: age, sex, primary renal disorder, age at initiation of CPD, and any existing comorbidities. Time-variant information—for example, anthropometric and laboratory data; current medications and dialysis prescription; ambulatory blood pressure and echocardiography findings; and the number, causes, and duration of hospitalizations—is requested every 6 months. Intercurrent events such as episodes of peritonitis or exit-site infection, catheter revisions, peritoneal equilibration tests, and clearance studies are entered when they occur. A termination entry is requested when a patient permanently discontinues CPD at the enrolling center because of kidney transplantation, transfer to HD, transfer to another center, death, or other reasons.
Data protection is ensured by pseudonymized data input. The data are automatically checked for plausibility and completeness. Approval for the registry project was obtained from local institutional review boards or ethics committees, and informed consent was obtained from the patients or their legal guardians, or both, when required by local review boards.
GROSS NATIONAL INCOME
The economic wealth of each participating country was classified by the per-capita GNI in 2010 based on purchasing power parity converted to international dollars, using the tables published by the World Bank (http://data.worldbank.org/indicator/NY.GNP.PCAP.PP.CD). An international dollar has the same purchasing power over GNI as a US dollar has in the United States. Table 1 gives GNI values by country. The countries were arbitrarily categorized into income groups according to annual per-capita GNI: low income (<$12 000), lower-middle income ($12 000 to <$18 000), upper-middle income ($18 000 to <$28 000), and high income ($28 000 or more). The country-specific reported dialysis prevalence (Table 1) was noted to increase sharply with GNI in the lowest economic stratum, but did not vary consistently when GNI exceeded $10 000.
DATA ANALYSIS
All available measurements were utilized for this exploratory analysis performed for descriptive purposes and the generation of new hypotheses. Measured values were divided by the number of measurements per patient to account for the variable number of repeated measurements available for each patient. Data are expressed as mean ± standard deviation for normally distributed data, and as median and interquartile range for non-normally distributed variables. Between-group differences in means were assessed by an analysis of variance followed by a Student Newman-Keuls test for multiple comparisons. Differences in proportions were assessed using chi-square tests.
A mixed linear model procedure was applied to analyze the effects of GNI and various factors known to affect height standard deviation score (SDS), making use of all observations in this mixed longitudinal dataset.
Actuarial patient and PD technique survival were calculated by the Kaplan-Meier life table method, and differences were assessed for significance by a log-rank test. For calculation of PD technique survival, patients discontinuing PD for reasons other than technique failure (for example, transplantation, family wish, transfer to an adult dialysis center) were censored at the time of termination from the registry. A Cox proportional hazards regression analysis was performed to assess the impact of GNI relative to known major risk factors such as young patient age and comorbidities. Height for age and body mass index (BMI) for height-age were normalized to SDS using the World Health Organization 2006 normative values for children up to age 5 years and the National Center for Health Statistics 2000 reference charts for older children (9,10). Data were analyzed using the SAS software application (version 9.2: SAS Institute, Cary, NC, USA).
RESULTS
PATIENT CHARACTERISTICS
By 1 March 2012, the registry had enrolled 1773 patients 0 - 19 years of age (median: 10.2 years; IQR: 3.6 - 14.7 years) from 86 pediatric dialysis centers in 33 countries (Table 1). According to our classification criteria, 47% of patients were from high-income, 11% from upper-middle-income, 34% from lower-middle-income, and 8% from low-income countries. By comparison, the countries contributing to the IPPN registry represent 3.863 billion of the world’s population, with 19% living in high-income, 2% in upper-middle-income, 7% in lower-middle-income, and 72% in low-income categories.
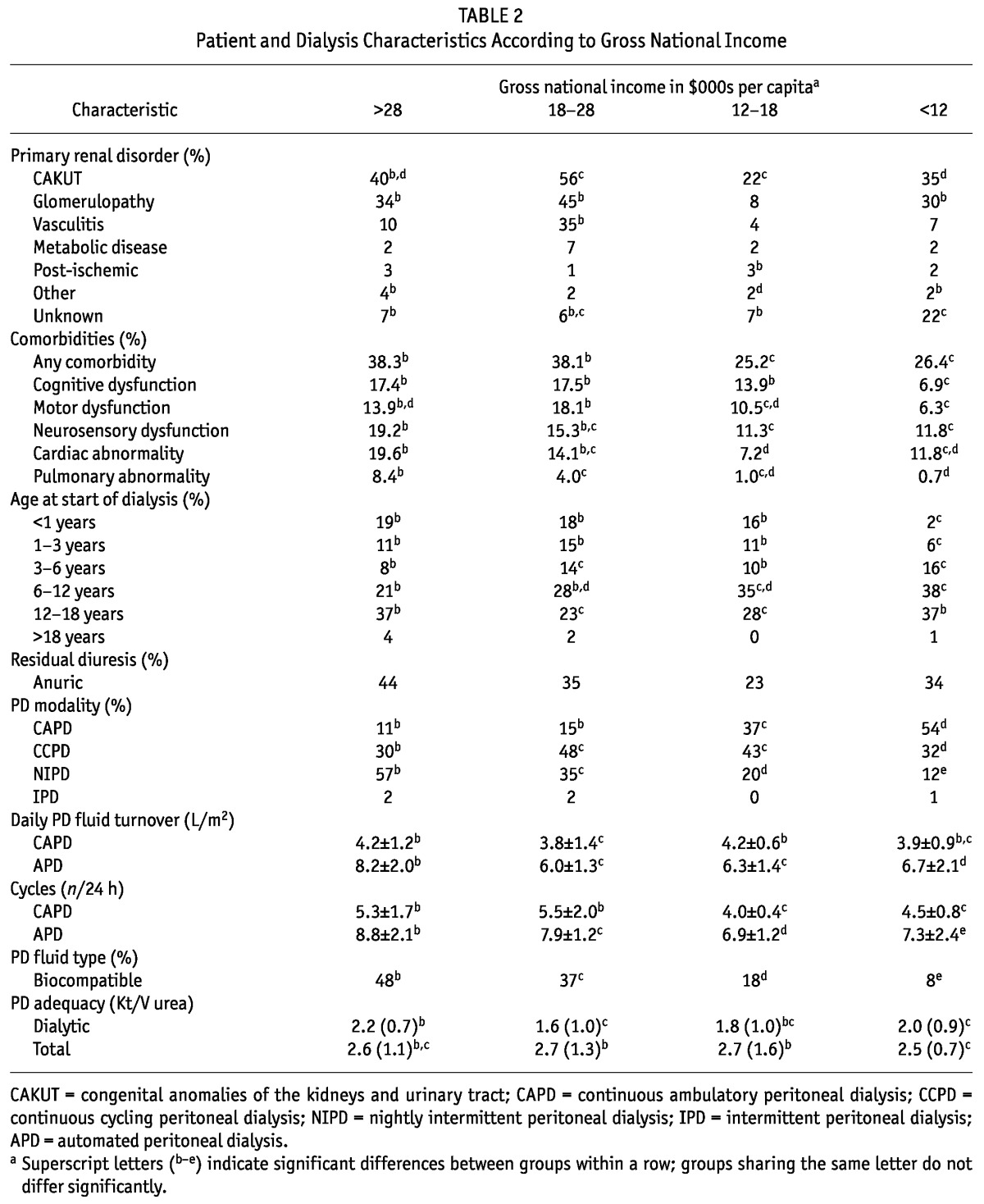
Several patient characteristics varied with the GNI (Table 2). In low-income countries compared with middle- and high-income countries, the dialysis population encompassed a significantly smaller fraction of infants younger than 3 years of age (8%) at dialysis initiation, but a higher proportion of 3- to 12-year-olds. With respect to the spectrum of underlying kidney disorders, the primary renal diagnosis was unknown in a much higher proportion of children from low-income countries (22%) than in those from countries in the other GNI groups. Extrarenal comorbidities were more commonly present in patients from high- and upper-middle-income countries than in those from lower GNI countries. Patients with cognitive and motor deficits were particularly less frequently found in the low-income countries; patients with neurosensory dysfunctions (vision or hearing deficits, or both) or cardiac abnormalities were more commonly found in high-income countries; and pulmonary hypoplasia or bronchopulmonary dysplasia were almost never reported in children from low-income and lower-middle-income countries.
TABLE 2.
Patient and Dialysis Characteristics According to Gross National Income
DIALYSIS CHARACTERISTICS
Major differences were also observed with respect to the PD modalities used. The use of continuous ambulatory PD was inversely correlated with GNI, but among patients treated with automated PD, the use of nightly intermittent PD increased with rising GNI. Among patients undergoing automated PD, total daily fluid turnover was higher in high-income than in lower-income countries because of the use of shorter, more frequent cycles in patients from the former country group.
Despite the differences in PD modality, the small-molecule clearances achieved showed only minor variations between countries and no consistent association with GNI. The use of neutral-pH PD solutions low in glucose degradation products (“biocompatible” solutions) increased progressively with GNI from 8% in the low-income countries to 48% in the high-income countries. Excluding patients from the United States, where such fluids are not available, the use of biocompatible PD fluids was even greater, at 68% of patients in the high-income economies.
PATIENT AND TECHNIQUE SURVIVAL
Follow-up was discontinued in 1027 patients because of kidney transplantation (60% of all dropouts), technique failure and switch to HD (20%), death (7%), partial recovery of renal function (2%), or loss of follow-up (11%—usually because of transfer to adult care).
Patient survival on PD was significantly better in patients from high-income countries (94.1% at 5 years) than in the middle- and low-income countries combined (88.7%, p = 0.01, Figure 1). In the regression analysis, a patient age of less than 1 year at study entry [hazard ratio (HR): 3.3; 95% confidence interval (CI): 2.0 to 5.46], the number of comorbidities present (HR: 1.54; 95% CI: 1.32 to 1.79), and $10 000 in GNI (HR: 0.70; 95% CI: 0.56 to 0.88) were identified as independent mortality risk factors. Compared with patients from countries with a GNI higher than $12 000, patients from the low-income countries tended to die more often from infections unrelated to PD (5 of 9 vs 15 of 61, p = 0.10).
Figure 1.
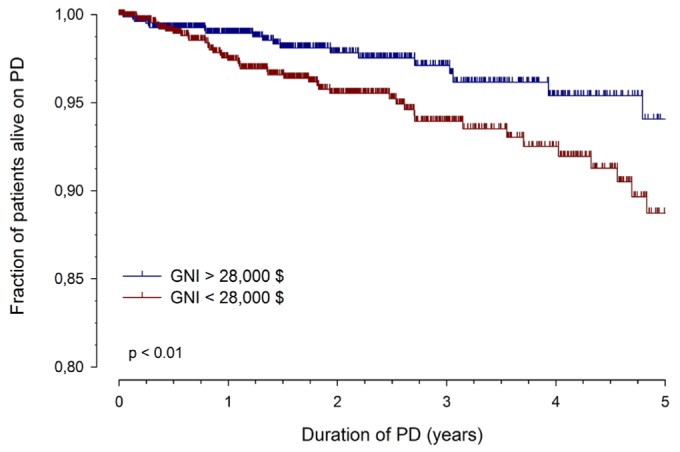
— Patient survival on peritoneal dialysis (PD) in countries with a per-capita gross national income (GNI) greater or less than $28 000 per year (international dollars). Lines represent unadjusted actuarial survival rates; vertical marks, censored observations.
Overall PD technique survival was not significantly affected by GNI. Patients from the low-income countries showed a slightly higher early technique failure rate (2-year functional survival: 89.2% vs 93.6% in the other strata, pooled), but technique survival at 5 years was identical (Figure 2).
Figure 2.
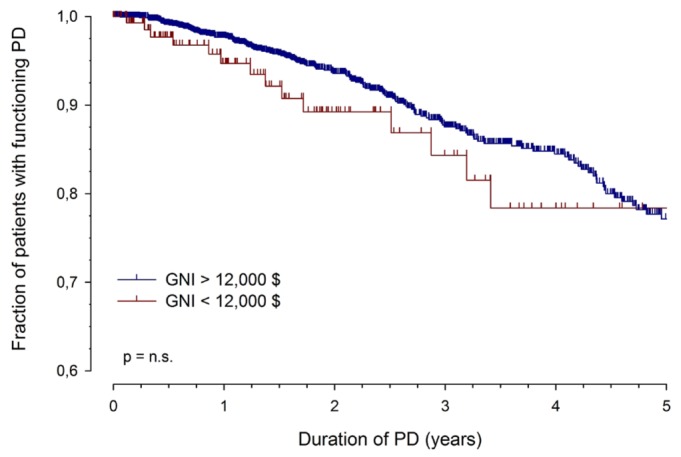
— Peritoneal dialysis (PD) technique survival in countries with a per-capita gross national income (GNI) greater or less than $12 000 per year (international dollars). Lines represent unadjusted actuarial survival rates; vertical marks, censored observations.
GROWTH AND NUTRITION STATUS
When heights of patients from countries in the lower economic strata were compared with international height reference data, those patients were found to be approximately 1 standard deviation smaller than patients from wealthier countries (Table 3, Figure 3). Recombinant growth hormone (rGH) therapy was used much less frequently (4% - 5% of growth-retarded children aged 1 - 16 years) in the low-income and lower-middle-income countries than in the high-income regions (35%). Multivariate analysis confirmed a highly significant positive effect of GNI on height SDS (effect size: 0.31 ± 0.03 per $10 000; p < 0.0001), which was independent of the effects of having a congenital renal disorder (-0.56 ± 0.08, p < 0.0001), being anuric (-0.11 ± 0.03, p < 0.005), age at the start of PD (0.03 ± 0.01, p < 0.0001), duration of PD (-0.05 ± 0.02 per year, p < 0.005), and serum PTH and rGH therapy (nonsignificant).
TABLE 3.
Treatment Outcomes According to Gross National Income
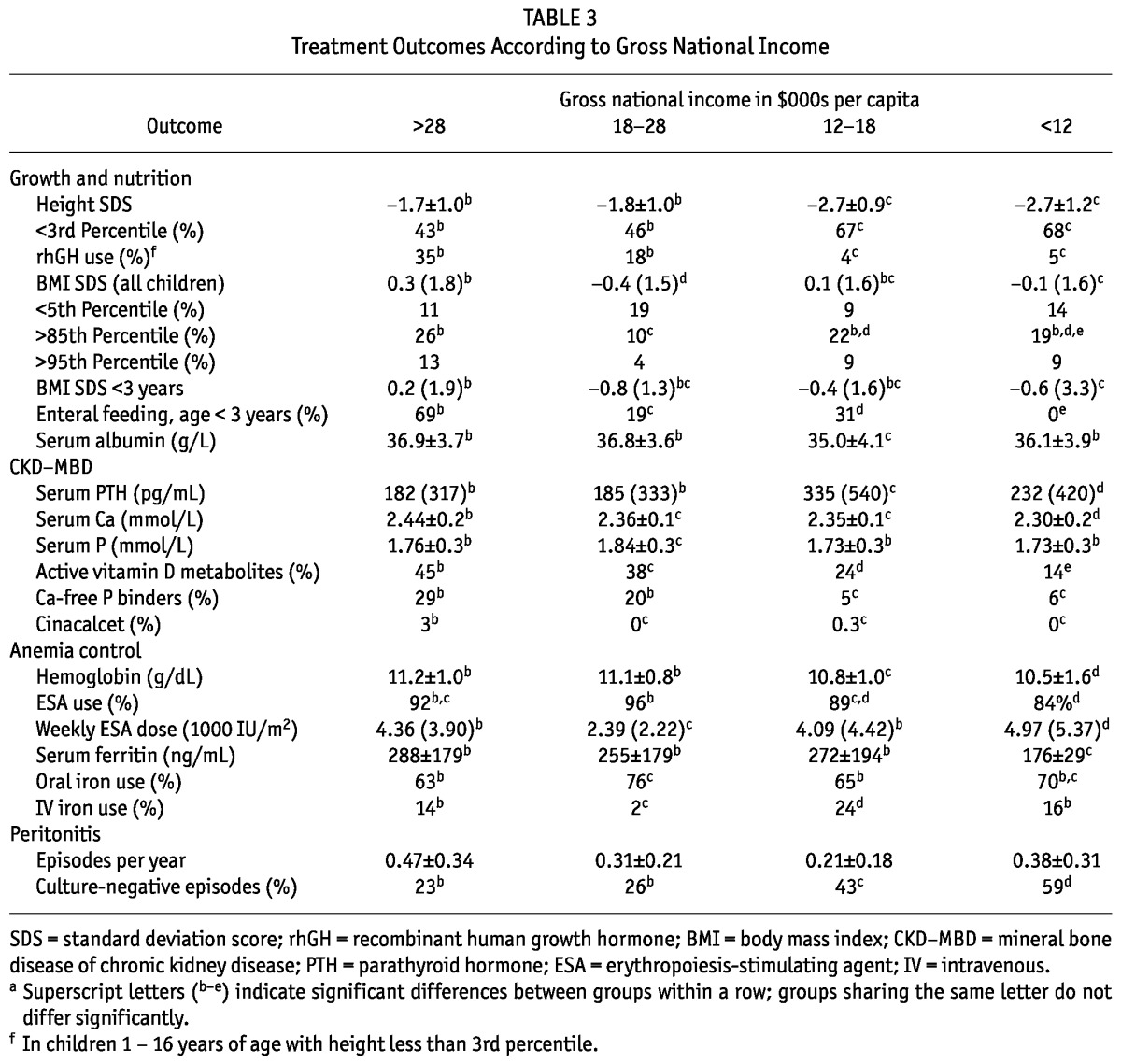
Figure 3.
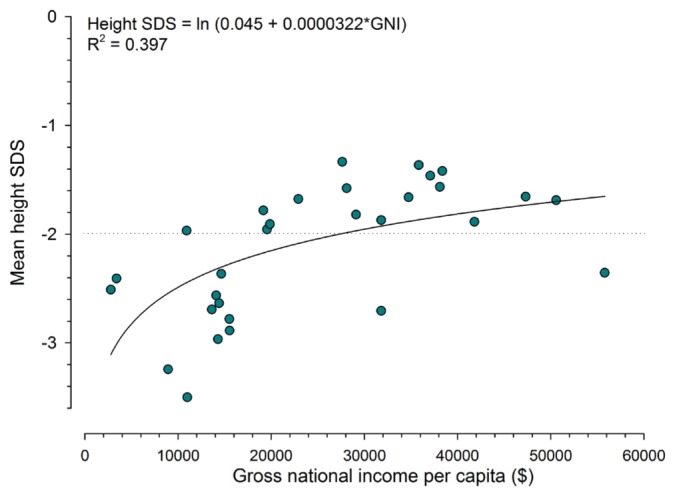
— Mean height standard deviation score (SDS) per country by per-capita gross national income (GNI). Country-specific values for mean height SDS were calculated from all measurements, adjusting for the number of measurements per subject. The dotted line indicates the 3rd height percentile.
The BMI SDS was significantly lower in the low-income group [-0.1 (IQR: 1.6)] than in the high-income group [0.3 (IQR: 1.8)], but lowest in the upper-middle-income group [-0.4 (IQR: 1.5)], which comprised mainly Eastern European countries. The fraction of overweight and obese patients did not differ between the high-income (26% obese) and low-income countries (19% obese). Differences in BMI were more accentuated when the infant group (age < 3 years) was considered, with a negative median SDS being observed in all but the high-income strata. Those differences partly mirrored variation in the prevalence of enteral feeding via nasogastric tube or gastrostomy, which was favored in the high-income countries (69%), but was not applied in any of the low-income countries and in only 19% of the Eastern European countries.
BONE AND MINERAL DISORDERS
Patients in the two lower economic strata showed higher PTH levels, which were accompanied by lower serum calcium levels and significantly lower use of active vitamin D metabolites (Table 3). The higher PTH levels were observed despite similar or even slightly lower serum phosphorus levels in patients from countries in the lower-income strata. The use of calcium-free phosphate binders was closely correlated with GNI, and cinacalcet was almost exclusively administered in high-income countries.
ANEMIA
Achieved hemoglobin levels were significantly lower in the lower-middle- and low-income countries. That finding coincided with lower use of ESAs and higher iron. In contrast, however, treated patients in the low-income countries received higher ESA doses and showed lower serum ferritin levels (Table 3).
INFECTIOUS AND NONINFECTIOUS PD COMPLICATIONS
The country-specific peritonitis incidence rates did not differ significantly between the GNI strata (Table 3). The incidence of culture-negative peritonitis episodes was markedly increased in the lower GNI groups. The distribution of cultured organisms did not depend on GNI.
DISCUSSION
The present study examines, for the first time at a global level, the relationship between economic wealth and CPD practices and outcomes in children with end-stage kidney disease. Pediatric renal replacement therapy is a very costly treatment that currently cannot be offered by health systems in most African and many Asian countries, including large parts of India and China. Indeed, 27 of the 33 countries contributing to the IPPN registry (and encompassing 92% of the patients in the registry) would be considered “high income” economies according to the official criteria of the World Bank (GNI > $12 276) (11). To differentiate group effects associated with relative economic wealth in the present analysis, we readjusted the partition values, defining countries below $12 000 in GNI as “low income” and only those with a GNI of $28 000 or more as “high income” countries.
Major technological and procedural advances in PD accomplished since the end of the 1980s have enabled pediatric nephrologists to offer PD in all age groups, starting in the neonatal period. The excellent long-term patient survival (>90% at 5 years) and the maintenance of PD function (close to 80% at 5 years) observed in the IPPN registry reflect that remarkable success. Nonetheless, the management of very young patients— who frequently present with significant comorbid conditions—remains particularly challenging and consumes substantial resources.
In this context, several notable demographic differences between the economic strata were observed. The primary renal disorder was unknown in more than a fifth of the children in the low-income countries. Difficulties in establishing a renal diagnosis may point to late referral practices and possibly a lack of resources and expertise to perform extensive diagnostic testing, including renal imaging and genetic screening. Furthermore, the age distribution of the children undergoing chronic dialysis markedly varied with economic wealth, with almost 1 of 5 patients in the high-income countries, but only 1 in 50 in the low-income countries, being younger than 12 months. Infants up to 3 years of age comprised 27% - 33% of dialyzed patients in countries with a GNI above $12 000, but only 8% in the low-income group. Likewise, the number of associated comorbidities was markedly lower in the low-income and lower-middle-income countries. These observations strongly suggest that, in a setting of severely limited resources, access to dialysis tends to be restricted to patients with isolated renal failure and a low risk of technical difficulties. The expected time on dialysis, which depends on the existence and efficacy of transplantation programs, may also play a role in the decision to initiate chronic renal replacement therapy in very young infants.
Not unexpectedly, we also observed major differences with respect to PD modalities and the choice of PD solutions. The use of automated PD techniques was closely correlated with GNI, which is not surprising given the (at least) 20% higher treatment costs with automated PD relative to classical continuous ambulatory PD because of higher fluid turnover and cycler expenses. Nonetheless, it is important to note that the prevalence of automated PD is approaching 50% even in the developing countries.
Less expected was the preferential use of nightly intermittent PD—that is, automated PD without a significant daytime dwell—in countries with a higher income, in conjunction with more frequent cycles and higher dialysate turnover. Those findings might be interpreted as being reflective of a greater focus on lifestyle-compatible PD characterized by rapid short cycles with a high fluid turnover in the high-income countries, as opposed to a preference for optimal utilization of PD fluid over 24 hours in the low-income countries. Notably, the different approaches did not result in any differences in the dialytic clearances achieved.
“Biocompatible” PD fluids with neutral pH and a low content of toxic glucose degradation products are increasingly used in children, given the potentially repeated need for a functioning peritoneal membrane for dialysis during a long prospective lifespan (12). Because those solutions are even more costly than conventional PD fluid, it is not surprising that their utilization remains low in the lower-income countries. In the high-income countries (with the exception of the United States, where biocompatible PD fluids are not yet approved), two thirds of children are now receiving such fluids.
The apparent preferential acceptance of “low-risk” patients to dialysis programs in the low-income countries affects the interpretation of patient and technique survival rates. When adjusting for patient age and number of comorbidities, the economic strata had a highly significant impact on mortality risk, lowering it by almost 30% per $10 000 of GNI. Regarding causes of death, it appears that fatal systemic infections unrelated to dialysis are slightly more common in patients from low-income countries. Communicable diseases are the leading cause of death in the developing world (13), and children undergoing PD in that environment may be at increased risk for infections with a fatal outcome. Although that finding may be related to impaired global immune defense, health management issues such as late diagnosis, remote place of living, limited availability of anti-infection drugs, high rates of nosocomial infections, and general hygiene issues are additional potential risk factors.
Given the critical role of infections unrelated to PD in patient survival, it was remarkable to observe peritonitis incidence rates well below 1 episode per 24 months around the globe, with no observable systematic effect of prevailing socio-economic conditions. The incidence of peritonitis in patients receiving CPD has steadily declined over the past several decades because of improved caregiver education and technological advances such as flush-before-fill, automated PD, and improved connection systems. Peritonitis incidence rates in children, while paralleling the downward trend observed in adult patients, have remained higher than those seen in adults, possibly because of problems specific to pediatrics, such as limited patient cooperation, immunologic immaturity, shorter tunnel configuration, and so on (14). Provided that the results observed here are not biased by gross underreporting, an excessive risk of peritonitis may no longer be considered a limitation to CPD even in low-income countries, in particular when offered by dedicated health care professionals with access to international clinical practice guidelines, such as the sites and clinicians participating in the IPPN. Unfortunately, guideline awareness does not preclude organizational problems in less advanced health care settings, as evidenced by the increased incidence of culture-negative peritonitis (23% in high-income countries compared with 59% in low-income countries), which confirms previous findings of the International Pediatric Peritonitis Registry and may reflect insufficient laboratory techniques or frequent initiation of antibiotic treatment before acquisition of samples for culturing (15).
In children with chronic kidney disease, statural growth is a common final pathway reflecting the impact of various endogenous, environmental, and disease-related factors on overall health and well-being (16). Growth failure is an independent risk factor for mortality in children with end-stage kidney disease (17). We identified a strong positive association of GNI with standardized height. By univariate analysis, GNI explained almost 40% of the variation in height SDS. In the multivariate analysis, the effect of GNI was independent of the nature of the underlying kidney disease, the duration of end-stage renal disease, age at initiation of dialysis, and residual renal function. Use of rGH was largely restricted to the high- and upper-middle-income strata, but a statistical association of this treatment with better growth could not be documented from our data because of a marked bias by indication—that is, smaller patients were more likely to receive growth hormone therapy.
It might be argued that the observed association of GNI and growth might be confounded by the variable “fit” of international reference standards with populations of different ethnic background. However, ethnic distributions were widely scattered even within the economic strata, and ethnicity did not affect the association of standardized height with GNI in the multivariate analysis. Hence, height SDS might be a useful surrogate marker of the impact of socio-economic factors on the overall health of dialyzed children.
The prevalence of malnutrition, one of the hallmarks of the uremic state, was low and largely unaffected by socio-economic conditions, except in young infants, who tended to be better nourished in the high-income countries. The difference in nutrition status in that age group is most likely explained by variation in the use of enteral tube feeding, which ranged from 0% of patients in the low-income countries to 69% of patients in the high-income countries. Interestingly, an increased prevalence of obesity was noted in many countries, largely regardless of economic strength, probably reflecting the current global trend towards overweight in the pediatric population (18).
Major differences between the economic strata were also observed with respect to the management of mineral bone disorders of chronic kidney disease. Active vitamin D metabolites were less commonly used in lower-income countries, probably explaining the lower serum calcium and higher serum PTH observed in those regions. On the other hand, phosphorus control was achieved exclusively with calcium-containing binders in 95% of the lower-income countries; that compares with an almost 30% share of the more costly calcium-free binders in the high-income economies. Although these different practices provided equally good phosphorus control, they likely prevented an even greater difference in serum calcium levels.
Finally, our survey revealed certain differences in anemia control related to economic conditions. Achieved hemoglobin levels tended to be somewhat lower in the lower-income countries, and were accompanied by a slightly lower fraction of patients receiving ESAs at any time point. Although that observation might indicate restricted ESA access in individual countries, the average ESA doses administered in the treated patients were highest in the low-income economies, suggesting a high prevalence of ESA resistance. In turn, serum ferritin in the low-income stratum was significantly lower despite a higher proportion of patients receiving iron supplementation, compatible with a higher incidence of (presumably nutrition-related) iron deficiency in the low-income countries.
CONCLUSIONS
Our global survey suggests that CPD is practiced successfully, albeit with major regional variation related to differences in economic wealth, in children around the globe. The variations encompass issues such as the acceptance of very young patients and those with associated comorbidities to chronic dialysis programs, the utilization of automated PD and expensive drugs, and the diagnostic management of peritoneal infections, but did not result in differences in technique survival. However, economic conditions do appear to affect mortality on dialysis and standardized height, a marker of global morbidity in children with chronic disease.
Considering the general health challenges to children in the developing world, and even allowing for the fact that CPD may be offered to only a limited proportion of eligible patients, pediatric nephrologists in the lower-income economies appear to perform remarkably well in providing maintenance PD once a treatment decision is made. The observed outcomes mirror the remarkable progress in establishing specialized pediatric dialysis units in many threshold countries during the past 10 - 15 years and raise hope that PD programs will soon become available and be managed successfully in more developing economies as their GNIs gradually increase. The global distribution of uniform dialysis equipment has certainly been key to this progress. Moreover, the universal availability of clinical practice guidelines, educational materials, and professional networks over the Internet has been instrumental in harmonizing standards of management on a global scale. The rapid adoption of the IPPN web portal by the pediatric PD community has been paradigmatic of that process.
The following principal investigators are contributing to the IPPN registry:
Argentina: L. Alconcher, Hospital Interzonal General, Bahia Blanca; E. Sojo, Hospital de Pediatria Garrahan, Buenos Aires; P.A. Coccia, Hospital Italiano de Buenos Aires; A. Suarez, Hospital de Niños Sor Maria Ludovica, La Plata; P.G. Valles, Hospital Pediatrico Humberto Notti, Mendoza; R. Salim, Rennius SA Salta. Belgium: K. van Hoeck, University Hospital Antwerp, Edegem. Brazil: V. Koch, Hospital das Clinicas FMUSP, Sao Paulo. Canada: J. Feber, Children’s Hospital of Eastern Ontario, Ottawa; D.A. Geary, Hospital for Sick Children, Toronto; C. White, BC Children’s Hospital, Vancouver. Chile: M. Valenzuela, Hospital Guillermo Grant Benavente, Concepcion; J. Villagra, Hospital Base, Osorno; F. Cano, Hospital Luis Calvo Mackenna, Santiago; M.A. Contreras, Roberto del Rio Hospital, Santiago; A. Vogel, Pontivicia Universidad Catolica de Chile, Santiago; P. Zambrano, Hospital Dr. Gonzales Cortes, Santiago; P. Berrocal, Hospital Sotero del Rio, Santiago. PR China: K.C. Tse, Princess Margret Hospital, Hong Kong; H. Xu, Children’s Hospital of Fudan University, Shanghai. Czech Republic: K. Vondrak, University Hospital Motol, Prague. Finland: K. Rönnholm, Hospital for Children and Adolescents, Helsinki. France: J. Harambat, Hôpital des Enfants, Bordeaux; B. Ranchin, Hôpital Femme Mère Enfant, Lyon; G. Roussey, CHU Nantes; T. Ulinski, Armand Trousseau Hospital, Paris; M. Fischbach, Children’s Dialysis Center, Strasbourg. Germany: R. Büscher, Children’s Hospital Essen; M. Kemper, University Medical Center, Hamburg; L. Pape, Medical School, Hannover; F. Schaefer and D. Borzych, Center for Pediatrics and Adolescent Medicine, Heidelberg; J. Misselwitz, Kidney Center for Children and Adolescents; G. Klaus, University Hospital, Marburg; D. Haffner, University Children’s Hospital, Rostock. Greece: F. Papachristou, Aristoteles University, Thessaloniki. Hungary: A. Szabo, 1st Department of Pediatrics, Semmelweis University, Budapest. India: A. Bagga, All India Institute of Medical Sciences, New Delhi; M. Kanitkar, Armed Forces Medical College, Pune. Italy: E. Verrina, G. Gaslini Institute, Genova; A. Edefonti, Fondazione Ospedale Maggiore Policlinico, Milano; E. Vidal, Pediatric Nephrology, Dialysis and Transplant Unit, Padova; G. Leozappa, Dipartimento Nefrologia-Urologia, Rome. Israel: D. Landau, Soroka Medical Center, Beer-Sheva. Korea: I.S. Ha, Dialysis Center for Children and Adolescents, Seoul; K.H. Paik, Samsung Medical Center, Seoul. Lebanon: B. Aoun, Rafik Hariri University Hospital, Beirut. Macedonia: E. Sahpazova, Pediatric Clinic, Skopje. Mexico: L. Sànchez Barbosa, Pediatric Hospital Medical Center SXXI, Cuahutemoc. New Zealand: W. Wong, Starship Children’s Hospital, Auckland. Netherlands: J.W. Groothoff, Academic Medical Center, Amsterdam. Nicaragua: Y. Silva, Hospital Infantil de Nicaragua, Managua. Peru: R. Loza Munarriz, Cayetano Heredia Hospital, Lima. Poland: A.M. Zurowska and D. Borzych, Medical University, Gdańsk; D. Drozdz, University Children’s Hospital, Krakow; M. Lipka, Children’s Memorial Health Institute, Warsaw; H. Ziolkowska, Public Paediatric Teaching Hospital, Warsaw; M. Sczepanska, Dialysis Division for Children, Zabrze. Romania: O. Brumariu, St. Maria Children’s Hospital, Iasi. Saudi Arabia: J. Kari, King AbdulAziz University Hospital, Jeddah. Singapore: H.K. Yap, Shaw-NKF-NUH Children’s Kidney Center. Spain: G. Ariceta, Hospital de Cruces, Baracaldo; F. Santos, Hospital Universitario Central de Asturias, Oviedo. Turkey: N. Besbas, Hacettepe University, Ankara; S. Bakkaloglu, Gazi University, Ankara; I. Bilge, Department of Pediatric Nephrology, Çapa-Istanbul; E. Serdaroglu, Dr. Behcet Children Research and Educational Hospital, Izmir; A. Bal, Tepecik Children and Research Hospital, Izmir; S. Mir, Ege University Faculty of Medicine, Izmir-Bornova. United Kingdom: L. Rees, Great Ormond Street Hospital, London; A.R. Watson, Children and Young People’s Kidney Unit, Nottingham. Uruguay: J. Grünberg, SE.N.NI.AD, Montevideo. United Arab Emirates: E. Simkova, Dubai Hospital. United States: L. Greenbaum, Children’s Healthcare Pediatric Dialysis Unit, Atlanta; A. Neu, Johns Hopkins Hospital, Baltimore; D. Askenazi, Children’s Hospital of Alabama, Birmingham; M. Ferris, University of North Carolina, Chapel Hill; H. Patel, Children’s Hospital, Columbus; S. Al-Akash, Driscoll Children’s Hospital, Corpus Christi; S. Pottoore, Children’s Medical Center, Dallas; V. Dharnidharka, Division of Pediatric Nephrology, Gainesville; T. Bunchman, Helen DeVos Children’s Hospital, Grand Rapids; A. Chua, Texas Children’s Hospital, Houston; B.A. Warady, Children’s Mercy Hospital, Kansas City; N. McAfee, Seattle Children’s Hospital; J. Zaritsky, UCLA Medical Center, Los Angeles.
DISCLOSURES
The authors have no financial conflicts of interest to declare.
Acknowledgments
The authors thank Anja Sander, biostatistician at the Institute of Medical Biometry and Informatics at Heidelberg University, for helpful statistical advice. We gratefully acknowledge the support of the International Society for Peritoneal Dialysis, Baxter Health Care, Fresenius Medical Care, Ipsen, Pfizer, IBM, and Sandoz. Last but not least, we appreciate the continued dedicated support of the IPPN by members of the medical and nursing staffs in all collaborating centers.
REFERENCES
- 1. White SL, Chadban SJ, Jan S, Chapman JR, Cass A. How can we achieve global equity in provision of renal replacement therapy? Bull World Health Organ 2008; 86:229–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grassmann A, Gioberge S, Moeller S, Brown G. ESRD patients in 2004: global overview of patient numbers, treatment modalities and associated trends. Nephrol Dial Transplant 2005; 20:2587–93 [DOI] [PubMed] [Google Scholar]
- 3. Hooi LS, Wong HS, Morad Z. Prevention of renal failure: the Malaysian experience. Kidney Int Suppl 2005; (94):S70–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jain AK, Blake P, Cordy P, Garg AX. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol 2012; 23:533–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Just PM, de Charro FT, Tschosik EA, Noe LL, Bhattacharyya SK, Riella MC. Reimbursement and economic factors influencing dialysis modality choice around the world. Nephrol Dial Transplant 2008; 23:2365–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. United States Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, U.S. Renal Data System (USRDS). 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: USRDS; 2011. [Available online at: http://www.usrds.org/atlas.aspx; accessed 10 May 2012] [Google Scholar]
- 7. van Stralen KJ, Tizard EJ, Verrina E, Schaefer F, Jager KJ. on behalf of the European Society for Paediatric Nephrology/European Renal Association-European Dialysis and Transplant Association (ESPN/ERA-EDTA) registry study group. Demographics of paediatric renal replacement therapy in Europe: 2007 annual report of the ESPN/ERA-EDTA registry. Pediatr Nephrol 2010; 25:1379–82 20177709 [Google Scholar]
- 8. Schaefer F, Warady BA. Peritoneal dialysis in children with end-stage renal disease. Nat Rev Nephrol 2011; 7:659–68 [DOI] [PubMed] [Google Scholar]
- 9. WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl 2006; 450:76–85 [DOI] [PubMed] [Google Scholar]
- 10. Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11 2002; (246):1–190 [PubMed] [Google Scholar]
- 11. The World Bank. How we classify countries [Web page]. Washington, DC: The World Bank; n.d. [Available online at: http://data.worldbank.org/about/country-classifications; accessed 10 May 2012] [Google Scholar]
- 12. Schmitt CP, Zaloszyc A, Schaefer B, Fischbach M. Peritoneal dialysis tailored to pediatric needs. Int J Nephrol 2011; 2011:940267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet 2003; 361:2226–34 [DOI] [PubMed] [Google Scholar]
- 14. Chadha V, Schaefer FS, Warady BA. Dialysis-associated peritonitis in children. Pediatr Nephrol 2010; 25:425–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schaefer F, Feneberg R, Aksu N, Donmez O, Sadikoglu B, Alexander SR, et al. Worldwide variation of dialysis-associated peritonitis in children. Kidney Int 2007; 72:1374–9 [DOI] [PubMed] [Google Scholar]
- 16. Schaefer F. Endocrine and growth disorders in chronic renal failure. In: Avner ED, Harmon WE, Niaudet P, Yoshikawa N, eds. Pediatric Nephrology. 6th ed. Berlin, Germany: Springer; 2009: 1714–53 [Google Scholar]
- 17. Furth SL, Stablein D, Fine RN, Powe NR, Fivush BA. Adverse clinical outcomes associated with short stature at dialysis initiation: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatrics 2002; 109:909–13 [DOI] [PubMed] [Google Scholar]
- 18. Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes 2006; 1:11–25 [DOI] [PubMed] [Google Scholar]


