Abstract
“Cardiorenal syndrome” is a term used to describe a dys-regulation of the heart affecting the kidneys, or vice versa, in an acute or chronic manner (1,2). Renal impairment can range from reversible ischemic damage to renal failure requiring short- or long-term renal replacement therapy (2). Patients who require mechanical circulatory support, such as a left ventricular assist device (LVAD), as definitive treatment for congestive heart failure or as a bridge to cardiac transplantation pose a unique challenge with respect to receiving dialysis, because they experience higher rates of morbidity and mortality from infection in the post-LVAD period (3-7). Acute dialysis access can pose an increased infection risk.
In this article, we present a patient who required renal replacement therapy and a LVAD for management of acute-on-chronic cardiorenal syndrome while awaiting heart transplantation. A literature review to determine whether peritoneal dialysis or hemodialysis is superior for patients with profound hemodynamic dysfunction and the need to minimize risk of infection did not offer clear guidance about which modality is superior in patients with advanced congestive heart failure. However, there is clear evidence of the superiority of peritoneal dialysis in reducing the risk of systemic infection secondary to acute dialysis access. Given the high risk of LVAD infection, we therefore conclude that, to decrease mortality secondary to systemic infection, peritoneal dialysis should strongly be considered in patients who require renal replacement therapy before or after LVAD placement.
Keywords: Bacteremia, cardiorenal syndrome, hemodialysis, LVAD
A 63-year-old Caucasian man with a history of hypertrophic cardiomyopathy, hypertension, ventricular fibrillation arrest, and chronic stage IV kidney disease presented to his outpatient cardiologist with a complaint of shortness of breath and orthopnea. Over the preceding 6 months, the symptoms had gradually worsened, and a repeat echocardiogram indicated a significant change in ejection fraction from 53% to 20%. He had experienced numerous firings of his implantable cardioverter defibrillator. Blood work at the time of his outpatient presentation revealed azotemia, with deterioration of kidney function. He was referred for hospitalization to manage decompensated heart failure and acute-on-chronic kidney injury.
On admission, the patient’s blood urea nitrogen was 121 mg/dL and his creatine was 3.7 mg/dL. He was started on dobutamine and a loop diuretic infusion that resulted in a net diuresis of 2 L daily. His renal function worsened, and the nephrology service was consulted. A renal biopsy (performed in anticipation of cardiacrenal transplantation) revealed changes consistent with type II cardiorenal syndrome, specifically ischemic glomerulopathy. With continued diuresis, the patient’s nonoliguric acute kidney injury failed to improve. A peritoneal dialysis (PD) catheter was placed, and PD was initiated a few days later.
Despite rapid-exchange nighttime PD, the patient did not experience significant clinical improvement, and so an intra-aortic balloon pump (IABP) was placed. The patient did well after insertion of the IABP. Renal function improved, and PD was held. However, the patient could not be weaned off the IABP, and therefore discussion began regarding insertion of a left ventricular assist device (LVAD) as a temporizing measure until heart transplantation.
Before implantation of the LVAD, a discussion between the cardiology, cardiothoracic surgery, and nephrology services ensued about whether the PD catheter should be removed to reduce the risk of infection. The patient had a minor exit-site infection that was being successfully treated with gentamicin cream, but no indications of peritonitis or tunnel tract infection were evident. A decision to maintain the PD catheter was ultimately made. The patient underwent LVAD placement without complications or postsurgical infection. Peritoneal dialysis for volume removal and clearance was required for 11 days post surgery. The patient subsequently suffered a severe thromboembolic stroke and received comfort care.
DISCUSSION
PD AND CONGESTIVE HEART FAILURE
The first challenge in the present case was the choice of modality for renal replacement. Considering the patient’s need for inotropic support, there was concern about his ability to tolerate the hemodynamic demands of hemodialysis (HD). Ultimately, PD was chosen based on the theory that it allows for gentle ultrafiltration and requires just one procedure for a permanent catheter, which has a lower risk of systemic infection than a HD catheter does. Moreover, the PD catheter could also be used to perform dialysis at home.
The merits of PD in patients with congestive heart failure (CHF) have previously been described (8). Krishnan and Oreopoulos outlined how PD provides a simple solution to the volume-overloaded sodium-retentive state of CHF, because filtration and volume removal are gentle and continuous, allowing for a more hemodynamically tolerated procedure. Those authors also noted that once patients are stabilized with volume removal, renal function is often regained, and response to diuretics is improved. A limitation of their review is the sample size of the available studies.
Despite such proposed benefits, previous studies had suggested that mortality rates are higher in end-stage renal disease patients with CHF treated with chronic PD than in those treated with HD (9). More recent literature exploring the relationship between mortality and dialysis modality suggests that outcomes are not equivalent for all patients (10). The study by Vonesh and colleagues to determine risk factors for mortality by dialysis modality illustrates the complexities. Their study subjects were stratified by age, modality, presence of diabetes mellitus, and presence of comorbidities, including heart failure. For all strata, risk of death was either equivalent or higher for subjects on HD except for older patients with diabetes (10). Similarly, in a recent publication by Mehrotra et al., survival based on modality was assessed for patients with and without diabetes, taking comorbidity into account. That study’s findings again suggest that the presence of diabetes mellitus accounts for the decreased survival in PD patients, and that the survival discrepancy has been narrowing over time (11). To further elucidate the relationship between heart failure and dialysis modality, Trespalacios et al. conducted a study to determine whether dialysis modality affects hospitalization for or occurrence of heart failure. Their study revealed that HD led to a greater incidence of hospitalization for heart failure, as well as a greater incidence of de novo heart failure (12). The foregoing studies were all focused on outcomes for patients receiving chronic PD, and thus were not directly applicable our clinical case. Yet it is important to consider longer-term outcomes, because one possible outcome of our case might have been longer-term reliance on LVAD therapy—and thus PD—while awaiting transplantation.
LVAD AND SYSTEMIC INFECTION
Once the decision was made to implant a LVAD in our patient, the next clinical decision was whether to remove the PD catheter to reduce infection risk before LVAD implantation. The concern to prevent systemic infection in the peri-LVAD hospital course is a significant one, because the incidence of bacteremia post-LVAD is well described in the literature (Table 1). The consequences and effects of bacteremia on management options and transplant candidacy are less well-defined.
TABLE 1.
Studies Describing the Infection Incidence During Therapy with a Left Ventricular Assist Device (LVAD)
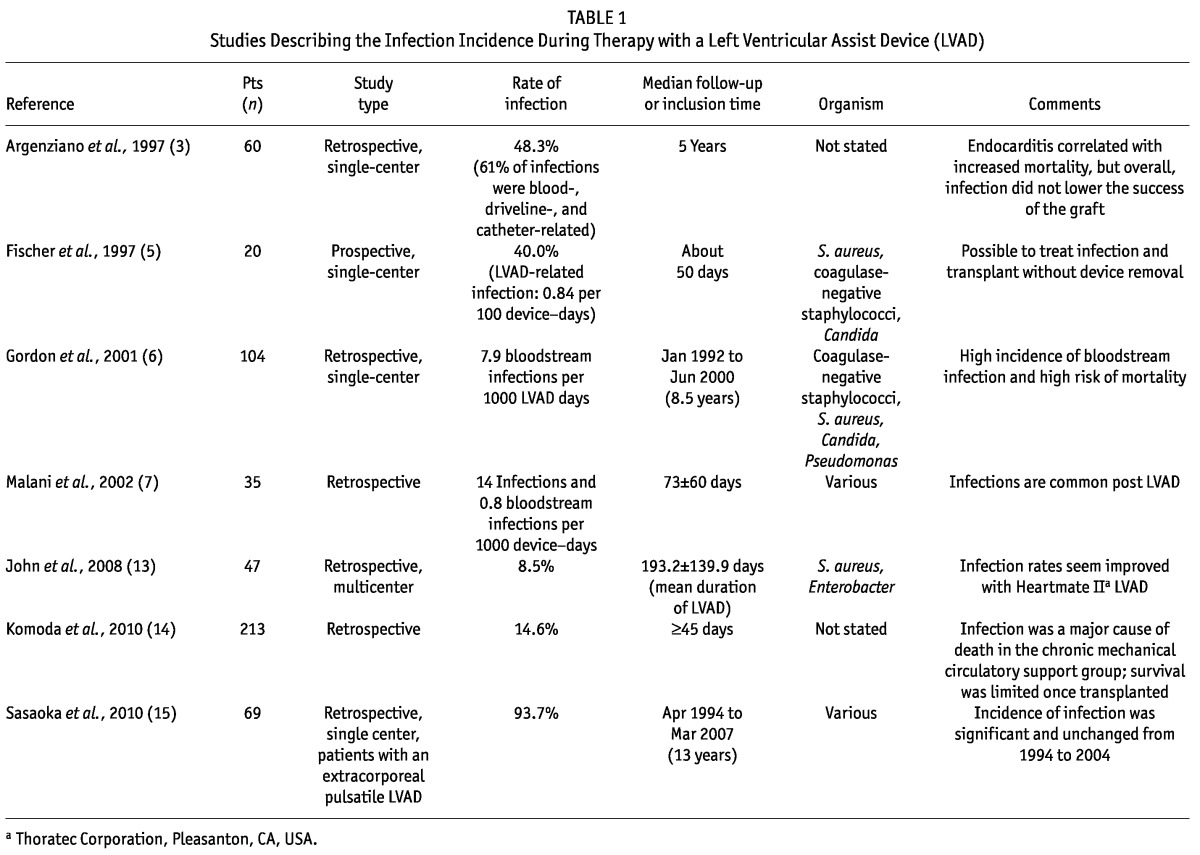
The study from 1997 conducted by Argenziano et al. describes a retrospective single-center analysis in which bloodstream infections occurred in half the patients implanted with LVADs in a 5-year period, but the author demonstrates that only endocarditis portended a poor survival prognosis (3). In the same year, Fischer et al. published a retrospective study of the first 20 LVADs placed at their institution and noted that, although 8 of the 20 patients developed bloodstream infections, a 6-week course of antibiotic therapy was successful in treating infection, and transplantation was ultimately achieved without an increase in post-transplant mortality (5).
In 2001, Gordon et al. published a study investigating nosocomial infections in patients that received an implantable LVAD at the Cleveland Clinic (6). In those 104 patients, 7.9 bloodstream infections occurred per 1000 LVAD days, with a first infection developing on average about 23 days post implantation. As had been reported by the Argenziano and Fischer groups, bloodstream infections did not portend a poorer survival prognosis post transplantation. Importantly, Gordon’s work did reveal a statistically significantly higher death rate in the group with bloodstream infections during LVAD treatment (6). In the same year, Malani et al. published on the incidence of nosocomial infections in LVAD recipients at a single institution over a 32-month period. Their aim was to better ascertain incidence and risk factors, and their research revealed frequent postsurgical infections ranging from pneumonia to bloodstream infections. In assessing prospective risk factors, the only positive risk factor was acute HD, which was a risk factor present in 77% of the patients who developed infections and in only 30% of those who did not, although the difference did not reach statistical significance (7).
Sasaoka et al. conducted a study in Japan to ascertain whether management of post-LVAD complications had improved between 1997 and 2004 (15). This group had a special interest in investigating complication rates in long-term LVAD treatment with changing LVAD technology, because the time on the wait list for a heart transplant in Japan can exceed 2 years. They discovered that the high incidence of systemic infection remained the same despite advances in technology, but that mortality rates had declined with improved and prompt initiation of treatment for infection (15).
In Germany, Komoda et al. performed a study to determine whether prioritization of candidates for heart transplantation needed to be revisited in light of a severe organ shortage. Infection was one of the indices studied to determine whether the incidence of infection should affect the priority of patients receiving mechanical circulatory support while awaiting transplantation (14). Infection was found to be a leading cause of death in the acute phase post LVAD insertion, and it was a leading cause of a change in status to “urgent” for those awaiting heart transplantation at 45 days post LVAD. The study illustrated that survival was superior for the group urgently transplanted secondary to bleeding or coagulation diathesis than secondary to infection. That finding led the group to question whether, compared with coagulation difficulties, infection should take a lower priority in the decision about who should be awarded a graft when both patients are classified as urgent (14).
Considering all of the foregoing findings, it can be concluded that systemic infection is a serious and common complication after LVAD implantation in acute and chronic LVAD therapy alike. Although many studies illustrate an ability to transplant effectively despite infection, the study by Gordon and colleagues indicates higher mortality secondary to infection while a patient is maintained on a LVAD. As seen in the work by the Sasoaka and Komoda groups, long-standing maintenance until transplantation can be the norm in an organ-scarce setting.
HD VERSUS PD: RISK OF INFECTION
Infection is second only to cardiovascular disease as the major cause of death in the chronic dialysis population, and it is the leading cause of hospitalization (16,17). In the acute setting, initiation of PD or HD involves either intravascular or intraperitoneal catheter placement, and therefore the infection risk is increased. Several studies in the literature explore the relationship between dialysis access type and consequent infection risk (Table 2).
TABLE 2.
Studies Describing Dialysis Access and Infection
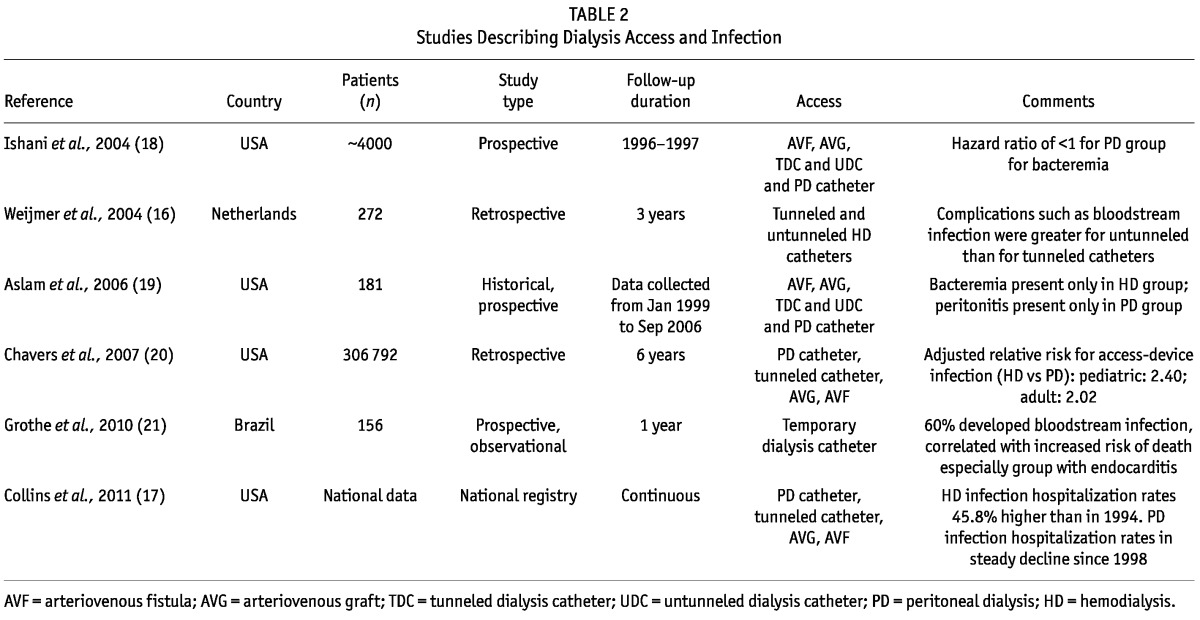
The most recent data from the US Renal Data System indicate that, compared with 1994, hospitalization rates for HD patients have risen by 45.8% (17). In contrast, PD hospitalization rates for infection have been declining steadily since 1994, although hospitalizations for bacteremia or sepsis have increased for both modalities (17). A recent publication by Chavers et al. assessed the incidence of hospitalization for infection in the pediatric and adult populations by modality. Study results revealed that, compared with the risk in PD groups, the risk for hospitalization secondary to device-related infection in both the adult and pediatric HD groups was more than doubled (20).
The prospective observational cohort study by Aslam et al. involved quantifying incident infection for patients newly initiated on dialysis within a 5-year period at a single outpatient dialysis unit (19). Of the 119 HD and 62 PD patients assessed, bacteremia developed only in the HD cohort. Within that cohort, the incidence of bacteremia clearly correlated with access type, the highest risk being associated with a temporary dialysis catheter and the second highest, with a tunneled cuffed dialysis catheter (19). Similarly, the prospective study reported in 2004 by Ishani et al. showed that the risk for development of bacteremia was significantly reduced in the PD population (18). Of the available HD access modalities, catheters were correlated with a significantly higher incidence of bacteremia and endocarditis than were grafts or fistulas (18).
Wejimer and colleagues performed a study in the Netherlands to assess whether risk of infection varied between tunneled and temporary HD access (16). Results indicated a lower incidence of infection in tunneled catheters and uncuffed subclavian catheters, with uncuffed internal jugular and uncuffed femoral catheters being correlated with much higher rates of infection (16). In Brazil, Grothe et al. showed that uncuffed catheters were associated with an extremely high rate of bloodstream infection and higher mortality rates, especially when the infection led to endocarditis (21).
From the foregoing studies, we conclude that acute HD access such as uncuffed catheters and tunneled cuffed catheters result in a significantly higher incidence of bacteremia. Catheters for PD primarily lead to exit-site infection, tunnel tract infections, and most severely, peritonitis. It is less common for peritonitis to lead to sepsis. The occasional case report exists, but the origin of the peritonitis in those instances is often the gastrointestinal tract, not primarily the catheter (22). We must also again raise the point that the conclusions from the studies discussed here cannot be directly related to our clinical scenario, because they deal with longer-term infection by modality, and our concern was primarily for perioperative LVAD infection risk.
CONCLUSIONS
Cardiorenal syndrome can lead to the need for renal replacement therapy, either for the purpose of clearance or to assist in volume management. Access placement for acute HD has been associated with increased incidences of systemic infection. This elevated risk is of particular concern in patients requiring a LVAD as bridge therapy to transplantation, or as definitive treatment for CHF, because LVAD infection can reduce survival.
The present review includes literature addressing the long-term risks of PD compared with HD for infection and mortality outcomes alike. Although not directly applicable (considering the acuity of our patient’s needs), chronicity was an important factor to consider because our patient’s wait time for transplantation was unknown, and he was expected to remain dialysis-dependent in the interim.
Despite a lack of definitive evidence for the efficacy of PD in the treatment of severe heart failure, PD has several significant advantages for patients with a LVAD. Most importantly, infections secondary to a peritoneal catheter seldom lead to bacteremia. Peritonitis is a significant infection, but the risk can be minimized with connectivity training and close patient monitoring. In addition, a PD catheter can be placed with conscious sedation and local anesthetic in the acute setting; it can be used soon after placement; and it allows the patient to perform dialysis at home. This last point is critical, because patients with significant cardiac impairment or a LVAD may not be candidates for outpatient management in a HD center because of their tenuous hemodynamics. Moreover, patients with a LVAD do not have a pulsatile blood pressure that can be recorded by standard devices, complicating the safety of outpatient dialysis. Thus, PD could potentially lead to quicker discharge because outpatient center arrangements would not have to be made if the patient remains dialysis-dependent. The availability of surgeons trained to non-invasively place PD catheters could be a limiting factor, but a solution exists in the possibility of interventional nephrologists or radiologists acquiring the ability to place the catheters.
Also, to avoid the scenario of a patient with significant heart disease and renal insufficiency requiring emergent dialysis secondary to cardiorenal syndrome, discussions should take place as soon as possible regarding placement of a permanent dialysis access, either as a fistula or a PD catheter. Again, a PD catheter would be a superior choice in this instance if the patient has a significant chance of regaining renal function or of receiving a graft, because the catheter can be removed.
Finally, PD has been known to preserve residual renal function longer than HD. In cardiorenal syndrome, there is a possibility that kidney function can return if hemodynamics stabilize for long enough in patients with viable renal parenchyma. Randomized controlled trials are needed to assess the outcomes of infection, morbidity, and mortality in patients with a LVAD who receive either HD or PD. Until further evidence from such trials is available, we assert that PD should be considered for patients who require renal replacement therapy and who are approaching the need for a LVAD.
DISCLOSURES
None of the authors of this manuscript claim any affiliations requiring disclosures for the present research.
REFERENCES
- 1. Roger VL. The heart failure epidemic. Int J Environ Res Public Health 2010; 7:1807–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, et al. on behalf of the Acute Dialysis Quality Initiative (ADQI) consensus group. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J 2010; 31:703–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Argenziano M, Catanese KA, Moazami N, Gardocki MT, Weinberg AD, Clavenna MW, et al. The influence of infection on survival and successful transplantation in patients with left ventricular assist devices. J Heart Lung Transplant 1997; 16:822–31 [PubMed] [Google Scholar]
- 4. Birks EJ, Yacoub MH, Banner NR, Khaghani A. The role of bridge to transplantation: should LVAD patients be transplanted? Curr Opin Cardiol 2004; 19:148–53 [DOI] [PubMed] [Google Scholar]
- 5. Fischer SA, Trenholme GM, Costanzo MR, Piccione W. Infectious complications in left ventricular assist device recipients. Clin Infect Dis 1997; 24:18–23 [DOI] [PubMed] [Google Scholar]
- 6. Gordon SM, Schmitt SK, Jacobs M, Smedira NM, Goormastic M, Banbury MK, et al. Nosocomial bloodstream infections in patients with implantable left ventricular assist devices. Ann Thorac Surg 2001; 72:725–30 [DOI] [PubMed] [Google Scholar]
- 7. Malani PN, Dyke DB, Pagani FD, Chenoweth CE. Nosocomial infections in left ventricular assist device recipients. Clin Infect Dis 2002; 34:1295–300 [DOI] [PubMed] [Google Scholar]
- 8. Krishnan A, Oreopoulos DG. Peritoneal dialysis in congestive heart failure. Adv Perit Dial 2007; 23:82–9 [PubMed] [Google Scholar]
- 9. Stack AG, Molony DA, Rahman NS, Dosekun A, Murthy B. Impact of dialysis modality on survival of new ESRD patients with congestive heart failure in the United States. Kidney Int 2003; 64:1071–9 [DOI] [PubMed] [Google Scholar]
- 10. Vonesh EF, Snyder JJ, Foley RN, Collins AJ. The differential impact of risk factors on mortality in hemodialysis and peritoneal dialysis. Kidney Int 2004; 66:2389–401 [DOI] [PubMed] [Google Scholar]
- 11. Mehrotra R, Chiu YW, Kalantar-Zadeh K, Bargman J, Vonesh E. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 2011; 171:110–18 [DOI] [PubMed] [Google Scholar]
- 12. Trespalacios FC, Taylor AJ, Agodoa LY, Bakris GL, Abbott KC. Heart failure as a cause for hospitalization in chronic dialysis patients. Am J Kidney Dis 2003; 41:1267–77 [DOI] [PubMed] [Google Scholar]
- 13. John R, Kamdar F, Liao K, Colvin-Adams M, Boyle A, Joyce L. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg 2008; 86:1227–35 [DOI] [PubMed] [Google Scholar]
- 14. Komoda T, Drews T, Hetzer R, Lehmkuhl HB. New prioritization of heart transplant candidates on mechanical circulatory support in an era of severe donor shortage. J Heart Lung Transplant 2010; 29:989–96 [DOI] [PubMed] [Google Scholar]
- 15. Sasaoka T, Kato TS, Komamura K, Takahashi A, Nakajima I, Oda N, et al. Improved long-term performance of pulsatile extracorporeal left ventricular assist device. J Cardiol 2010; 56:220–8 [DOI] [PubMed] [Google Scholar]
- 16. Weijmer MC, Vervloet MG, ter Wee PM. Compared to tunnelled cuffed haemodialysis catheters, temporary untunnelled catheters are associated with more complications already within 2 weeks of use. Nephrol Dial Transplant 2004; 19:670–7 [DOI] [PubMed] [Google Scholar]
- 17. Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, et al. US Renal Data System 2010 Annual Data Report. Am J Kidney Dis 2011; 57(Suppl 1):A8,e1-526 [DOI] [PubMed] [Google Scholar]
- 18. Ishani A, Collins AJ, Herzog CA, Foley RN. Septicemia, access and cardiovascular disease in dialysis patients: the USRDS Wave 2 study. Kidney Int 2005; 68:311–18 [DOI] [PubMed] [Google Scholar]
- 19. Aslam N, Bernardini J, Fried L, Burr R, Piraino B. Comparison of infectious complications between incident hemodialysis and peritoneal dialysis patients. Clin J Am Soc Nephrol 2006; 1:1226–33 [DOI] [PubMed] [Google Scholar]
- 20. Chavers BM, Solid CA, Gilbertson DT, Collins AJ. Infection-related hospitalization rates in pediatric versus adult patients with end-stage renal disease in the United States. J Am Soc Nephrol 2007; 18:952–9 [DOI] [PubMed] [Google Scholar]
- 21. Grothe C, da Silva Belasco AG, de Cássia Bittencourt AR, Vianna LA, de Castro Cintra Sesso R, Barbosa DA. Incidence of bloodstream infection among patients on hemodialysis by central venous catheter. Rev Lat Am Enfermagem 2010; 18:73–80 [DOI] [PubMed] [Google Scholar]
- 22. Morduchowicz G, van Dyk DJ, Wittenberg C, Winkler J, Boner G. Bacteremia complicating peritonitis in peritoneal dialysis patients. Am J Nephrol 1993; 13:278–80 [DOI] [PubMed] [Google Scholar]


