Abstract
♦ Background, Objectives, and Methods: Hospitalization and mortality rates in pediatric dialysis patients remain unacceptably high. Although studies have associated the presence of comorbidities with an increased risk for death in a relatively small number of pediatric dialysis patients, no large-scale study had set out to describe the comorbidities seen in pediatric dialysis patients or to evaluate the impact of those comorbidities on outcomes beyond the newborn period. In the present study, we evaluated the prevalence of comorbidities in a large international cohort of pediatric chronic peritoneal dialysis (CPD) patients from the International Pediatric Peritoneal Dialysis Network registry and began to assess potential associations between those comorbidities and hospitalization rates and mortality.
♦ Results: Information on comorbidities was available for 1830 patients 0 - 19 years of age at dialysis initiation. Median age at dialysis initiation was 9.1 years [interquartile range (IQR): 10.9], median follow-up for calculation of hospitalization rates was 15.2 months (range: 0.2 - 80.9 months), and total follow-up time in the registry was 2095 patient-years. At least 1 comorbidity had been reported for 602 of the patients (32.9%), with 283 (15.5%) having cognitive impairment; 230 (12.6%), motor impairment; 167 (9.1%), cardiac abnormality; 76 (4.2%), pulmonary abnormality; 212 (11.6%), ocular abnormality; and 101 (5.5%), hearing impairment. Of the 150 patients (8.2%) that had a defined syndrome, 85% had at least 1 nonrenal comorbidity, and 64% had multiple comorbidities. The presence of at least 1 comorbidity was associated with a higher hospitalization rate [hospital days per 100 observation days: 1.7 (IQR: 5.8) vs 1.2 (IQR: 3.9), p = 0.001] and decreased patient survival (4-year survival rate: 73% vs 90%, p < 0.0001).
♦ Conclusions: Nearly one third of pediatric CPD patients in a large international cohort had at least 1 comorbidity, and multiple comorbidities were frequently reported among patients with a defined syndrome. Preliminary analysis suggests an association between comorbidity and poor outcome in those patients. As this powerful international registry matures, further multivariate analyses will be important to more clearly define the impact of comorbidities on hospitalization rates and mortality in pediatric CPD patients.
Keywords: Pediatric, comorbidities, syndrome, outcomes, hospitalization, mortality
Although significant improvements have been realized in the care of children with end-stage kidney disease (ESKD), hospitalization and mortality rates remain unacceptably high (1-5). Efforts to improve outcomes in pediatric ESKD patients have appropriately focused on optimizing cardiovascular status and minimizing the risk for infection, which are two of the leading causes of hospitalization and death in pediatric ESKD patients (2,3,5).
The comorbidities associated with an increased risk for poor outcomes in adult ESKD patients—including diabetes mellitus, chronic obstructive lung disease, peripheral vascular disease, and cerebrovascular disease—are not common in pediatric patients. However, children with ESKD may have other comorbidities, such as neurocognitive impairment and congenital heart disease, that may independently influence outcomes. To date, no study had set out to define the presence of such comorbidities in a large cohort of pediatric dialysis patients. The International Pediatric Peritoneal Dialysis Network (IPPN) is a voluntary global registry that collects data, including data about comorbidities, on children maintained on chronic peritoneal dialysis (CPD). The objectives of the present study were to characterize the prevalence of comorbidities in this multicenter international cohort and to begin to evaluate potential associations between comorbidity and hospitalization rates and mortality.
METHODS
The IPPN was established in 2007 and currently collects comprehensive patient, clinical, and laboratory information on children maintained on CPD at 87 centers in 33 countries around the world. Data input to the IPPN registry is voluntary and performed exclusively using an Internet-based web platform (http://www.pedpd.org). The patient characteristics collected at entry to the registry are age, sex, cause of ESKD, age at initiation of CPD, and presence of comorbidities, including a defined syndrome, cognitive impairment, motor impairment, cardiac abnormalities, pulmonary abnormalities, ocular abnormalities, and hearing impairment. A separate yes-or-no response is requested to indicate the presence or absence of each comorbidity. If a comorbidity is indicated as present, free-text responses are solicited to describe the comorbidity. Patient updates, including clinical and laboratory data, are requested at 6-month intervals. Data requested at each update include the number of hospitalizations and the number of hospital days in the preceding 6 months. Termination forms are requested when a patient permanently discontinues CPD at the enrolling center because of kidney transplantation, transfer to hemodialysis, transfer to another center, death, or other reason.
Data protection is ensured by pseudonymized data input. The data are automatically checked for plausibility and completeness. Approval for the registry project was obtained from local institutional review boards or ethics committees and informed consent was obtained from the patients or their legal guardians, or both, when required by local review boards.
To define the prevalence of comorbidities, the study cohort included all patients enrolled in the registry for whom at least 1 comorbidity was indicated as present or absent, and who had been 0 to less than 20 years of age at the time of dialysis initiation. For the purposes of the study, cognitive abnormalities were grouped into 10 subcategories: severe cognitive impairment, mild cognitive impairment, cognitive impairment without specified severity, learning disability, behavioral or psychiatric abnormalities, pervasive developmental disorder, central nervous system malformations, hypoxic or ischemic central nervous system injury, seizures, and other secondary or unspecified impairment. Motor abnormalities were grouped into 7 subcategories: severe motor impairment, mild motor impairment, motor impairment without specified severity, neuropathy or undefined hypotonia, central nervous system malformations, skeletal malformations, and other secondary or unspecified motor delay. Cardiac abnormalities included 6 subcategories: complex congenital heart disease, mild congenital heart disease, valvular disease, heart transplant, cardiomyopathy, and other or unspecified abnormality. Pulmonary abnormalities included 5 subcategories: pulmonary hypoplasia, bronchopulmonary dysplasia, obstructive sleep apnea, recurrent infection or inflammatory disease, and other abnormality. Ocular abnormalities included 11 subcategories: optic nerve abnormalities, strabismus, ametropia, nystagmus, retinal abnormalities, cranial nerve abnormalities, cataracts, other corneal abnormalities, blindness, and other or unspecified abnormality. Hearing abnormalities were categorized as deafness or hearing impairment.
Clinical characteristics of patients identified as having any comorbidity were compared with characteristics of those having no comorbidity. The variables compared included age at dialysis initiation, duration of dialysis at study enrollment, and cause of ESKD. These characteristics and the presence of other comorbidities were also compared in the cohorts of patients with and without a defined syndrome, cognitive abnormality, cardiac abnormality, or pulmonary abnormality. For analytic purposes, the causes of ESKD were categorized as congenital anomalies of the kidney and urinary tract (CAKUT) and others.
Hospitalization rates are presented as number of hospital days per 100 patient-days at risk and were calculated by dividing the total number of hospital days during the observation period by the number of days in the observation period for the patient, multiplied by 100. The observation period was counted as the number of days from the first update for the patient through the most recent update. Data entry for the present analysis was closed on 4 April 2012. Patients without 2 clinical updates were excluded from the hospitalization analysis. Survival rates were calculated from time of study entry until termination because of death. Patients were censored at the time of termination from the registry.
Data are expressed as median and interquartile range (IQR). Differences in group medians were assessed using a Wilcoxon signed-rank test, and differences in proportions were assessed using the chi-square test. The survival analysis used Kaplan-Meier curves and log-rank tests to compare mortality in patients with and without any comorbidity, and with and without cognitive, cardiac, or pulmonary comorbidity. Data were analyzed using the SAS software application (version 9.2: SAS Institute, Cary, NC, USA).
RESULTS
Baseline demographic data were available for 1844 patients, 5 of whom had missing comorbidity data. At the time of dialysis initiation, 9 patients were 20 years or older, and so the final cohort included 1830 patients. Median age of the cohort at dialysis initiation was 9.1 years (IQR: 10.9 years) and median duration of dialysis at registry entry was 0.5 years (IQR: 1.2 years). Of the 1830 patients, 1014 (55.4%) were male, and CAKUT was identified as the underlying cause of ESKD in 815 patients (44.5%).
Of the 1830 patients, 602 (32.9%) were reported to have at least 1 comorbidity. Table 1 shows the percentage of patients reported in each of the comorbidity categories and subcategories. Some form of cognitive abnormality was identified for 283 patients (15.5%), with 117 (6.4%) having severe impairment and 107 (5.8%) having mild impairment. Cardiac abnormalities were reported in 167 patients (9.1%), including complex congenital heart disease in 22. Pulmonary abnormalities were reported in 76 (4.2%) patients.
TABLE 1.
Prevalence of Comorbidities in an International Pediatric Peritoneal Dialysis Network Cohort of 1830 Patients
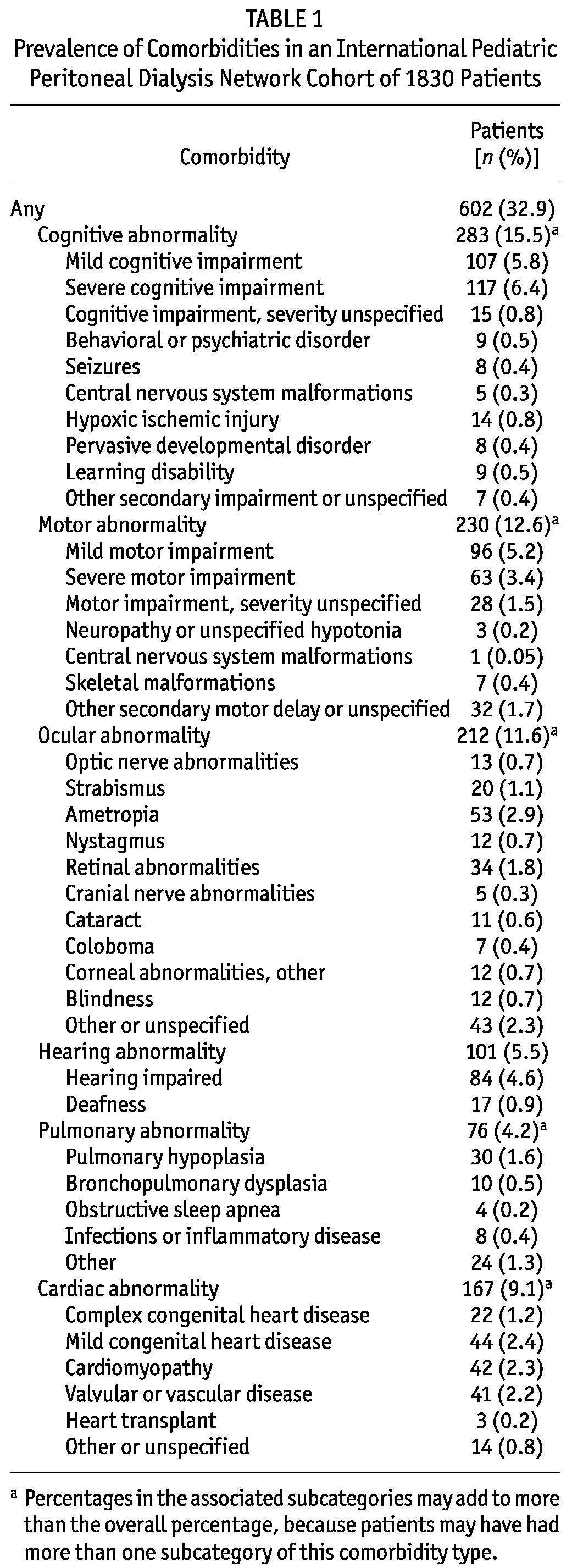
Compared with patients not having a comorbidity, patients with a comorbidity were significantly younger at dialysis initiation [7.9 years (IQR: 11.7 years) vs 9.5 years (IQR: 10.4 years), p < 0.001], had a longer duration of dialysis at entry into the registry [0.5 years (IQR: 1.3 years) vs 0.4 years (IQR: 1.2 years), p = 0.04], and were more likely to have CAKUT as the cause of ESKD (48.2% vs 42.8%, p = 0.028).
An identified syndrome was reported in 150 patients (8.2%). Table 2 lists syndromes that were found in at least 2 patients in the cohort. An additional 29 syndromes were present in a single patient each. Age at dialysis initiation [9.1 years (IQR: 8.9 years) vs 9.1 years (IQR: 11.0 years), p = 0.356], duration of dialysis at study entry [0.5 years (IQR: 1.4 years) vs 0.4 years (IQR: 1.2 years), p = 0.125], and percentage of patients with CAKUT as the cause of ESKD (40.7% vs 44.9%, p = 0.320) were not different between the syndromic and nonsyndromic patients.
TABLE 2.
Syndromes Present in 150 Pediatric Chronic Peritoneal Dialysis Patients Registered in the International Pediatric Peritoneal Dialysis Network
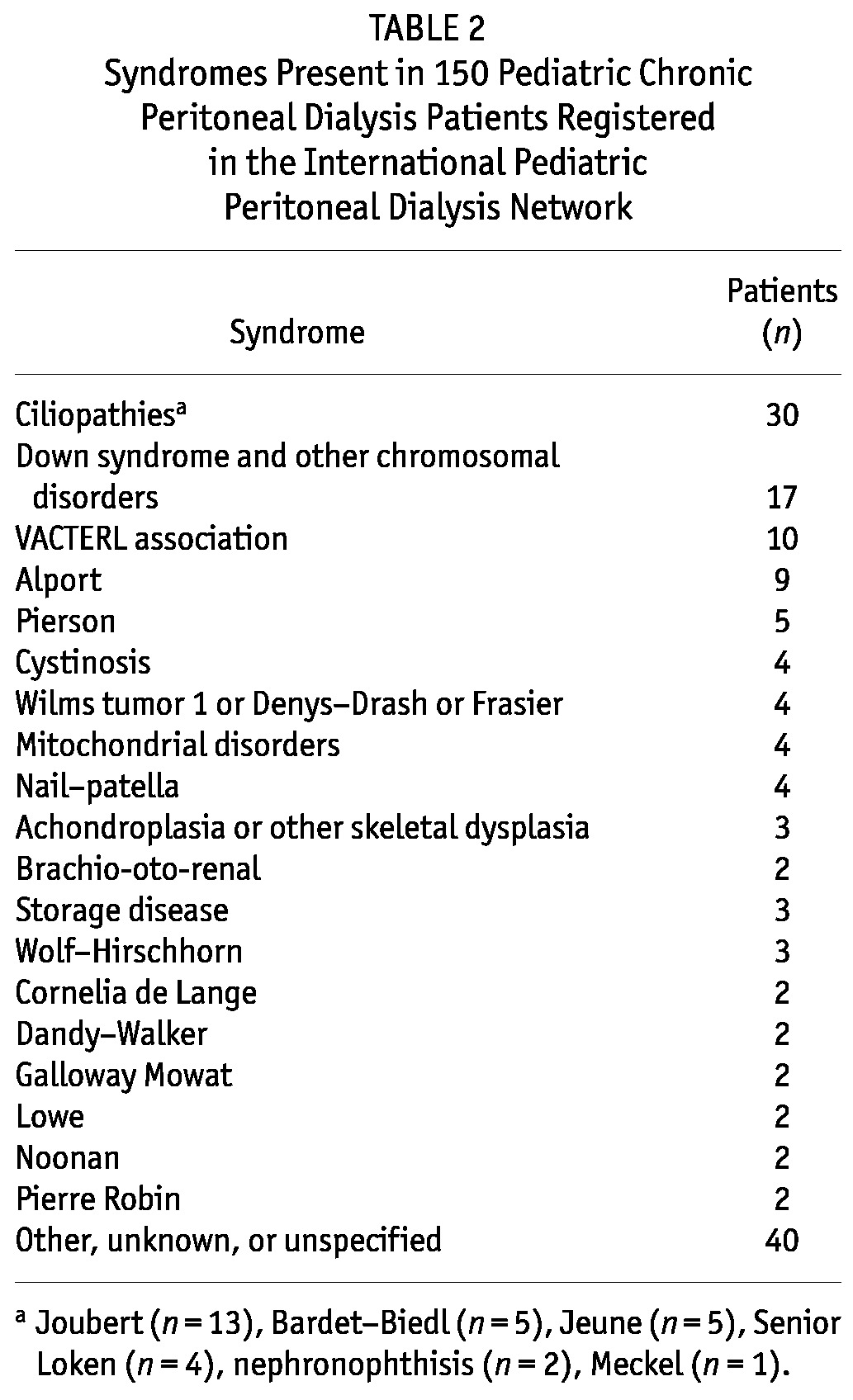
Table 3 shows, for the various comorbidity categories, the percentage of patients having the comorbidity in isolation or associated with a syndrome. Each of the comorbidity categories was significantly more likely to be reported in patients with a defined syndrome than in patients without.
TABLE 3.
Prevalence of Comorbidity in Pediatric Chronic Peritoneal Dialysis Patients With and Without a Defined Syndrome
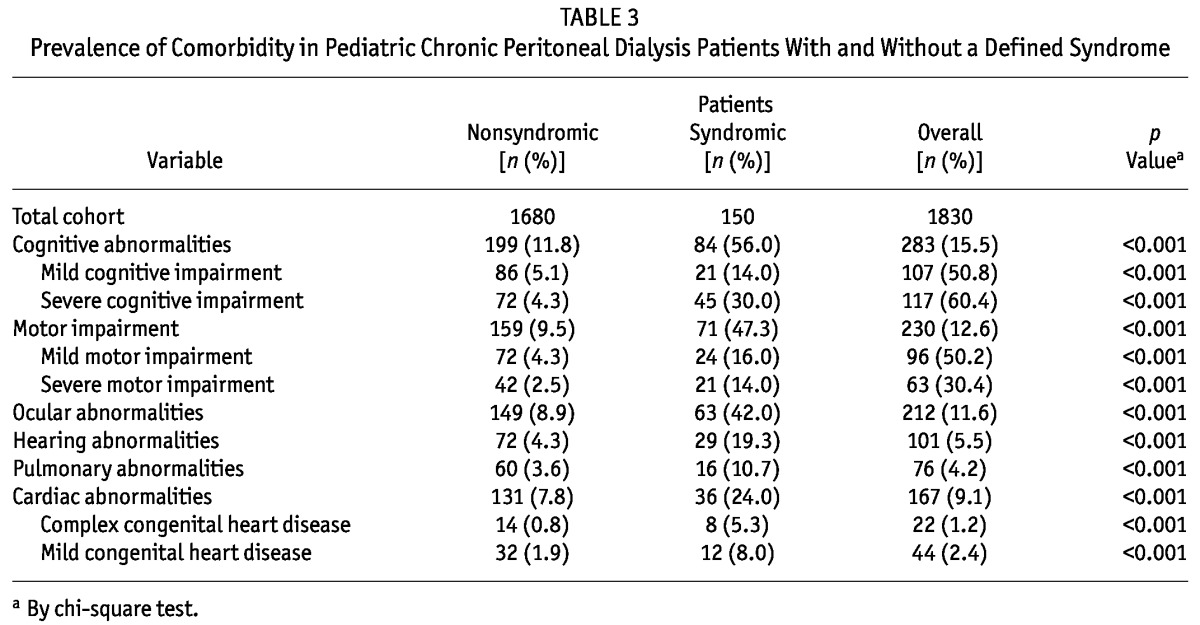
Figure 1 shows the percentage of patients with multiple comorbidities in the entire cohort and in the groups with and without an identified syndrome, and with CAKUT as the cause of ESKD. Of the 150 patients with a syndrome, 128 (85%) had at least 1 nonrenal comorbidity, and 96 (64%) had multiple comorbidities.
Figure 1.
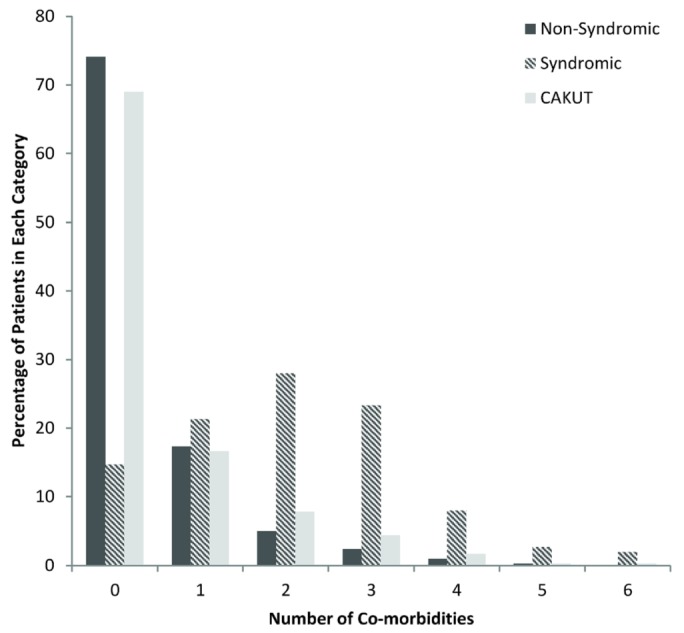
— Percentage of patients having multiple comorbidities with and without a syndrome and having CAKUT (congenital anomalies of the kidney and urinary tract) as the cause of end-stage kidney disease.
A comparison of patients having a reported cognitive abnormality with the remainder of the cohort revealed that patients with a cognitive abnormality were significantly younger at dialysis initiation [6.5 years (IQR: 10.7 years) vs 9.4 years (IQR: 10.9 years), p < 0.001], had a longer duration of dialysis at study entry [0.6 years (IQR: 1.5 years) vs 0.4 years (IQR: 1.2 years), p = 0.003], were more likely to have a cardiac or pulmonary abnormality (27.6% vs 9.1%, p < 0.001), and were more likely to have CAKUT as the underlying cause of ESKD (55.1% vs 42.6%, p < 0.001). For patients with cardiac abnormalities, median age at dialysis initiation was also significantly lower than it was in patients without a cardiac abnormality [7.4 years (IQR: 11.7 years) vs 9.2 years (IQR: 10.6 years), p = 0.014], and CAKUT was more commonly the cause of ESKD (52.1% vs 43.8%, p = 0.039). However, duration of dialysis at study entry was not different between the groups [0.5 years (IQR: 1.1 years) vs 0.5 years (IQR: 1.3 years), p = 0.842]. Similarly, patients with pulmonary abnormalities were younger at dialysis initiation [2.8 years (IQR: 10.7 years) vs 9.2 years (IQR: 10.6 years), p < 0.001] and more likely to have CAKUT (59.2% vs 43.9%, p = 0.009), but duration of dialysis at study entry was not significantly different between patients with and without pulmonary abnormalities [0.5 years (IQR: 1.3 years) vs 0.5 (IQR: 1.2 years), p = 0.859].
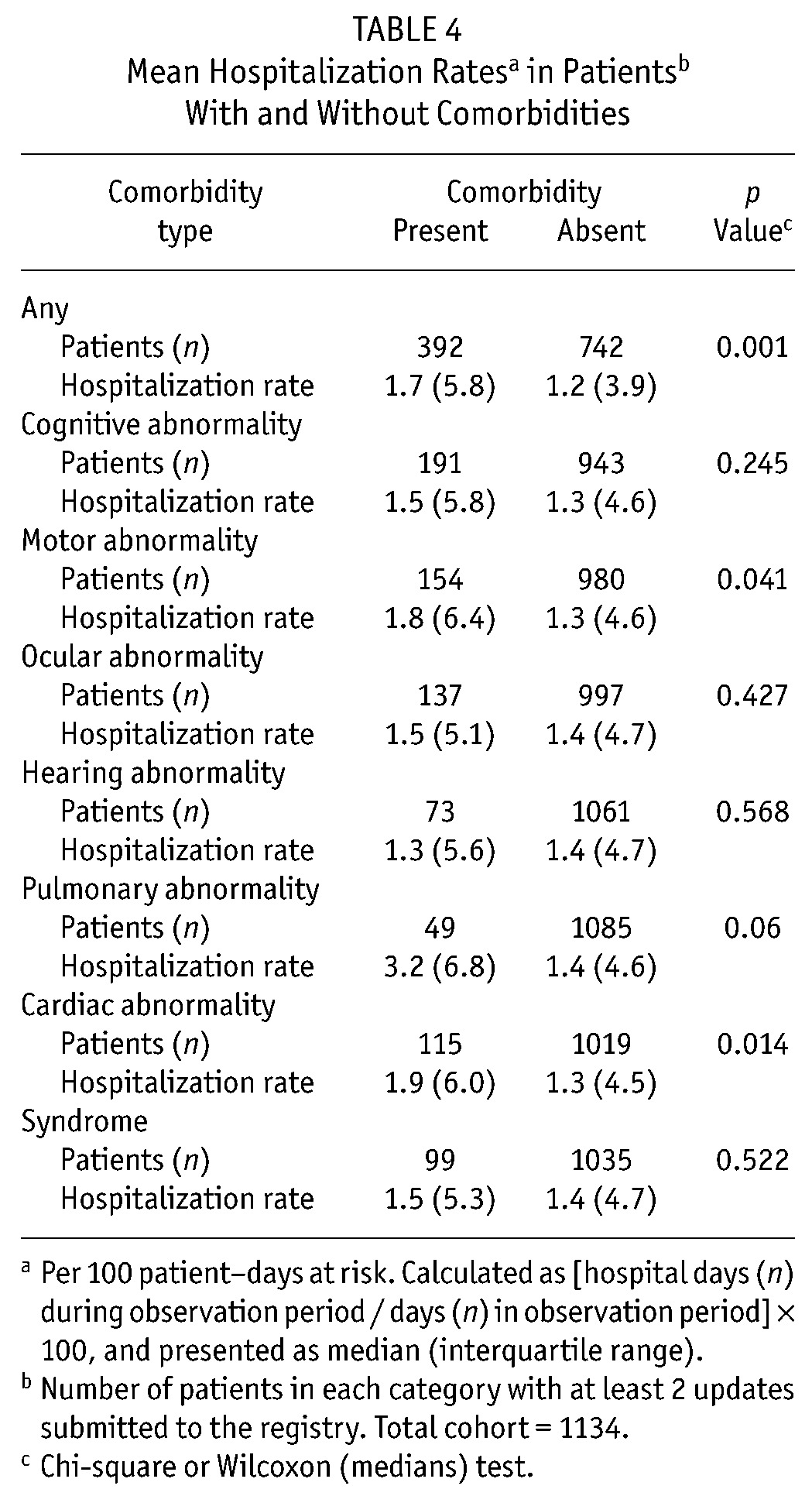
Of the 1830 patients, 1134 (62%) had at least 2 updates submitted and were included in the hospitalization analysis. The median duration of follow up was 15.2 months (range: 0.2 - 80.9 months). The median hospitalization rate (days per 100 patient-days at risk) for the 1134 patients was 1.4 (IQR: 4.7). Table 4 compares hospitalization rates for patients with and without a comorbidity. Patients with any comorbidity had a higher hospitalization rate than did patients without a comorbidity. In addition, patients with a motor, pulmonary, or cardiac abnormality had a higher hospitalization rate than did patients without those comorbidities.
TABLE 4.
Mean Hospitalization Ratesa in Patientsb With and Without Comorbidities
Among the 1830 patients in the cohort, 71 were reported to have died. The most frequent causes of death were infections (31%) and cardiac causes (25%), with 10 deaths (14.1%) being attributed to dialysis-related infections; 4 (5.6%), to noninfectious dialysis complications; 2 (2.8%), to malignancy; and 15 (21.1%), to other or unknown causes. For the survival analysis, total follow-up time in the registry was 2095 patient-years. Figure 2 reveals that, compared with patients not having a comorbidity, those with any comorbidity had a significantly lower survival rate during the observation period (73% vs 90%, p < 0.0001). The same was true for patients with cognitive abnormalities (63% vs 90%, p < 0.0001), cardiac abnormalities (73% vs 84%, p < 0.001), and pulmonary abnormalities (50% vs 85%, p < 0.0001). Patients with comorbidities were more likely to die from infections than were patients without comorbid conditions (24 of 43 vs 8 of 28, p < 0.05).
Figure 2.
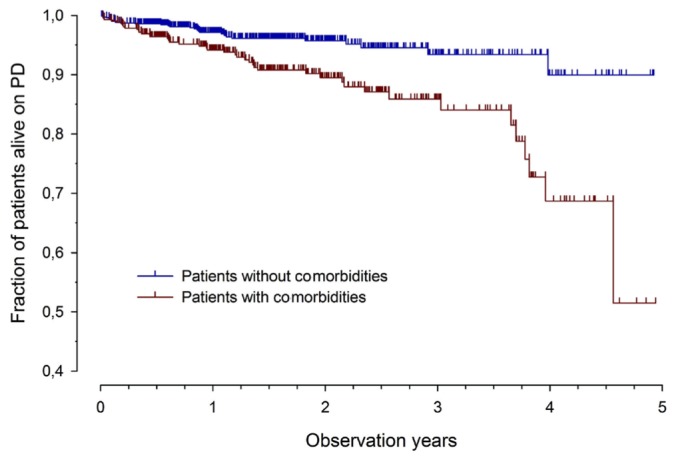
— Cumulative survival by absence or presence of at least 1 comorbidity (log-rank p < 0.0001).
DISCUSSION
The care of children with ESKD has seen significant improvements over the past several decades, and survival rates in pediatric patients are typically superior to those seen in adult ESKD patients (1-5). This difference is often attributed to the fact that pediatric patients have fewer comorbidities. Although the specific comorbidities that contribute to higher hospitalization rates and mortality in adults may not be common in children with ESKD, other comorbidities—including those associated with syndromes—are certainly present. Our study demonstrates that a third of pediatric CPD patients in a large international cohort have at least 1 reported comorbidity.
The most common comorbidity was cognitive impairment, with 15.5% of patients having some cognitive abnormality. For 9% of the patients, a cardiac abnormality was reported, and for 4%, a pulmonary abnormality. Of the 150 patients with an identified syndrome (8.2%), a large proportion had at least 1 nonrenal comorbidity. However, comorbidities were not limited to syndromic patients. Patients with a comorbidity were more likely than those without to have CAKUT as the cause of their ESKD, and although some of the comorbidities may be related to the oligohydramnios or early development of chronic kidney disease often seen with CAKUT, co-existence of these comorbidities may also support the notion that genetic factors might be implicated in both kidney malformation and other organ dysfunction or malformation in a significant number of patients (6,7).
The data reported here reinforce the results of earlier small studies that emphasized the impact of comorbidities on outcomes in children with ESKD (8-10). In one of the studies, which reviewed the course of 137 children initiating dialysis at less than 6 years of age, the presence of pulmonary disease or hypoplasia (or both) was significantly associated with an increased risk for death (8). In a more recent retrospective study of 52 patients who initiated CPD during infancy and who were treated at a single center over a 25-year period, the patients with comorbidities (including pulmonary hypoplasia, central nervous system disease, or a recognized syndrome) also had a significantly increased risk for mortality (9). Both of the foregoing studies focused on the outcomes of infants on dialysis, because data from large multi-center registries have consistently demonstrated that, among children on dialysis, the youngest age group has the highest risk for mortality (1-5). In a single-center study from Taiwan of 29 pediatric dialysis patients, including older children and adolescents, Tsai et al. demonstrated in univariate analysis that the presence of comorbid disease was associated with an increased risk for mortality; however, in the multivariate analysis of that relatively small cohort, only low serum albumin, and not comorbidity, was independently associated with mortality (10).
Although several studies have used large registries and databases to demonstrate important associations between clinical parameters (including low albumin, anemia, and poor linear growth) and poor outcomes in pediatric dialysis patients, the registries involved do not routinely collect data concerning the types of comorbidities typically seen in pediatric ESKD patients (11-16). The IPPN is the only multicenter registry to capture data on the presence of those comorbidities, making it possible, for the first time, to study the potential impact of comorbidities on outcomes in pediatric CPD patients beyond the newborn period. In fact, in a univariate analysis, the data demonstrate that the presence of a comorbidity (compared with an absence of comorbidities) is associated with a significantly higher hospitalization rate and a higher rate of mortality. The data are largely descriptive and multivariate analyses are clearly required to better define the impact of the comorbidities on outcomes. In particular, the effects of age, regional variations in determining eligibility for dialysis and indications for hospitalization, and other clinical parameters associated with increased risk for poor outcomes will need to be accounted for. As the registry matures, additional data on hospitalization and mortality may support such analyses. Notably, regional variations in the prevalence of comorbidities among patients in the IPPN are discussed in an accompanying article in this issue of Peritoneal Dialysis International (17).
Our study has several other limitations. The registry is voluntary, and therefore patient enrollment and submission of patient updates and terminations may not be complete. In fact, nearly 700 patients did not have an at least 2 updates at the close of the data entry for the present study, and they were excluded from the hospitalization analysis. Enrollment in the study was not limited to incident patients, and therefore patients who died before a center joined the registry or before consent to enroll could be obtained would not have been captured. In addition, deaths that occurred after termination because of transfer to hemodialysis, kidney transplantation, or transfer to another facility were also not captured. The survival analysis therefore focused on survival only beginning at the time of entry into the registry, and patients were censored at termination. In addition, the presence of comorbidities may have been underreported. However, to minimize underreporting, the online data entry is structured to prompt for a response—either “yes” or “no”—in each comorbidity category; and in fact, only 5 of the 1844 patients entered into the registry at the time of the present analysis did not have data entered for at least 1 comorbidity. Finally, although participation in the IPPN is open to any center caring for pediatric CPD patients, participation is not universal, and the demographics and characteristics (including comorbidities) of the patients cared for at the participating centers may not be representative of all children with ESKD. Clearly, the present study does not include children on hemodialysis or with a functioning kidney graft. However, the study does represent the largest cohort of pediatric CPD patients about whom comorbidity data have been collected. Additional strengths of the analysis are the international nature of the registry and the rigorous nature of the data validation that is performed, including additional checks for plausibility for all variables included in the current analyses.
CONCLUSIONS
Our analysis demonstrates that up to one third of pediatric patients receiving CPD in pediatric centers around the globe have at least 1 co-existing nonrenal comorbidity and that many have multiple comorbidities. The presence of comorbidities appears to be associated with a higher hospitalization rate and a lower patient survival rate. Additional data collection and analyses should more clearly define the impact of comorbidities compared with those of modifiable clinical parameters (including dialysis adequacy and hemoglobin, fluid, and blood pressure control) on outcomes in this complex patient population.
The following principal investigators are contributing to the IPPN registry:
Argentina: L. Alconcher, Hospital Interzonal General, Bahia Blanca; E. Sojo, Hospital de Pediatria Garrahan, Buenos Aires; P.A. Coccia, Hospital Italiano de Buenos Aires; A. Suarez, Hospital de Niños Sor Maria Ludovica, La Plata; P.G. Valles, Hospital Pediatrico Humberto Notti, Mendoza; R. Salim, Rennius SA Salta. Belgium: K. van Hoeck, University Hospital Antwerp, Edegem. Brazil: V. Koch, Hospital das Clinicas FMUSP, Sao Paulo. Canada: J. Feber, Children’s Hospital of Eastern Ontario, Ottawa; D.A. Geary, Hospital for Sick Children, Toronto; C. White, BC Children’s Hospital, Vancouver. Chile: M. Valenzuela, Hospital Guillermo Grant Benavente, Concepcion; J. Villagra, Hospital Base, Osorno; F. Cano, Hospital Luis Calvo Mackenna, Santiago; M.A. Contreras, Roberto del Rio Hospital, Santiago; A. Vogel, Pontivicia Universidad Catolica de Chile, Santiago; P. Zambrano, Hospital Dr. Gonzales Cortes, Santiago; P. Berrocal, Hospital Sotero del Rio, Santiago. PR China: Kei-Chiu Tse, Princess Margret Hospital, Hong Kong; H. Xu, Children’s Hospital of Fudan University, Shanghai. Czech Republic: K. Vondrak, University Hospital Motol, Prague. Finland: K. Rönnholm, Hospital for Children and Adolescents, Helsinki. France: J. Harambat, Hôpital des Enfants, Bordeaux; B. Ranchin, Hôpital Femme Mère Enfant, Lyon; G. Roussey, CHU Nantes; T. Ulinski, Armand Trousseau Hospital, Paris; M. Fischbach, Children’s Dialysis Center, Strasbourg. Germany: R. Büscher, Children’s Hospital Essen; M. Kemper, University Medical Center, Hamburg; L. Pape, Medical School, Hannover; F. Schaefer and D. Borzych, Center for Pediatrics and Adolescent Medicine, Heidelberg; J. Misselwitz, Kidney Center for Children and Adolescents; G. Klaus, University Hospital, Marburg; D. Haffner, University Children’s Hospital, Rostock. Greece: F. Papachristou, Aristoteles University, Thessaloniki. Hungary: A. Szabo, 1st Department of Pediatrics, Semmelweis University, Budapest. India: A. Bagga, All India Institute of Medical Sciences, New Delhi; M. Kanitkar, Armed Forces Medical College, Pune. Italy: E. Verrina, G. Gaslini Institute, Genova; A. Edefonti, Fondazione Ospedale Maggiore Policlinico, Milano; E. Vidal, Pediatric Nephrology, Dialysis and Transplant Unit, Padova; G. Leozappa, Dipartimento Nefrologia-Urologia, Rome. Israel: D. Landau, Soroka Medical Center, Beer-Sheva. Korea: I.S. Ha, Dialysis Center for Children and Adolescents, Seoul; K.H. Paik, Samsung Medical Center, Seoul. Lebanon: B. Aoun, Rafik Hariri University Hospital, Beirut. Macedonia: E. Sahpazova, Pediatric Clinic, Skopje. Mexico: L. Sànchez Barbosa, Pediatric Hospital Medical Center SXXI, Cuahutemoc. New Zealand: W. Wong, Starship Children’s Hospital, Auckland. Netherlands: J.W. Groothoff, Academic Medical Center, Amsterdam. Nicaragua: Y. Silva, Hospital Infantil de Nicaragua, Managua. Peru: R. Loza Munarriz, Cayetano Heredia Hospital, Lima, Peru. Poland: A.M. Zurowska and D. Borzych, Medical University, Gdańsk; D. Drozdz, University Children’s Hospital, Krakow; M. Lipka, Children’s Memorial Health Institute, Warsaw; H. Ziolkowska, Public Paediatric Teaching Hospital, Warsaw; M. Sczepanska, Dialysis Division for Children, Zabrze. Romania: O. Brumariu, St. Maria Children’s Hospital, Iasi. Saudi Arabia: J. Kari, King AbdulAziz University Hospital, Jeddah. Singapore: H.K. Yap, Shaw-NKF-NUH Children’s Kidney Center. Spain: G. Ariceta, Hospital de Cruces, Baracaldo; F. Santos, Hospital Universitario Central de Asturias, Oviedo. Turkey: N. Besbas, Hacettepe University, Ankara; S. Bakkaloglu, Gazi University, Ankara; I. Bilge, Department of Pediatric Nephrology, Çapa-Istanbul; E. Serdaroglu, Dr. Behcet Children Research and Educational Hospital, Izmir; A. Bal, Tepecik Children and Research Hospital, Izmir; S. Mir, Ege University Faculty of Medicine, Izmir-Bornova. United Kingdom: L. Rees, Great Ormond Street Hospital, London; A.R. Watson, Children and Young People’s Kidney Unit, Nottingham. Uruguay: J. Grünberg, SE.N.NI.AD, Montevideo. United Arab Emirates: E. Simkova, Dubai Hospital. United States: L. Greenbaum, Children’s Healthcare Pediatric Dialysis Unit, Atlanta; A. Neu, Johns Hopkins Hospital, Baltimore; D. Askenazi, Children’s Hospital of Alabama, Birmingham; M. Ferris, University of North Carolina, Chapel Hill; H. Patel, Children’s Hospital, Columbus; S. Al-Akash, Driscoll Children’s Hospital, Corpus Christi; S. Pottoore, Children’s Medical Center, Dallas; V. Dharnidharka, Division of Pediatric Nephrology, Gainesville; T. Bunchman, Helen DeVos Children’s Hospital, Grand Rapids; A. Chua, Texas Children’s Hospital, Houston; B.A. Warady, Children’s Mercy Hospital, Kansas City; N. McAfee, Seattle Children’s Hospital; J. Zaritsky, UCLA Medical Center, Los Angeles.
DISCLOSURES
The authors have no financial conflicts of interest to report.
Acknowledgments
The authors gratefully acknowledge the support of the International Society for Peritoneal Dialysis, Baxter Health Care, Fresenius Medical Care, Ipsen, Pfizer, IBM, and Sandoz. We also appreciate the continued dedicated support of the IPPN by the members of the medical and nursing staffs in all collaborating centers.
REFERENCES
- 1. McDonald SP, Craig JC. on behalf of Australian and New Zealand Paediatric Nephrology Association. Long-term survival of children with end-stage renal disease. N Engl J Med 2004; 350:2654–62 [DOI] [PubMed] [Google Scholar]
- 2. United States Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, U.S. Renal Data System (USRDS). 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: USRDS; 2011. [Available online at: http://www.usrds.org/atlas.aspx; accessed 10 May 2012] [Google Scholar]
- 3. North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS). 2011 Annual Dialysis Report. Rockville, MD: EMMES Corporation; 2011. [Available online at: https://web.emmes.com/study/ped/annlrept/annualrept2011.pdf; accessed 10 May 2012] [Google Scholar]
- 4. Samuel SM, Tonelli MA, Foster BJ, Alexander RT, Nettel-Aguirre A, Soo A, et al. on behalf of the Pediatric Renal Outcomes Canada Group. Survival in pediatric dialysis and transplant patients. Clin J Am Soc Nephrol 2011; 6:1094–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harambat J, van Stralen KJ, Kim JJ, Tizard EJ. Epidemiology of chronic kidney disease in children. Pediatr Nephrol 2012; 27:363–73 [Erratum in: Pediatr Nephrol 2012; 27:507] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tabatabaeifar M, Schlingmann KP, Litwin M, Emre S, Bakkaloglu A, Mehls O, et al. on behalf of ESCAPE Trial Group. Functional analysis of BMP4 mutations identified in pediatric CAKUT patients. Pediatr Nephrol 2009; 24:2361–8 [DOI] [PubMed] [Google Scholar]
- 7. Weber S, Moriniere V, Knüppel T, Charbit M, Dusek J, Ghiggeri GM, et al. Prevalence of mutations in renal developmental genes in children with renal hypodysplasia: results of the ESCAPE study. J Am Soc Nephrol 2006; 17:2864–70 [DOI] [PubMed] [Google Scholar]
- 8. Wood EG, Hand M, Briscoe DM, Donaldson LA, Yiu V, Harley FL, et al. on behalf of the North American Pediatric Renal Transplant Cooperative Study. Risk factors for mortality in infants and young children on dialysis. Am J Kidney Dis 2001; 37:573–9 [PubMed] [Google Scholar]
- 9. Hijazi R, Abitbol CL, Chandar J, Seeherunvong W, Freundlich M, Zilleruelo G. Twenty-five years of infant dialysis: a single center experience. J Pediatr 2009; 155:111–17 [DOI] [PubMed] [Google Scholar]
- 10. Tsai HL, Yang LY, Chin TW, Wang HH, Liu CS, Wei CF, et al. Outcome and risk factors for mortality in pediatric peritoneal dialysis. Perit Dial Int 2010; 30:233–9 [DOI] [PubMed] [Google Scholar]
- 11. Warady BA, Ho M. Morbidity and mortality in children with anemia at initiation of dialysis. Pediatr Nephrol 2003; 18:1055–62 [DOI] [PubMed] [Google Scholar]
- 12. Amaral S, Hwang W, Fivush B, Neu A, Frankenfield D, Furth S. Association of mortality and hospitalization with achievement of adult hemoglobin targets in adolescents maintained on hemodialysis. J Am Soc Nephrol 2006; 17:2878–85 [DOI] [PubMed] [Google Scholar]
- 13. Amaral S, Hwang W, Fivush B, Neu A, Frankenfield D, Furth S. Serum albumin level and risk for mortality and hospitalization in adolescents on hemodialysis. Clin J Am Soc Nephrol 2008; 3:759–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong CS, Hingorani S, Gillen DL, Sherrard DJ, Watkins SL, Brandt JR, et al. Hypoalbuminemia and risk of death in pediatric patients with end-stage renal disease. Kidney Int 2002; 61:630–7 [DOI] [PubMed] [Google Scholar]
- 15. Furth SL, Hwang W, Yang C, Neu AM, Fivush BA, Powe NR. Growth failure, risk of hospitalization and death for children with end-stage renal disease. Pediatr Nephrol 2002;17:450–5 [DOI] [PubMed] [Google Scholar]
- 16. Furth SL, Stablein D, Fine RN, Powe NR, Fivush BA. Adverse clinical outcomes associated with short stature at dialysis initiation: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatrics 2002; 109:909–13 [DOI] [PubMed] [Google Scholar]
- 17. Schaefer F, Borzych-Duzalka D, Azocar M, Loza Munarriz R, Sever L, Aksu N, et al. on behalf of the IPPN investigators. Impact of global economic disparities on practices and outcomes of chronic peritoneal dialysis in children: insights from the International Pediatric Peritoneal Dialysis Network registry. Perit Dial Int 2012; 32:399–409 [DOI] [PMC free article] [PubMed] [Google Scholar]


