Peritoneal dialysis (PD)-related peritonitis is a common morbid complication of PD. Culture-negative peritonitis should not represent more than 20% of episodes (1). In our hospital, the ratio of culture-negative peritonitis has been declining in recent years. Here, we discuss the reasons for that improvement.
METHODS
The hospital records of all patients who underwent peritoneal dialysis (PD) catheter placement from February 2004 to January 2011 were retrospectively analyzed. The 228 patients who developed PD-related peritonitis were included in the study. The diagnosis of PD-related peritonitis was based on standard criteria—that is, the presence of 2 or 3 of these signs: peritoneal inflammation; positive gram stain or culture of PD effluent (or both); and an effluent cell count with white blood cells exceeding 100/mm3 (after a dwell time of at least 2 hours), with at least 50% polymorphonuclear neutrophilic cells (2). Patients with peritonitis received an empirical intraperitoneal antibiotic regimen according to the International Society for Peritoneal Dialysis guideline (3), specifically: intermittent amikacin 2 mg/kg (in 1 daily exchange), intermittent cefazolin 15 mg/kg (in 1 daily exchange), or vancomycin 15 mg/kg every 5 days. Prophylaxis to prevent exit-site infections involved the application of mupirocin to the exit site twice daily for 5 - 7 days, and in all patients, the application of gentamicin cream daily at the exit site after cleaning. Additionally, we used a single dose of intravenous vancomycin (1 g) at the time of catheter placement to decrease the risk of subsequent infection.
Infections were classified as relapse or recurrent peritonitis. Peritonitis relapse was defined as an episode occurring within 4 weeks of completion of antibiotic therapy (or within 5 weeks if intermittent vancomycin therapy had been used) for a prior episode with the same organism or 1 culture-negative episode. Peritonitis-related death was recorded if the patient’s death was directly attributable to peritonitis in the clinical opinion of the treating nephrologist. A peritonitis episode was considered “cured” by antibiotics alone if the patient was symptom free, the PD effluent was clear, and the episode was not complicated by relapse, catheter removal, or death (4).
The peritonitis rate was calculated by dividing the sum of the number of months each patient spent on dialysis by the total number of peritonitis episodes experienced by all patients and is reported as the interval in months between episodes. Relapses were not counted as 2 separate episodes, but as 1 episode (5).
Of the 228 patients, 161 (70.6%) were treated with continuous ambulatory PD, and 67 (29.4%), with automated PD. The PD catheters had been placed using percutaneous technique in 69 patients (30.3%) and using conventional surgical technique in 159 patients (69.7%).
RESULTS
Of the 228 patients, 118 (51.8%) were men, and 110 (48.2%) were women. The mean age of the patients was 50.3 ± 14.4 years. The causes of end-stage renal disease were diabetes mellitus in 71 patients (31.1%), hypertension in 39 (17.1%), amyloidosis in 8 (3.5%), glomerulonephritis in 5 (2.2%), polycystic kidney disease in 5 (2.2%), others in 14 (6.1%), and unknown in the remaining patients.
These 228 PD patients developed 491 peritonitis episodes (51 patients had no episodes of peritonitis during the study period). Initial cure was achieved in 452 of the 491 peritonitis episodes (92.1%). Peritonitis was culture-negative in 28 episodes (20.2%) with use of antibiotics during the preceding 30 days. Six patients (1.2%) recovered with antibiotic treatment plus simultaneous PD catheter removal and replacement, with maintenance of PD. Catheter removal without simultaneous replacement was performed in 28 patients (5.7%), with 24 of them (4.9%) being switched to hemodialysis; the other 4 patients (0.8%) died during the course of their treatment for PD-related peritonitis. Another 5 patients (1.0%) died without catheter removal—that is, 9 patients in total (1.8%) died during the course of treatment for PD-related peritonitis. Recurrence of PD-related peritonitis was observed in 6 patients (1.2%).
Seasonally, 126 peritonitis episodes (25.7%) developed in spring; 129 (26.3%), in summer; 85 (17.3%), in autumn; and 151 (30.7) in winter. The overall peritonitis rate was 1 episode in 25.6 patient-months.
Table 1 shows the organisms causing PD-related peritonitis. Gram-positive bacteria are the most common. After separating the episodes into two groups, culture-positive and culture-negative, we observed no significant differences between the groups in terms of relapse, catheter removal rate, season, or death (Table 2).
TABLE 1.
Causative Organisms in Peritoneal Dialysis-Related Peritonitis
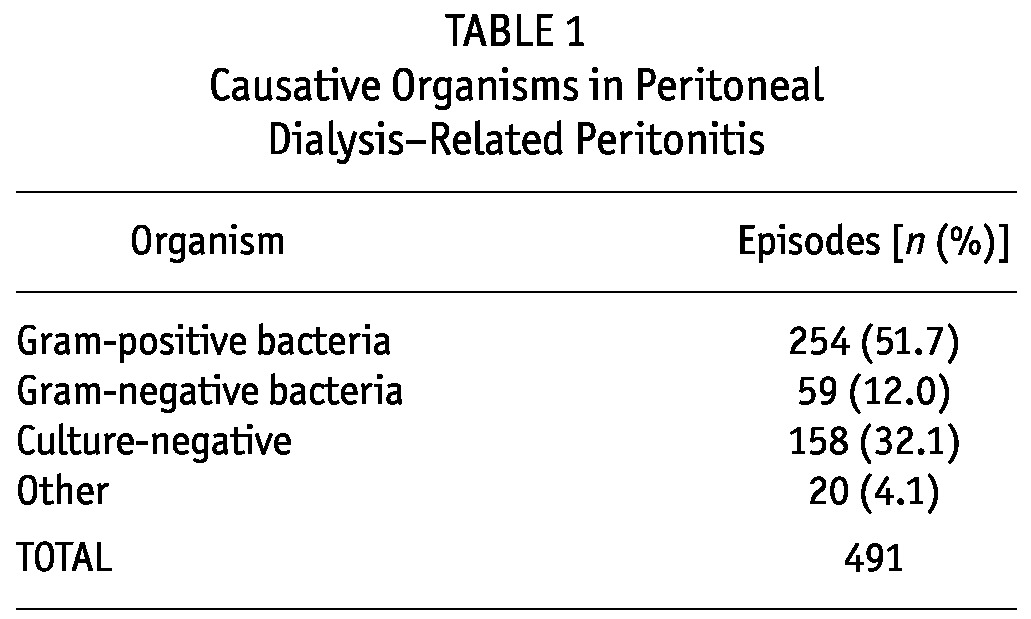
TABLE 2.
Comparison of Culture-Negative and Culture-Positive Peritonitis Episodes
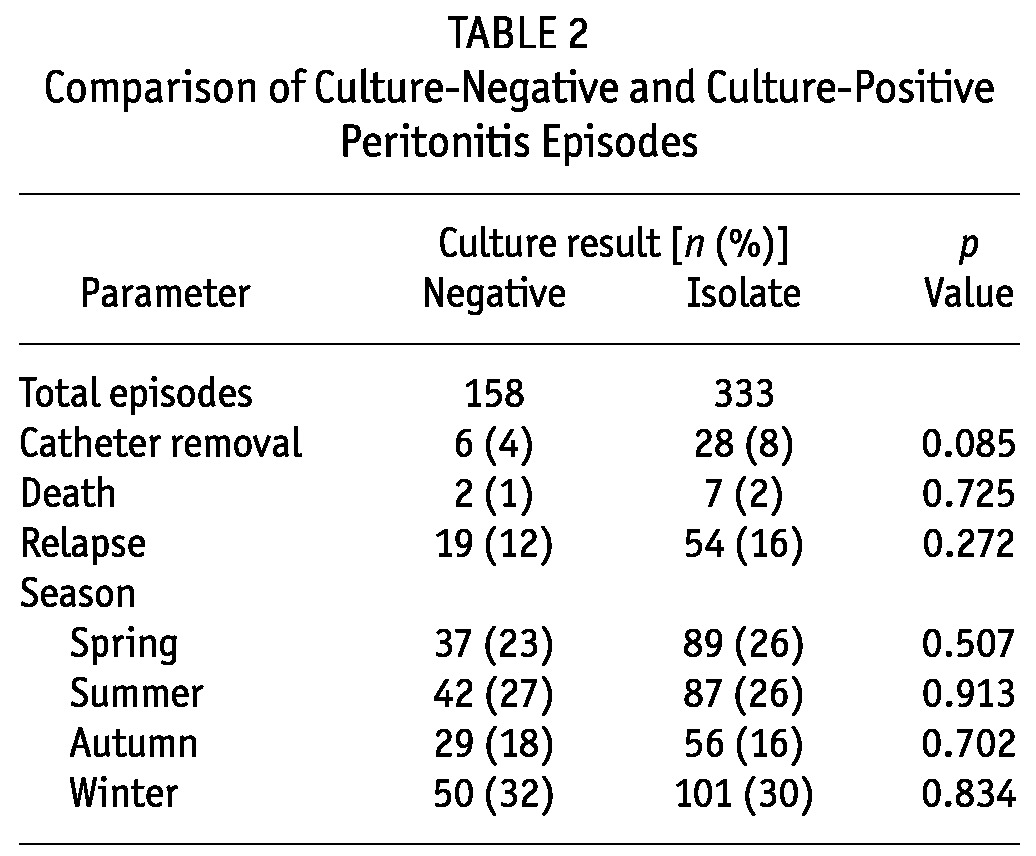
Figure 1 shows the ratio of culture-negative peritonitis episodes during 2004 - 2010. In 2004, it was 40.5%; by 2010, it had declined to 18.8%. There was also a significant difference in the culture-negative peritonitis rate before and after 2006: 88 of 220 episodes (40%) compared with 70 of 271 episodes (26%) respectively (p < 0.001).
Figure 1.
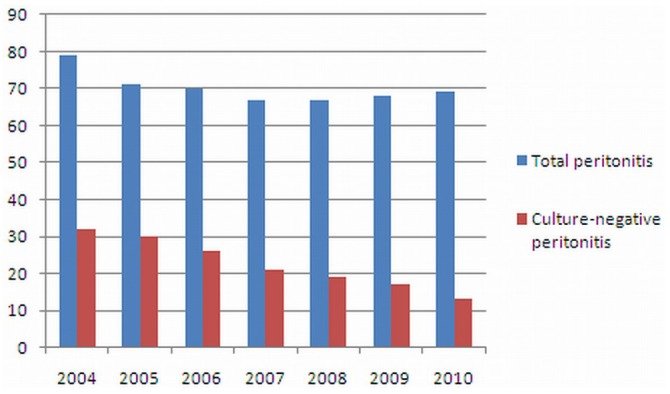
— Culture-negative peritonitis episodes compared with total peritonitis episodes, 2004 - 2010.
DISCUSSION
Culture-negative peritonitis may be the result of either infectious or noninfectious causes. Infectious culture-negative peritonitis may occur after recent antibiotic exposure, secondary to suboptimal sample collection or culture methods, or because of unusual organisms such as fungi; mycobacteria; Legionella, Campylobacter, or Ureaplasma species; Mycoplasma; or enteroviruses (3). Noninfectious culture-negative peritonitis may reflect chemical irritation (caused, for example, by icodextrin), collection of PD fluid from a “dry” abdomen, or other noninfectious causes of cloudy dialysate such as chylous ascites or effluent eosinophilia (3,6).
One reason for the observed fall in culture-negative PD-related peritonitis cases might be improvements in the laboratory environment. It is conceivable that, because of fewer samples and perhaps a lack of microbiology expertise specific to this area, smaller PD centers might be culturing PD effluent suboptimally, thereby increasing the culture-negative peritonitis rate. The importance of culture technique in improving the micro-organism identification rate was shown first by Sewell et al. (7), who found no significant difference between Bactec (Becton-Dickinson, Mountain View, CA, U.S.A.) blood culture bottle-based techniques (with or without effluent centrifugation) and large-volume dialysate culture (94% vs 82% vs 88% respectively); however, they showed that direct inoculation of sediment from centrifuged PD effluent onto plated media produced a significantly lower culture-positive rate (65%, p < 0.05). By contrast, Lye et al. (8) showed that a significantly higher identification rate was achieved using Bactec blood culture bottles inoculated with 5 mL well-mixed effluent compared with direct inoculation of sediment from 50 mL centrifuged PD effluent onto plated media (75% vs. 58%, p = 0.05).
After 2005, the standard culture technique—using blood culture bottles, but a large-volume sample (the sediment from centrifuging 50 mL effluent)—was recommended. We started using that technique in 2006, and the change might have further improved the recovery of micro-organisms.
We also found that, although we did not recommend use of an antibiotic before samples were collected, patients with abdominal pain were administering antibiotics to themselves. The antibiotics were usually left over from a previous peritonitis episode. The technique for acquiring cultures was changed right after the 2005 guideline. However, the self-administration of antibiotics by patients remained a problem that took time to solve and for which we increased the frequency of patient education programs. In the education programs, we focused on this particular issue, and the clinic nurses visited the patients’ homes more often thereafter. The members of the clinic staff, especially the renal nurses, were also educated about culture acquisition techniques, and their attention to culture-negative peritonitis episodes increased. In addition, the number of nurses attending to the patients has also increased over the years.
In a study Szeto et al. (9), 26.4% of the peritonitis episodes had a history of antibiotic use in the preceding 30 days; in the study by Fahim et al., the rate was 19.7%. In the present study, 20.2% of peritonitis episodes were preceded by antibiotic therapy in the 30 days before the episode, which accords with other findings the literature.
CONCLUSIONS
Most cases of culture-negative peritonitis in PD patients can be explained by recent antibiotic therapy or by technical problems during effluent culture. Here, we report a decline in culture-negative peritonitis over 7 years that is thought to be related to a decrease in antibiotic use before cultures are obtained and an increase in staff and patient consciousness about the issue.
DISCLOSURES
The authors declare that no financial conflicts of interest exist.
REFERENCES
- 1. Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30:393–423 [Erratum in: Perit Dial Int 2011; 31:512] [DOI] [PubMed] [Google Scholar]
- 2. Rüger W, van Ittersum FJ, Comazzetto LF, Hoeks SE, ter Wee PM. Similar peritonitis outcome in CAPD and APD patients with dialysis modality continuation during peritonitis. Perit Dial Int 2011; 31:39–47 [DOI] [PubMed] [Google Scholar]
- 3. Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, Holmes C, et al. Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int 2005; 25:107–31 [PubMed] [Google Scholar]
- 4. Fahim M, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Culture-negative peritonitis in peritoneal dialysis patients in Australia: predictors, treatment, and outcomes in 435 cases. Am J Kidney Dis 2010; 55:690–7 [DOI] [PubMed] [Google Scholar]
- 5. Liakopoulos V, Stefanidis I, Dombros NV. Peritoneal dialysis glossary 2009. Int Urol Nephrol 2009; 42:417–23 [DOI] [PubMed] [Google Scholar]
- 6. Rocklin MA, Teitelbaum I. Noninfectious causes of cloudy peritoneal dialysate. Semin Dial 2001; 14:37–40 [DOI] [PubMed] [Google Scholar]
- 7. Sewell DL, Golper TA, Hulman PB, Thomas CM, West LM, Kubey WY, et al. Comparison of large volume culture to other methods for isolation of microorganisms from dialysate. Perit Dial Int 1990; 10:49–52 [PubMed] [Google Scholar]
- 8. Lye WC, Wong PL, Leong SO, Lee EJ. Isolation of organisms in CAPD peritonitis: a comparison of two techniques. Adv Perit Dial 1994;10:166–8 [PubMed] [Google Scholar]
- 9. Szeto CC, Wong TY, Chow KM, Leung CB, Li PK. The clinical course of culture-negative peritonitis complicating peritoneal dialysis. Am J Kidney Dis 2003; 42:567–74 [DOI] [PubMed] [Google Scholar]


