The number of patients with a longer peritoneal dialysis (PD) duration is currently growing because of improved survival rates worldwide. To our knowledge, a longer PD duration is associated with alterations of the peritoneal membrane and peritoneal macrophages (1), potentially decreasing the immune defense against peritonitis. However, the current evidence on this issue is conflicting. Earlier studies have reported that a longer PD duration predicts worse (2,3) or better (4) peritonitis outcomes or has no effect (5,6).
Notably, recent studies have indicated that first and subsequent peritonitis episodes represent distinct clinical entities (7,8). First episodes of peritonitis have better outcomes than do subsequent episodes—particularly episodes of recurrent peritonitis (7). We hypothesized that different proportions of first and subsequent peritonitis episodes have possibly led to the paradoxical findings across PD centers in earlier studies.
We therefore aimed to determine whether the influence of PD therapy duration on peritonitis outcomes is different for first and subsequent peritonitis episodes.
METHODS
Our study was performed at the PD center of Peking University First Hospital. All consecutive PD patients who presented with PD-associated peritonitis between 1 January 2008 and 30 April 2011 were recruited. Peritonitis was defined as the presence of at least two of the following conditions (9):
Abdominal pain or tenderness
Presence of white blood cells (100/mL or more) in peritoneal effluent, with at least 50% polymorphs
Positive effluent culture results
All patients had undergone Tenckhoff catheter insertion and were dialyzed by continuous ambulatory PD using lactate-buffered glucose dialysate and a twin-bag connection system (Baxter Healthcare, Guangzhou, China).
We recorded patient age, sex, diabetes status, duration of PD before the onset of peritonitis, residual renal function (RRF), biochemical parameters (such as serum albumin and hemoglobin) measured no more than 3 months before the onset of peritonitis, PD effluent cell count, and causative micro-organisms.
Peritonitis episodes were treated using the standard antibiotic protocol at our center, modified from the International Society for Peritoneal Dialysis guidelines (9). Routine follow-up visits were scheduled on days 1, 3, 5, 7, 14, and 21 after initiation of treatment for peritonitis. Treatment success was defined as complete resolution of peritonitis (effluent white blood cells below 100/mL and clinical resolution for the patient) without the need for Tenckhoff catheter removal. Conversely, treatment failure included patients who either died from a peritonitis episode or were transferred to hemodialysis.
After determining the median PD duration for patients experiencing a 1st, 2nd, 3rd, and 4th-to-8th peritonitis episode (26.7 months, 34.8 months, 46.1 months, and 50 months respectively), we allocated the patients to one of two groups depending on whether they had a shorter PD duration (shorter than the median) or a longer PD duration (longer than median) before a 1st episode (n = 62 and n = 62 respectively) or a subsequent episode of peritonitis [2nd episode, n = 28 and n = 29 respectively; 3rd episode, n = 17 and n = 17 respectively; and 4th-to-8th episode (analyzed together because of the relatively small sample size), n = 16 and n = 17 respectively].
The study was approved by the Medical Ethical Committee of Peking University. Written informed consent was obtained from each patient.
RESULTS
Between 1 January 2008 and 30 April 2011, 248 episodes of peritonitis occurred, including 124 first peritonitis episodes and 124 subsequent peritonitis episodes (including 57 second episodes, 34 third episodes, 15 fourth episodes, 11 fifth episodes, 4 sixth episodes, 2 seventh episodes, and 1 eighth episode). Over this period, the peritonitis rate at our center was 1 episode in 55.5 patient-months.
In 1st peritonitis episodes, members of the longer-duration group were, compared with the shorter-duration group, less likely to be male (32.2% vs 50%, p = 0.045) or diabetic (25.8% vs 53.2%, p = 0.002), and they had lower RRF (0.07 mL/min vs 0.49 mL/min, p < 0.001). Mean age, hemoglobin, serum albumin, total Kt/V, spectrum of pathogenic bacteria, and effluent white cell counts on each follow-up day were not significantly different between the groups. The dropout rate was also not significantly different between the longer-duration group and the shorter-duration group (25.8% vs 17.7%, p = 0.28). After adjustments for age, sex, albumin, causative organisms, presence of diabetes, and RRF, the longer-duration group still had a dropout risk similar to that of the shorter-duration group [adjusted odds ratio (OR): 1.17; 95% confidence interval (CI): 0.35 to 3.95; p = 0.80; Table 1). If PD duration was treated as continuous variable (to avoid the bias produced when duration was arbitrarily divided into “shorter” and “longer”), it still could not predict PD outcome (adjusted OR: 1.00 for every 1-month increase in PD duration; 95% CI: 0.98 to 1.02; p = 0.98).
TABLE 1.
Dropout Rate at First and Subsequent Peritonitis Episodes by Duration (Shorter or Longer) of Peritoneal Dialysis (PD)
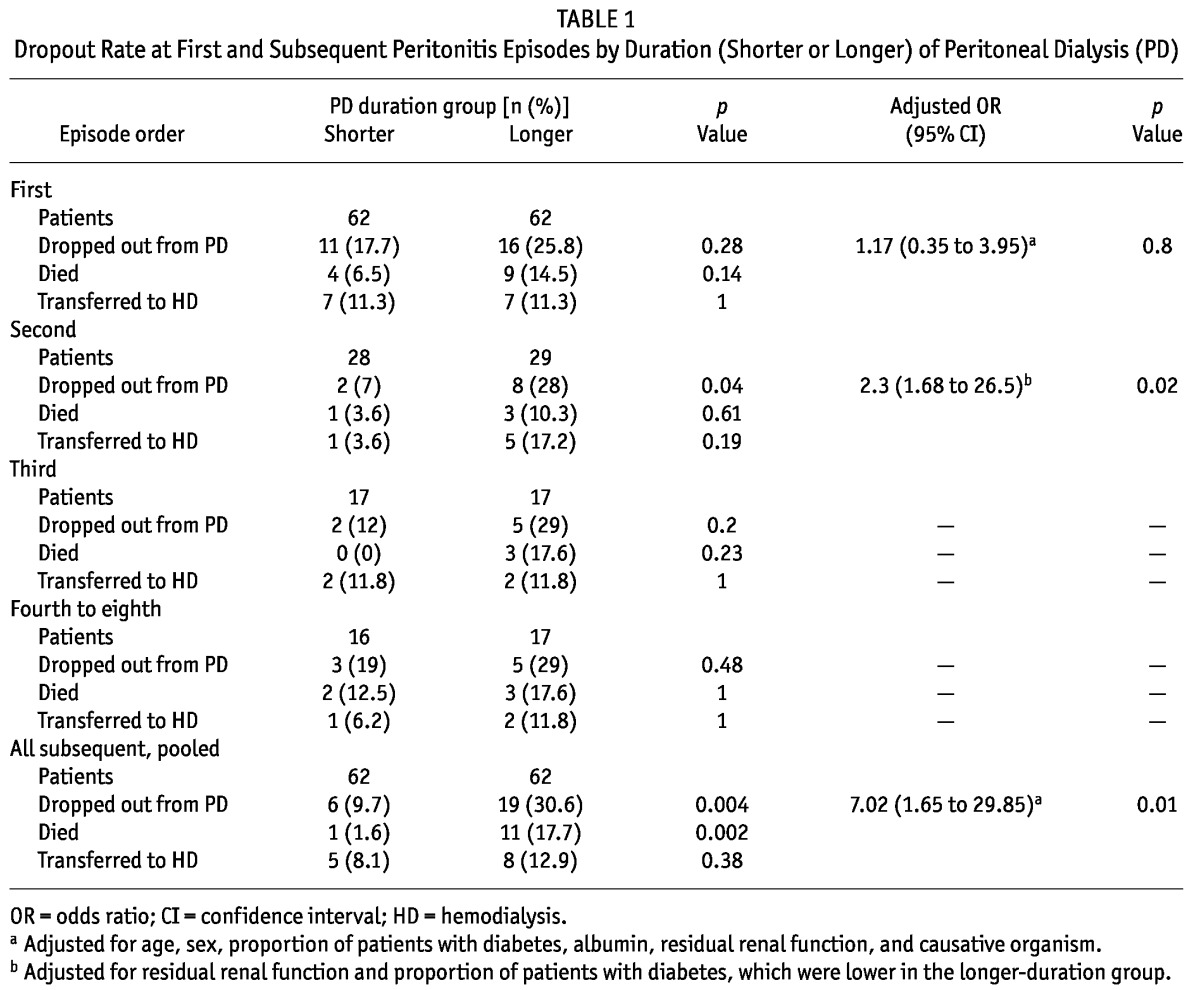
In 2nd episodes, the members of the longer-duration group were, compared with the shorter-duration group, less likely to be diabetic (17.2% vs 67.9%, p < 0.001), and they had lower RRF (0.00 mL/min vs 0.29 mL/min, p < 0.05). In 4th-to-8th episodes, the members of the longer-duration group were less likely to be diabetic (47.1% vs 81.2%, p = 0.04), and they had lower hemoglobin (10.4 g/dL vs 11.9 g/dL, p = 0.02) and RRF (0.00 mL/min vs 0.16 mL/min, p = 0.04). The mean age, proportion of men and women, serum albumin, total Kt/V, spectrum of pathogenic bacteria, and effluent white cell counts on each follow-up day were not significantly different between the shorter-duration and longer-duration groups in all subsequent episodes. In 2nd episodes, the longer-duration group had a significantly higher dropout rate (28% vs 7%, p = 0.04) even after adjustment for the lower proportion of diabetic patients and the lower RRF (adjusted OR: 2.3; 95% CI: 1.68 to 26.5; p = 0.02; Table 1). In the 3rd and 4th-to-8th episode groups, the dropout rate was not significantly higher in the “longer duration” group (29% vs 12%, p = 0.20, and 29% vs 19%, p = 0.48, respectively). The sample sizes were insufficient to calculate an adjusted risk for PD dropout for the 3rd and 4th-to-8th episode groups.
If subsequent peritonitis episodes were analyzed as a whole (at a median PD duration of 43.2 months), the longer-duration group had a significantly higher dropout rate (30.6% vs 9.7%, p = 0.004) even after adjustment for age, sex, albumin, causative organisms, presence of diabetes, and RRF (adjusted OR: 7.02; 95% CI: 1.65 to 29.85; p = 0.01; Table 1). If PD duration was treated as a continuous variable, it was still an independent predictor for PD dropout (adjusted OR: 1.029 for every 1 month increase in PD duration; 95% CI: 1.006 to 1.052; p = 0.013).
DISCUSSION AND CONCLUSIONS
Earlier studies have produced conflicting findings in terms of the impact of PD duration on peritonitis outcomes (2-6). We suspected that the heterogeneity of peritonitis episodes across studies might represent a potential bias. The present analysis shows that the impact of longer PD duration on peritonitis outcomes varied when we separated first and subsequent peritonitis episodes. Although a longer PD duration was observed to be associated with a higher dropout rate for subsequent peritonitis, the same association was not present for first peritonitis episodes. Also, it is reasonable to speculate that longer PD duration would probably predict worse outcome in 3rd peritonitis episodes, 4th episodes, and so on, if a sufficient sample size were to be available.
The potential mechanisms for this phenomenon are not clear. It could not be explained by the severity of causative organisms, because the distribution of organisms was not significantly different between the groups. Whether the host defenses of patients with a longer PD duration worsen as the patients experience subsequent episodes of peritonitis needs to be clarified. The present study may contribute to individual tailoring of therapeutic strategies for peritonitis in patients with different PD durations.
DISCLOSURES
The authors have no financial conflicts of interest to declare.
Acknowledgments
The authors express their appreciation to the patients and staff of the PD center of First Hospital, Peking University, for their participation in this study. The study was supported in part by the Homecoming Investigator Award from Peking University, the Ketosteril Research Award from Fresenius Kabi Deutschiand GmbH, and the Baxer Clinical Research Award from Baxter Corporation, China.
REFERENCES
- 1. McGregor SJ, Topley N, Jörres A, Speekenbrink AB, Gordon A, Gahl GM, et al. Longitudinal evaluation of peritoneal macrophage function and activation during CAPD: maturity, cytokine synthesis and arachidonic acid metabolism. Kidney Int 1996; 49:525–33 [DOI] [PubMed] [Google Scholar]
- 2. Krishnan M, Thodis E, Ikonomopoulos D, Vidgen E, Chu M, Bargman JM, et al. Predictors of outcome following bacterial peritonitis in peritoneal dialysis. Perit Dial Int 2002; 22:573–81 [PubMed] [Google Scholar]
- 3. Pérez Fontan M, Rodríguez-Carmona A, García-Naveiro R, Rosales M, Villaverde P, Valdés F. Peritonitis-related mortality in patients undergoing chronic peritoneal dialysis. Perit Dial Int 2005; 25:274–84 [PubMed] [Google Scholar]
- 4. Troidle L, Gorban-Brennan N, Kliger AS, Finkelstein FO. Effect of duration of chronic peritoneal dialysis therapy on the development of peritonitis. Perit Dial Int 1999; 19:376–9 [PubMed] [Google Scholar]
- 5. Kofteridis DP, Valachis A, Perakis K, Maraki S, Daphnis E, Samonis G. Peritoneal dialysis-associated peritonitis: clinical features and predictors of outcome. Int J Infect Dis 2010; 14:e489–93 [DOI] [PubMed] [Google Scholar]
- 6. Zalunardo NY, Rose CL, Ma IW, Altmann P. Higher serum C-reactive protein predicts short and long-term outcomes in peritoneal dialysis-associated peritonitis. Kidney Int 2007; 71:687–92 [DOI] [PubMed] [Google Scholar]
- 7. Szeto CC, Kwan BC, Chow KM, Law MC, Pang WF, Chung KY, et al. Recurrent and relapsing peritonitis: causative organisms and response to treatment. Am J Kidney Dis 2009; 54:702–10 [DOI] [PubMed] [Google Scholar]
- 8. Szeto CC, Kwan BC, Chow KM, Law MC, Pang WF, Leung CB, et al. Repeat peritonitis in peritoneal dialysis: retrospective review of 181 consecutive cases. Clin J Am Soc Nephrol 2011; 6:827–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30:393–423 [Erratum in: Perit Dial Int 2011; 31:512] [DOI] [PubMed] [Google Scholar]


